
Recovery of Mn2+, Co2+ and Ni2+ from manganese nodules by redox leaching and solvent extraction
SHEN Yong-feng(申勇峰)1, 2, 3, XUE Wen-ying(薛文颖)4, LI Wei(李 薇)3,LI Shan-de(李禅德)2, LIU Xiang-hua(刘相华)4
1. Key Laboratory for Anisotropy and Texture of Materials (Ministry of Education), Northeastern University, Shenyang 110004, China;
2. Jinchuan Nonferrous Metals Complex, Jinchang 737100, China;
3. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
4. State Key Laboratory of Rolling and Automation, Northeastern University, Shenyang 110004, China
Received 8 November 2006; accepted 16 April 2007
Abstract:
The recovery of Mn, Co and Ni from deep-sea manganese nodules was conducted by acid oxidative leaching and solvent extraction. The results indicate that pyrrhotite used during leaching can effectively facilitate the leaching out of manganese, cobalt and nickel. The leaching behaviors of Mn, Ni and Co were determined and the influences of temperature, leaching time and sulphuric acid concentration on leaching rate were also investigated. Co and Ni are precipitated from the leaching liquor by adding sodium sulfide into solution with agitation for 2 h at 50 ℃, and the manganese sulphate is obtained by concentrating the resulting solution. By re-dissolving the precipitates of cobalt and nickel, the separation of cobalt and nickel is performed using di(2-ethylhexyl) phosphoric acid (D2EHPA) for impurities elimination with 8 stages at organic-to-aqueous(O/A) volume ratio of 3?5, and 2- ethylhexyl phosphonic acid mono-2-ethylhexyl ester (known as PC88A or P507) for cobalt extraction with 3 stages counter-current operations at O/A volume ratio of 2?3 followed by their scrubbings and strippings, respectively. The final maximum recovery rates for manganese, cobalt and nickel are 85%, 75% and 78%, respectively.
Key words:
sea nodule; pyrrhotite; extraction; nickel; cobalt; manganese;
1 Introduction
Nickel and cobalt as well as their respective alloys, due to their excellent characteristics such as corrosion resistance, high melting point and unusual magnetic strength, are used for various purposes in industry. However, during the past several decades, an ever- increasing demand for some important nonferrous metals such as cobalt and nickel has lead to a fast depletion of their rich sources. On the other hand, manganese is used to form many important alloys. In steel, manganese improves the rolling and forging qualities, strength, toughness, stiffness, wear resistance, hardness, and hardenability. Manganese sulphate, as a byproduct of cobalt extraction from deep-sea manganese nodules, has wide utilities in medicine, fertilizer, chemical, and catalyzer as well as printing and dyeing [1-2]. The market price of manganese metal (99.95%) is about 500$/kg at present. Therefore, it is worthwhile exploiting leaner sources such as sea nodule or the secondary resource such as rechargeable batteries to recovery valuable metals. Sea manganese modules that contain mainly manganese dioxide and hydrated iron oxide are found beneath the sea floor in parts of all the deep oceans of the world except the Arctic. The presence of other valuable metals such as nickel, cobalt and copper has prompted the researchers to focus on the exploration of processing routes for potential application and the extraction of valuable metals from these nodules as future resource[3-4].
A variety of approaches, including selective leaching by dissolved SO2[5], microorganisms[6], hydrochloric acid[7], electrochlorination[8-9], pyrochemical extraction[10], have been proposed for the metals extraction from deep-sea nodules. A wide variety of organic solvents or their mixtures have been tested for the extraction of nickel(Ⅱ) and cobalt(Ⅱ) from aqueous solutions and cobalt from nickel. Among the acidic organo-phosphoric compounds, di(2-ethylhexyl) phosphoric acid (D2EHPA) and extractant 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (EHEHPA, commercially known as PC88A or P507), have been widely used for separation of Co from Ni[11]. Recently, the simultaneous extraction and separation of cobalt and nickel from manganese sulfate solutions have been carried out using the thio-organophosphinic extractant Cyanex 301 diluted in kerosene[12].
In our pervious investigation, the sulfur dioxide was successfully used to extract cobalt and nickel as well as manganese from sea nodules. However, the potential menace to environment and the relatively expensive cost inhabited SO2 as leachant in production, despite the higher leaching percentages. An alternative strategy is to use the cheap pyrrhotite as leachant because iron dissolved into the solution is continually precipitated, and it is believed that the processes have significant impact performance of the leaching in terms of the acid consumed and then metals are re-leased via reactions involving iron in various oxidation states in both solid and aqueous phases, i.e., iron has a catalytic effect on the leaching of metals[13-15]. For the purpose of scientific interest and commercial need, we conducted the study and the main objective is to present a whole hydrometallurgical process, in which pyrrhotite is used as leachant accompanied with H2SO4, for extraction manganese, cobalt and nickel from deep-sea manganese nodule. The effects of temperature, time and charge ratio on the leaching results are also explored. At the same time, the effect of organic reagents D2EHPA and P507 for separation and purification the solution containing cobalt and nickel is investigated.
2 Experimental
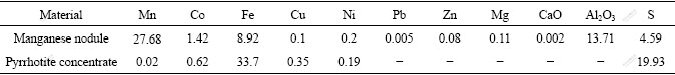
The deep-sea manganese nodule sample was collected from the south sea of China and the pyrrhotite concentrate sample was obtained from the flotation of the nodule that was also collected from the same site but contained lower concentration of cobalt. The nodule consists of black manganese, limonite, and trihydrate alumina as well as a little pyrolusite. The majority of cobalt entrained in the nodules as isomorphism. Chemical compositions of these samples used in the experiments are listed in Table 1. The sulphuric acid solutions used in leaching experiments were kept in concentration range of 0.5-2.0 mol/L organo-phosphoric compounds D2EHPA and P507 were respectively used as extractants for impurities removal and cobalt extraction in this study. The other chemicals used were of analytical grade.
Table 1 Chemical compositions of manganese nodule and pyrrhotite concentrate (mass fraction, %)
In the leaching tests, feed and H2SO4 solution were contacted in a 5 L round bottom glass flask with three openings serving for a feed tube, a thermometer and an overhead stirrer driven by a variable-speed motor. In the end of leaching (pH=1.0-1.5), the air was driven into the bottom of reactor to facilitate the precipitation of iron. The precipitation and re-dissolution experiments were performed in a 5 L beaker with an overhead stirrer.
The organic solution for impurities removal was prepared by dissolving appropriate 10%D2EHPA in 90% distilled kerosene, while the organic phase used for cobalt extraction was prepared by dissolving 25%P507 in 75% distilled kerosene. Selective cobalt extraction was carried out in 125 mL glass flasks at the desired organic to aqueous(O/A) volume ratios. The phases were mixed in sealed flasks and shaken for 5 min using a wrist action shaker at room temperature. The aqueous was separated from the organic phase after settling mixture for 5 min. The stripping experiments of cobalt from the loaded organic phase were performed with 3 mol/L HCl.
The analysis of metal ions in the solution and solid samples was done with the Perkin-Elmer Model 800 atomic absorption spectroscopy(AAS). The analysis of the organic phase was carried out wherever necessary, in which the organic phase was filtered through phase separation paper and a suitable aliquot was stripped with 0.5 mol/L hydrochloric acid followed by dilution and analysis using AAS.
3 Results and discussion 3.1 Leaching out of valuable metals
3.1.1 Chemical background
Pyrrhotite concentrate has been used as reductant for sea manganese nodules leaching. The cobalt that is presented in pyrrhotite is in fact transferred into solution during leaching. In this way, the leaching efficiency of cobalt is increased and this is the additional advantage of using pyrrhotite in this process. The chemical mechanism of the leaching process can be summarized as follows:
FeS+H2SO4=FeSO4+H2S (1)
H2S+MnO2+H2SO4=MnSO4+S+2H2O (2)
3MnO2+2FeS+6H2SO4=3MnSO4+Fe2(SO4)3+6H2O+2S (3)
MnO2+2FeSO4+2H2SO4=MnSO4+Fe2(SO4)3+2H2O (4)
Fe2(SO4)3+4H2O=2FeOOH↓+3H2SO4 (5)
FeS+Fe2(SO4)3=3FeSO4+S (6)
2FeSO4+O2+H2O=Fe2O3↓+2H2SO4 (7)
CoO+H2SO4=CoSO4+H2O (8)
NiO+H2SO4=NiSO4+H2O (9)
Co3O4+4H2SO4+2FeSO4=3CoSO4+4H2O+Fe2(SO4)3 (10)
Co2O3+3H2SO4+2FeSO4=2CoSO4+3H2O+Fe2(SO4)3 (11)
Initially, as observed in pressure oxidation leaching of industrial production[16], acid attacks the pyrrhotite and then the direct oxidation by dissolved oxygen quickly takes over and dominates the dissolution process. The H2S gas initially formed is further oxidized to elemental sulphur in the presence of oxygen and the resulting sulphur is absorbed on the mineral surface, leading to a retarding effect in the process of the reaction. Eventually, the ferric ions attack pyrrhotite as well as nodules to bring cobalt, nickel and manganese into solution. Iron dissolved into the solution is continually precipitated in a form of hematite according to Eqn.(9). It is believed that the processes have significant effect on the leaching in terms of the acid consumed and then metals are re-leased via reactions involving Fe in various oxidation states in both solid and aqueous phases, i.e., Fe has a catalytic effect on the leaching of Ni and Co in this system[13-15]. Simultaneously, most of valuable metals in the nodules dissolve by direct leaching according to Eqns.(10)-(13). Above processes are also consistent with the previous leaching investigations conducted by HAVLIK et al[4] and FILIPPOU et al[15]. At the end of leaching (pH=1.0-1.5), the air was driven into the reactor to facilitate the precipitation of iron (Eqns.(4), (7) and (9)) and enhance the agitation. Thus, the iron concentration in leaching liquor is restrained, which is favorable for the subsequent separation and extraction processes.
3.1.2 Effect of charge ratio
The experiments were conducted to determine the effect of charge ratio that represented the ratio of manganese nodule to pyrrhotite concentrate on metal extractions rates under the following leaching conditions: liquid to solid ratio(L/S) of 2?1; H2SO4 concentration of 1.5 mol/L; temperature of 85 ℃; time of 4 h and stirring speed of 300 r/min. It can be seen from Fig.1 that the manganese extraction rate decreases from 83% to 68% with increasing charge ratio from 1 to 4. In contrast, the extraction rates of cobalt and nickel increase with increase in charge ratio approaching a maximum at charge ratio of 3?2, and then decrease with the increasing charge ratio further. Therefore, under these leaching conditions, maximum leaching rates amount to 73% and 70% for nickel and cobalt, respectively.
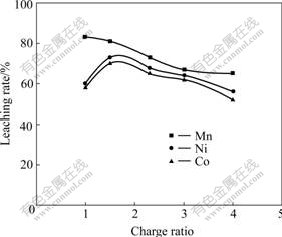
Fig.1 Effect of charge ratio on metals extraction
3.1.3 Effect of leaching temperature
To determine the effect of temperature on extraction rate of metals, leaching experiments were conducted with varying temperature from 70 ℃ to 100 ℃ under the following conditions: L/S of 2?1; charge ratio of 2?1; H2SO4 concentration of 1.5 mol/L; time of 4 h and stirring speed of 300 r/min accompanied by the agitation of airflow. The results indicate that the leaching temperature significantly affects the extractions of cobalt and nickel as well as manganese, especially for the extraction of Mn. Fig.2 shows the extraction rates of Mn, Co and Ni as a function of leaching temperature. For instance, the extraction is 46%, 50% and 53% for Mn, Co and Ni, respectively, at 70 ℃, but they respectively increase to 89%, 77% and 79% at 100 ℃.
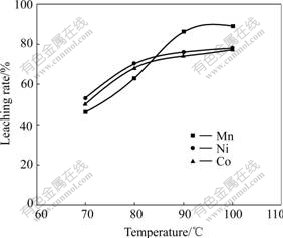
Fig.2 Effect of leaching temperature on metals extraction
3.1.4 Effect of initial H2SO4 concentration
Initial acid concentration was varied from 0.5 to 2.0 mol/L under the following leaching conditions: L/S of 2?1; charge ratio of 2?1; temperature of 85 ℃; time of 4 h and stirring speed of 300 r/min accompanied by the agitation of airflow. Fig.3 shows that the extraction rates of cobalt, nickel and manganese gradually increase with the increase in initial acid concentration in solution. It can be seen that the extraction extent is directly related to H2SO4 concentration at the whole range of acid concentrations investigated, although the metal extraction rates approach a plateau with sulphuric acid concentration higher than 1.25 mol/L. At the same time, it is also observed that the iron concentration in solution unfavorably increases with increase in acid concentration whereas the low acid concentration can lead to a decreased leaching rate of valuable metal as well as the formation of iron hydrolyte, which makes the filtration of slurry difficult. In order to achieve a higher valuable metal extraction and operable processing, a moderate acid concentration of 1.25 mol/L is preferable and the resulting extraction rates of Mn, Ni and Co are 80%, 73% and 68%, respectively.
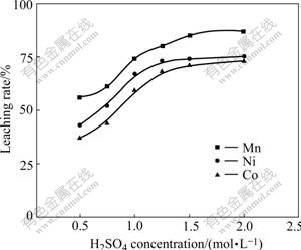
Fig.3 Effect of H2SO4 concentration on metals extraction
3.1.5 Effect of leaching time
In order to determine the effect of leaching time on metal extraction rate, further leaching experiments were carried out with the leaching time varying in the range of 90 min to 5 h. Fig.4 shows the effect of leaching time using 1.25 mol/L H2SO4 solution and liquid to solid ratio of 2?1 at 85 ℃ and stirring speed of 300 r/min on the extraction rates of manganese, nickel and cobalt. It can be seen that the leaching rates of Mn, Co and Ni are similar, i.e., longer leaching time gives high leaching efficiency, although cobalt extraction rate is slightly lower than that of nickel and manganese in the whole range of time investigated. It can also be observed that only slight increase in extraction rate takes place as the leaching time is more than 4 h, which is unfavorable due to the higher energy consumption. Under these conditions, the maximum extraction rates of manganese, nickel and cobalt are 88%, 81% and 80%, respectively.
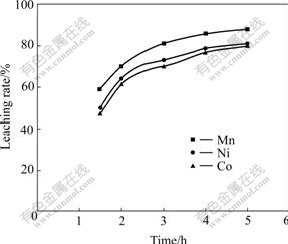
Fig.4 Effect of leaching time on metals extraction
3.1.6 34 factorial design of experiment
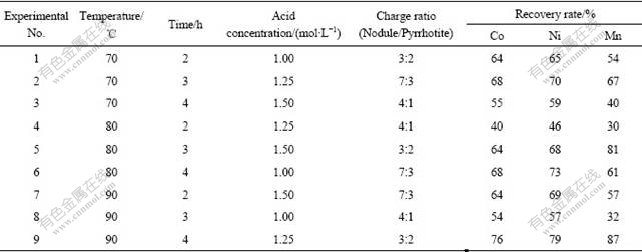
To optimize the conditions such as temperature, acid concentration, leaching time and charge ratio, a series of leaching experiments were further conducted with varying temperatures from 70 ℃ to 90 ℃. The lower and upper levers of acid concentration were chosen as 1.0 and 1.5 and those of leaching time were kept as 2 h and 4 h, respectively. The charge ratio was adjusted from 3?2 to 4?1 (Table 2). All the experiments were carried out at a constant L/S ratio of 2?1 and stirring speed of 300 r/min. The slurry obtained was filtered and the filtrate was analyzed for the contents of Co, Ni and Mn. The results are listed in Table 2. The extraction rates of cobalt under these conditions vary between 40% and 76%, while those of Ni and Mn vary from 46% to 79% and from 30% to 87%, respectively. It is observed from these experiments that the charge ratio gives a dominant effect on the extraction of metals and the maximum extraction of cobalt takes place under the conditions described as follows: temperature of 90 ℃; time of 4 h; H2SO4 concentration of 1.25 mol/L; charge ratio of 3?2; L/S of 2?1 and stirring speed of 300 r/min. Consequently, these conditions are termed as preferable conditions for dissolution of cobalt, nickel and manganese. In order to facilitate the separation of cobalt and nickel from manganese, the leach liquor is concentrated and the average composition is found to be (in g/L) 65.93 Mn, 2.82 Co, 0.25 Ni, 0.64 Fe, 1.37 Al and pH=2.0.
Table 2 Results of extraction rates of metals using 34 factorial design of experiment
3.2 Separation of Mn2+ and Co2+ as well as Ni2+ by precipitation
Before separating manganese from cobalt and nickel, elimination of iron and aluminum by precipitation was carried out by heating the solution up to 90 ℃ with agitation for 2 h. It is essential to use sodium carbonate solution as neutralizer and ferric ion Fe3+ as oxidizer so that the separation of iron from manganese and cobalt is possible. In the presence of a number of aluminium-rich sodium-hydronium jarosites, hydrolysis of iron and aluminum yields alunite/jarosite and it is also noticed that increased pH value results in decreased iron and aluminum concentrations, which is consistent with results obtained by WHITTINGTON et al[17-18]. The precipitation reaction occurred is
3(FeAl)2(SO4)3+Na2SO4+12H2O=2Na(Fe, Al)3(SO4)2(OH)6+6H2SO4 (12)
The resulting solution contains (in g/L) 65.93 Mn, 2.82 Co, 0.25 Ni, 0.001 Fe, 0.08 Al and pH=3.0. It can be observed that the content of manganese is thirty-fold more than that of cobalt in solution. However, it is a challenge to separate cobalt from manganese using solvent extraction due to the fact that most of organic solvents would rather select manganese than cobalt. Based on the difference in Ksp of MnS and CoS as well as NiS, which are 2.8×10-20, 1.8×10-22, 3.1×10-15, respectively, the cobalt and nickel are separated from manganese due to the formation of cobalt sulfide and nickel sulfide by adding stoichiometric sodium sulfide into solution with agitation for 2 h at 50℃. The resulting slurry is filtrated and the corresponding solution contains (in g/L) 63.89 Mn, 0.004 Co, 0.005 Ni, 0.64 Fe, 0.007 Al and pH=3.0. About 99.85% of cobalt is precipitated as cobalt sulfide and nickel is also removed from the solution by the similar method at the same time. Thus, the separation of manganese from cobalt and nickel is successfully achieved and the resulting residue is used to extract cobalt and nickel in the next process.
By adding the sodium hydroxide solution into the filtration to adjust the pH valve to 7.5, the manganese is subsequently precipitated with the addition of sodium carbonate solution. The residue obtained is washed using hot water to eliminate the entrained sodium and the resulting manganese carbonate is subsequently dissolved using 0.5 mol/L H2SO4 solution. As a result, manganese sulphate crystal is obtained by concentrating the resulting solution (Fig. 5).
3.3 Separation of Co2+ and Ni2+
As D2EHPA is proved to be an efficient extract extractant for elimination of impurities such as Mn, Fe, Zn and Cu in the presence of Ni and Co from sulphate solution, the number of counter-current stages required are predicted using the Kremser equation[16-20] and it can be calculated that the 8 stages extraction tests must be carried out in order to achieve an extraction efficiency of 99.9% for the impurities. This is confirmed by carrying out the counter-current extraction tests using organic phase consisting of 15% D2EHPA+85% Kerosene (volume fraction) with [H+] of 0.1 mol/L. The operations are performed accompanied with 3 stages scrubbing using 1 mol/L H2SO4 solution. The chemical compositions of raffinate obtained are found to be (in g/L) 21.36 Co, 1.41 Ni, 0.000 4 Fe, 0.000 4 Cu, 0.003 Mn, 0.000 4 Zn, 0.000 1 Pb, 0.007 Al, 0.002 Ca and 0.006 Mg. It can be found that high impurity extraction efficiency is achieved accompanied with a high recovery for both cobalt and nickel.
Subsequently, the separation of cobalt and nickel is carried out using the raffinate resulted from the process of impurities removal. Initial aqueous feed pH is kept at 3.5 and the extractant P507 neutralised to 70% is used for separation of Co and Ni with the organic phase composition of 25% P507 in 75% kerosene having [H+] of 0.2 mol/L. The counter-current extraction tests are conducted with 3 stages extraction at O/A of 2:3 and 5 stages scrubbing using 1.2 mol/L H2SO4 at O/A of 10?1. The loaded organic is stripped for 3 stages with 3 mol/L HCl solution. The resulting cobalt chloride solution contains (in g/L) 114.39 Co, 0.000 3 Ni, 0.01 Fe, 0.01 Cu, 0.01 Mn, 0.001 6 Zn, 0.000 1 Pb, 0.02 Al, 0.12 Ca and 0.02 Mg. The chemical compositions of raffinate are found to be (in g/L) 4.13 Ni, 0.004 Co, 0.000 4 Fe, 0.000 3 Cu, 0.000 5 Mn, 0.000 3 Zn, 0.000 1 Pb, 0.003 Al, 0.002 Ca and 0.003 Mg. All the extraction operations are conducted at room temperature (27 ℃).
It can be seen that the extraction rate of cobalt is 99.9% and the recovery of nickel is more than 99.5% in this process. The nickel sulphate solution and the stripped cobalt can be used for the further precipitation as respective salt. The overall recovery rates achieved for Ni and Co are 95% and 99%, respectively. The very low concentrations of all metals in the final raffinate and the cobalt stripping solution indicate that the present extraction system can be successfully used for separation of nickel and cobalt came from leach liquor of deep-sea nodules.
The resulting cobalt chloride solutions are diluted using distilled water to keep the content of cobalt at about 50 g/L and then are contacted with the mixed solution of oxalic acid and ammonium oxalate at 65 ℃. The precipitation process is completed as the content of cobalt in solution decreases to less than 0.5 g/L. The cobalt oxide is obtained by subsequently roasting the resulting solids at 700 ℃.
Based on the present study, the entire operation for recovery manganese and nickel as well as cobalt can be presented in Fig.5.
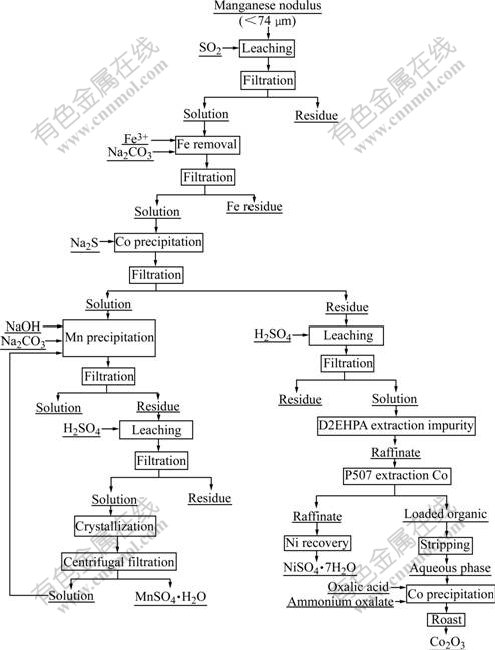
Fig.5 Flowsheet of process for recovery of cobalt, nickel and manganese from sea nodule using oxidative leaching and solvent extraction
4 Conclusions
1) Pyrrhotite can be effectively used as leachant for extraction of valuable metals such as manganese, cobalt and nickel from nodules in sulphuric acid solution.
2) Several factors such as charge ratio, leaching time, leaching temperature and sulphuric acid concentration significantly affect the leaching rate of metals. The preferable leaching conditions explored for extraction manganese, cobalt and nickel can be summarized as: temperature of 90 ℃; time of 4 h; H2SO4 concentration of 1.25 mol/L; charge ratio of 3?2; liquid to solid ratio of 2?1 and stirring speed of 300 r/min.
3) By precipitation, cobalt and nickel can be separated from manganese sulphate solution. The further operations, such as crystallization and solvent extraction as well as precipitation, can result in obtaining different products including manganese sulphate and nickel sulphate as well as cobalt oxide. The final maximum recovery rates for manganese, cobalt and nickel are 85%, 75% and 78%, respectively.
Acknowledgements
The authors would like to express their gratitude to Jinchuan Nonferrous Metals Complex for supplying financial support and the material as well as the experiments equipments and sites for this study.
References
[1] Complete Works of Chemical Fertilizer Industry Editorial Board. Complete works of chemical fertilizer industry [M]. Beijing: Chemical Industry Press, 1988: 342-346. (in Chinese)
[2] Handbook of a Concise Chemical Reagent Organizing Groups. Handbook of a concise chemical reagent [M]. Shanghai: Shanghai Science and Technology Press, 1988: 467-472. (in Chinese)
[3] GUPTA B, DEEP A, SINGH V, TANDON S N. Recovery of cobalt, nickel, and copper from sea nodules by their extractant with alkylphosophines [J]. Hydrometallurgy, 2003, 70: 121-129.
[4] HAVLIK T, LAUBERTOVA M, MISKUFOVA A, KONDAS J, VRANKA F. Extraction of copper, zinc, nickel and cobalt in acid oxidative leaching of chalcopyrite at the presence of deep-sea manganese nodule as oxidant [J]. Hydrometallurgy, 2005, 77: 51-59.
[5] KHALAPALLA S E, PAHLMAN J E. Selective extraction of metals from pacific sea nodules with dissolved sulfur-dioxide [J]. Journal of Metals, 1981, 33: 37-42.
[6] KHAN S, GUPTA R, SAXENA R K. Bioleaching of copper from ferromanganese sea nodules of Indian ocean [J]. Current Science, 1997, 73: 602-605.
[7] JANA R K, SINGH D D N, ROY S K. Alcohol-modified hydrochloric acid leaching of sea nodules [J]. Hydrometallurgy, 1995, 38: 289-298.
[8] JANA R K, SINGH D D N, ROY S K, SIRCAR S C. Electrochlorination of sea nodules to recover copper, nickel and cobalt [J]. Hydrometallurgy, 1993, 32: 21-38.
[9] JANA R K, SINGH D D N, ROY S K. Electrowinning of metals using buffered sodium-chloride electrolyte during electrochlorination of sea nodules [J]. Hydrometallurgy, 1994, 36: 295-314.
[10] VON WINBUSH S, MARONI V A. Pyrochemical extraction of transition metals from pacific ocean deep sea nodules [J]. Separation Science and Technology, 1987, 22: 1135-1148.
[11] SARANGI K, REDDY B R, DAS R P. Extraction studies of cobalt and nickel from chloride solutions using Na-Cyanex 272: Separation of Co(II)/Ni(II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures [J]. Hydrometallurgy, 1999, 52: 253-265.
[12] TSAKIRIDIS P E, AGATZINI S L. Simultaneous solvent extraction of cobalt and nickel in the presence of manganese and magnesium from sulfate solutions by Cyanex 301 [J]. Hydrometallurgy, 2004, 72: 269-278.
[13] DUTRIZAC J E, CHEN T T. A mineralogical study of the phases formed during the CuSO4-H2SO4-O2 leaching of nickel-copper matte [J]. Can Metall Q, 1987, 26: 265-276.
[14] PROVIS J L, VAN DEVENTER J S J, RADEMAN J A M, LORENZEN L. A kinetic model for the acid-oxygen pressure leaching of Ni-Cu matte [J]. Hydrometallurgy, 2003, 70: 83-99.
[15] FILIPPOU D, KONDURU R, DEMOPOULOS G P. A kinetic study on the acid pressure leaching of pyrrhotite [J]. Hydrometallurgy, 1997, 47: 1-18.
[16] SHEN Y F, XUE W Y, LI W, TANG Y L. Selective recovery nickel and cobalt from cobalt enrichment Ni-Cu matte by two stages counter-current leaching [J]. Separation and Purification Technology, 2007, 58: 167-173.
[17] WHITTINGTON B I, JOHNSON J A, QUAN L P, MCDONALD R G, MUIR D M. Pressure acid leaching of arid-region laterite ore (II): Effect of ore type [J]. Hydrometallurgy, 2003, 70: 47-62.
[18] WHITTINGTON B I, JOHNSON J A. Pressure acid leaching of arid-region laterite ore (III): Effect of process water on nickel losses in the residue [J]. Hydrometallurgy, 2005, 78: 256-263.
[19] KREMSER A. Theoretical analysis of absorption process [J]. National Petroleum News, 1930, 22: 42-52.
[20] SHENOYA U V, FRASERB D M. A new formulation of the Kremser equation for sizing mass exchangers [J]. Chemical Engineering Science, 2003, 58: 5121-5124.
Corresponding author: SHEN Yong-feng; Tel: +86-24-83681425; Fax: +86-24-23906472; E-mail: shenyf@smm.neu.edu.cn


