DOI:10.19476/j.ysxb.1004.0609.2019.01.11
xLi3V2(PO4)3·LiVPO4F/C复合正极材料的合成及储锂性能
李实,梁叔全,曹鑫鑫,罗志高,周江
(中南大学 材料科学与工程学院,长沙 410083)
摘 要:
用乙炔碳作为碳源,采用机械活化辅助碳热还原两步法合成xLi3V2(PO4)3·LiVPO4F/C复合正极材料。采用XRD、SEM、TEM等技术对样品的晶体结构和微观形貌进行了表征,采用循环伏安法和恒流充放电等测试方法对合成样品的电化学性能进行分析研究。结果表明:xLi3V2(PO4)3·LiVPO4F/C复合正极材料兼备了Li3V2(PO4)3的循环稳定性好、倍率性能佳的优点和LiVPO4F能量密度高的优势,此外还弥补了Li3V2(PO4)3在3~4.7 V电压范围充放电时放电电压平台缺失的缺陷。该材料在3~4.7 V之间的循环稳定性较好,在1C倍率下最高放电比容量为119.7 mA·h/g,循环300圈后为97.5 mA·h/g。其倍率性能较好,在0.1C倍率下充放电可获得高达152 mA·h/g的放电比容量,倍率升高到8C时仍能保持100 mA·h/g的放电比容量。
关键词:
锂离子电池;正极材料;碳热还原法;xLi3V2(PO4)3·LiVPO4F/C;
文章编号:1004-0609(2019)-01-0091-09 中图分类号:TM912.9;O646.54 文献标志码:A
随着信息化进程的高速推进、移动互联网技术的迅猛发展,人们对二次化学电源的需求越来越高。目前,电池的比容量与快速充放电性能甚至成为移动互联网设备持续高速发展的瓶颈。随着环境恶化越来越严重,雾霾、干旱、沙尘暴等极端天气的持续升温,电动汽车(EVs)和混合动力汽车(HEVs)行业广受关注,发展高功率特性的清洁锂离子动力二次电池具有战略意义[1-2]。正极材料是锂离子电池中最关键也是成本最高的部分。正极材料作为锂离子电池的核心部件之一,已成为了当今世界的研究热点。目前已经商用的锂离子电池正极材料主要有层状结构LiCoO2、尖晶石型LiMn2O4、橄榄石型LiFePO4及三元正极材料等[3-7]。但是LiCoO2的实际比容量仅为理论比容量的50%左右,且世界钴资源有限,我国的钴资源非常稀缺,导致钴的价格昂贵[3]。LiMn2O4对环境污染较小,且合成成本较低,但是其能量密度较低、高温稳定性较差[5]。LiFePO4对环境无污染,原料丰富,价格低廉,但其自身电子电导率较低,橄榄石型结构中Li+的一维扩散通道极大地限制了其在高功率动力电池方面的应用[5]。目前,三元正极材料以其能量密度高、循环寿命长等优势被认为是最有前景的动力电池正极材料,但其成本和安全性问题有待进一步解决[6-7]。
橄榄石型结构LiMPO4(M为Fe、Co、Ni、Mn等过渡金属)以其成本低、Li+脱嵌可逆性好及极好的热稳定性成为一种广为关注的正极材料[8]。由PO4四面体与MO6八面体共用顶点形成开放式NASICON结构材料比LiFePO4具有更高的锂离子传导性,其一般通式为LixM2(PO4)3。此外,聚阴离子开放式框架结构加快了离子迁移,导致其氧化还原电位更高、安全性能更好。Li3V2(PO4)3作为正极材料,其三维框架结构可以提供3个锂离子位置,当Li3V2(PO4)3充放电到4.8 V(vs Li/Li+)时,每个单元中会有3个锂离子或者电子在正负极之间进行迁移。这种情况下其理论容量可达197 mA·h/g[9-11]。因此,Li3V2(PO4)3是一种很有前途的锂离子电池正极材料。
BARKER等[12]最先报道了三斜晶系结构的氟磷酸钒锂(LiVPO4F)用作锂离子电池正极材料的合成方法及电化学性能。由于结构中氟离子与PO43-聚阴离子诱导效应的影响,LiVPO4F中V3+/V4+氧化还原对处在一个较高的电位,约为4.2V。与同样的聚阴离子结构的Li3V2(PO4)3中V3+/V4+氧化还原转变相比,LiVPO4F的电压平台更高。此外,与传统的磷酸盐LiFePO4、LiMnPO4和LiVOPO4等相比,LiVPO4F具有更好的电子导电性[13]。WANG等[14-16]采用掺杂、复合、包覆及表面改性等手段系统地研究了影响LiVPO4F正极材料电化学性能的因素,并且通过高效短流程合成方法制备出一系列综合性能优异的LiVPO4F正极材料。
考虑到Li3V2(PO4)3和LiVPO4F各自的优缺点,Li3V2(PO4)3在2.5~4.7 V之间进行充放电时,虽然其理论容量较高,但电压平台不明显[17],而LiVPO4F具有良好的4.2 V级放电电压平台,但其容量欠缺、倍率性能较差[18]。根据最近的报道[19-22],两种或多种纯物质的混合物可以有效提升材料的电化学性能。此外,纯相的Li3V2(PO4)3和LiVPO4F由于其内在特定的结构使之具有电子电导率较低的缺点,而碳包覆是一种改善电极材料电导率的有效方法[23-24]。
本文采用机械活化辅助碳热还原法合成了碳包覆的xLi3V2(PO4)3·LiVPO4F/C复合正极材料,并利用XRD、SEM和TEM等技术对样品的晶体结构和微观形貌进行了表征,采用循环伏安法、恒流充放电、倍率性能测试等方法对合成样品的电化学性能进行了分析研究。
1 实验
1.1 材料的合成
本实验采用了机械活化辅助碳热还原两步法合成了xLi3V2(PO4)3·LiVPO4F/C复合正极材料。第一步,称取化学计量比的V2O5(分析纯,99%,质量分数),NH4H2PO4(分析纯,99%,质量分数)和乙炔碳(25%,质量分数,过量),用玛瑙研钵研磨均匀后置于250 mL不锈钢球磨罐,装入一定比例直径为1 mm和10 mm的不锈钢磨球(控制球料质量比约为4:1)。以1200 r/min的速度进行高能球磨,每次5 min,每球磨两次后用小勺把粘在球磨罐内壁的样品刮下,共磨60 min。将混合粉末压成直径1 cm、厚度约2 mm的圆片,置于通有流动Ar的温度自动控制管式炉中于300 ℃预处理4 h,释放出NH3、H2O等,接着以5 ℃/min的速度升至800 ℃保温8 h得到中间体VPO4/C。第二步,按照1:1.05(摩尔比)的比例称取VPO4(减去碳的含量)和LiF,高能球磨60 min,压片后在通有92%Ar+8%H2 (体积分数)混合气的管式炉中于750 ℃保温一定时间,快速冷却即可得到LiVPO4F/C、xLi3V2(PO4)3·LiVPO4F/C和Li3V2(PO4)3/C复合材料样品。
1.2 材料的分析与表征
采用日本理学D/max-2500 型X射线粉末衍射仪(Cu Kα靶,单色器波长为λ=1.54178 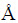 )对粉末样品进行物相鉴定,以8 (°)/min的速度从10°扫描到80°。用扫描电子显微镜(SEM, FEI Nova NanoSEM 230)来观察样品的形貌、粒径和均匀性。用透射电子显微镜(TEM, JEOL JEM2100 F)进一步表征样品的微观结构。采用同步热分析仪(NETZSCH SAT449C)在空气氛围下以10 ℃/min的升温速度对样品进行热分析实验,扫描温度范围为25~800 ℃,可以准确地分析出样品中的碳含量。
)对粉末样品进行物相鉴定,以8 (°)/min的速度从10°扫描到80°。用扫描电子显微镜(SEM, FEI Nova NanoSEM 230)来观察样品的形貌、粒径和均匀性。用透射电子显微镜(TEM, JEOL JEM2100 F)进一步表征样品的微观结构。采用同步热分析仪(NETZSCH SAT449C)在空气氛围下以10 ℃/min的升温速度对样品进行热分析实验,扫描温度范围为25~800 ℃,可以准确地分析出样品中的碳含量。
1.3 电池的组装与测试
按照质量比7:2:1称取活性物质、乙炔碳和PVDF粘结剂,充分研磨30 min,随后加入N-甲基吡咯烷酮(NMP)分散剂调成浆状物,其中,NMP与PVDF的质量比为0.02326。将浆状物用磁力搅拌器搅拌24 h后,用涂布器均匀的涂覆在铝箔集流体被腐蚀的面上。室温静置一段时间,在真空干燥箱中于80 ℃下干燥过夜。用直径为14 mm的冲片器冲成圆形正极片。用金属锂片为参比电极,微孔聚丙烯膜作为隔膜,将1 mol/L的LiPF6溶于混合有机溶剂EC+DMC+EMC (体积比1:1:1)作为电解液,在充满高纯氩气的手套箱(Mbraun,Germany)中组装成CR2016扣式电池。将组装好的电池用封口机封好后在室温下静置12 h后进行电化学性能测试。电池的循环伏安(CV)曲线采用三电极CHI604E电化学工作站测试,扫描速率为0.1 mV/s。恒流充放电实验在多通道电池测试系统(LAND,CT2001A)上进行,所测得的容量均基于活性物质的质量,实验中1C的电流密度为150 mA/g。
2 结果与讨论
2.1 样品的X射线衍射分析
图1所示为在氩气氛围中于800 ℃烧结8 h得到的中间体VPO4/C的XRD谱,与标准PDF卡片(PDF#76-2023)峰形一致,没有杂相生成,加入的多余碳以无定形状态存在。VPO4与正交晶系CrVO4(空间群Cmcm)结构相同,VPO4的框架结构由PO4四面体与VO6八面体共用氧原子构建而成。
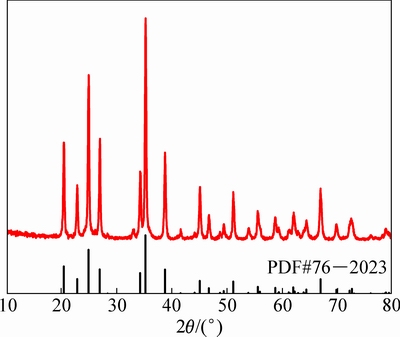
图1 800 ℃烧结8 h合成VPO4/C的XRD谱
Fig. 1 XRD patterns of VPO4/C synthesized at 800 ℃ for 8 h
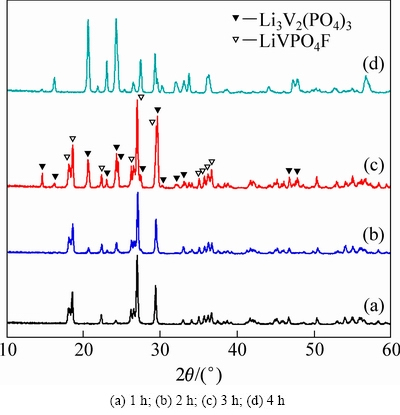
图2 750 ℃不同烧结时间合成样品的XRD谱
Fig. 2 XRD patterns of samples formed for different sintering time
图2所示为VPO4/C与LiF混合物在氢氩混合气体氛围中于750 ℃烧结1~4 h合成样品的XRD谱。在750 ℃短暂烧结1 h快速冷却得到样品A,从其XRD谱可以看出,已经生成纯相材料LiVPO4F/C,且结晶性良好,无其他杂相生成。经分析可知,其XRD谱与LiFePO4(OH)(PDF#41-1376)的相同,为属于P-1空间群的三斜晶系结构[25]。当烧结温度为2 h时合成得到样品为B,从其XRD谱中可以看出,已经出现 Li3V2(PO4)3杂相峰,但此时杂相峰还比较微弱,说明此时杂相Li3V2(PO4)3相对含量还较少。随着烧结时间的延长,Li3V2(PO4)3杂相峰越来越明显,在 14.7°,16.29°、20.72°、23.14°和24.44°等处尤为明显,峰强逐渐升高。说明随着保温时间的延长,LiVPO4F会逐渐分解,晶体结构发生重构生成杂相Li3V2(PO4)3,此猜想最初是由BARKER等提出的[18]。发生如下化学反应:
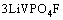 →
→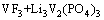 (1)
(1)
在750 ℃烧结3 h快速冷却得到样品C。从其XRD谱可以看出,没有其他LiF、V2O3等杂相的衍射峰,且峰形尖锐、半峰宽较窄。分析发现,这些相由结晶良好的三斜晶系LiVPO4F和单斜晶系Li3V2(PO4)3复合而成。继续延长烧结时间到4 h,样品完全转化成单斜晶系Li3V2(PO4)3。经过观察发现,在样品的XRD谱中均没有观察到多余的碳,说明包覆的碳含量较少或者碳以无定形态存在。包覆碳的具体含量采用同步热分析仪来协助测定。
2.2 样品的扫描电镜分析
图3(a)~(f)所示分别为保温1、3和4 h后得到的LiVPO4F/C、xLi3V2(PO4)3·LiVPO4F/C和Li3V2(PO4)3/C这3种复合材料的SEM像。由图3可以看出,不同保温时间得到样品的粒径范围均为0.5~3 μm,样品同时存在细小的一次颗粒和由一次颗粒团聚而成的二次大颗粒,微粒的结晶性良好且出现了些微的团聚现象。随着保温时间的延长,样品中大颗粒的比例增加,说明保温时间的延长会加剧样品的团聚。
2.3 样品C的透射电镜分析
图4(a)所示为合成的xLi3V2(PO4)3·LiVPO4F/C复合材料(样品C)的TEM像。合成的样品C一次颗粒大小约为几百纳米,颗粒之间有明显的空隙,有助于电解液的渗透和锂离子的扩散。图4(b)所示为样品C的HRTEM像,可见样品C颗粒表面包覆着无定形碳层,材料表面包覆的无定形碳也有助于改善其电化学性能。结晶相的晶面间距为0.303 nm和0.43 nm,分别对应于三斜晶系LiVPO4F的(110)晶面和单斜晶系Li3V2(PO4)3的(1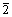 1)晶面。且两相的晶面界限清晰,表明样品C是由尺度为晶粒级的异质粒子复合而成,与传统的物理混合不同。
1)晶面。且两相的晶面界限清晰,表明样品C是由尺度为晶粒级的异质粒子复合而成,与传统的物理混合不同。
2.4 样品C的热分析
图5所示为750 ℃烧结3 h得到的样品C的TG-DSC谱。测试温度范围为25~800 ℃,升温速度为10 ℃/min。由图5可知,在150 ℃之前的区间有少量的质量损失,并伴随微弱吸热峰,这可能是样品中的吸附水、结晶水等物理水的脱出。从360 ℃到580 ℃之间质量剧减6.3%,并伴随强烈的放热峰,这是样品中的碳在空气中被氧化成气体挥发的结果。在600 ℃之后,有几个微弱的放热峰,这可能是LiVPO4F分解成Li3V2(PO4)3或者被氧化成LiVOPO4[22, 25],具体的反应机理有待进一步研究。
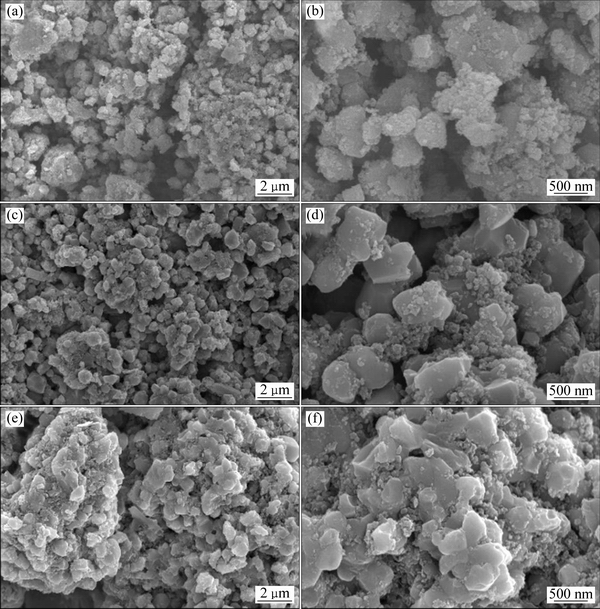
图3 LiVPO4F/C,xLi3V2(PO4)3·LiVPO4F/C和Li3V2(PO4)3/C复合材料的SEM像
Fig. 3 SEM images of LiVPO4F/C ((a), (b)), xLi3V2(PO4)3·LiVPO4F/C ((c), (d)), Li3V2(PO4)3/C ((e), (f)) composites
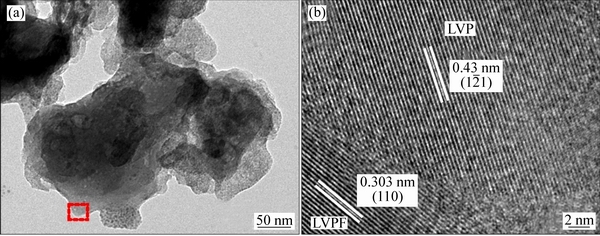
图4 xLi3V2(PO4)3·LiVPO4F/C复合材料的TEM和HRTEM像
Fig. 4 TEM image (a) and HRTEM image (b) of xLi3V2(PO4)3·LiVPO4F/C composite
2.5 样品的恒流充放电测试
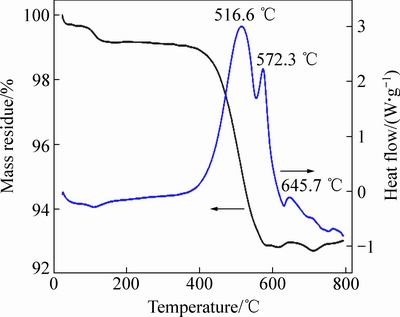
图5 xLi3V2(PO4)3·LiVPO4F/C复合材料的TG-DSC谱
Fig. 5 TG-DSC curves of xLi3V2(PO4)3·LiVPO4F/C composite
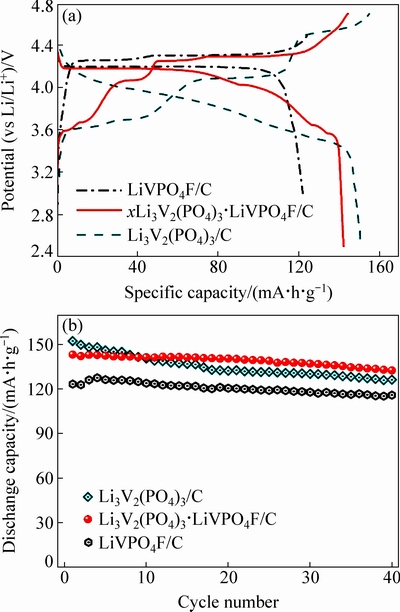
图6 LiVPO4F/C、xLi3V2(PO4)3·LiVPO4F/C和Li3V2(PO4)3/C复合材料在0.2C倍率下的充放电曲线和循环性能图
Fig. 6 Charge-discharge curves (a) and cycle performances (b) of LiVPO4F/C, xLi3V2(PO4)3·LiVPO4F/C, and Li3V2(PO4)3/C composites at 0.2C
图6所示为保温1、3和4 h得到的LiVPO4F/C、xLi3V2(PO4)3·LiVPO4F/C和Li3V2(PO4)3/C这3种复合材料在0.2C倍率下的充放电曲线和循环性能图。由图6(a)可见,LiVPO4F/C虽然具有完整的4.2 V级的放电电压平台,但其放电比容量较低。Li3V2(PO4)/C电极在充到高达4.7 V的电压时,其晶体结构不稳定,导致放电电压平台缺失[7]。xLi3V2(PO4)3·LiVPO4F/C复合电极保持了良好的充放电电压平台和较高的充放电比容量。在0.2C的倍率下循环40圈后,LiVPO4F/C、xLi3V2(PO4)3·LiVPO4F/C和Li3V2(PO4)/C的放电比容量分别为115.9、132.4和126.2 mA·h/g,对应的容量保持率分别为93.4%、92.5%和82.9。
图7所示为750 ℃烧结3 h合成的样品C以0.2C(30 mA/g)和1C(150 mA/g)的倍率在3~4.7 V电压范围内的充放电曲线和循环性能图。由图7(a)、(c)可以明显看出,有6个充电电压平台和4个放电电压平台,在4.2、4.0、3.6和3.5 V附近出现的放电电压平台与LiVPO4F和Li3V2(PO4)3中锂离子嵌入电位相对应。充电曲线中第一圈和后面几圈有一定偏差,可能与首次充电时电解液分解及其他不可逆反应有关。从图7(c)可以看出,以0.2C的倍率充放电时,虽然放电比容量存在一定的波动,但整体都保持在较高的水平。最高放电比容量为142.9 mA·h/g,循环100圈后为125.5 mA·h/g,是最高放电比容量的88%。从图7(b)和(d)可以看出,以1C的倍率充放电时,最初放电比容量为119.7 mA·h/g,循环300圈后还剩97.5 mA·h/g,循环300圈的容量衰减率仅为18%。可见,样品C在3~4.7 V电压范围内的电化学性能优异,而且与纯相的Li3V2(PO4)3[7-9]和LiVPO4F[13, 26]相比,经过复合后其电化学性能得到了较大的提高。
2.6 样品C的循环伏安分析
图8所示为750 ℃烧结3 h后合成的样品C的循环伏安图,扫描电压范围为3~4.7 V,扫描速率为0.1 mV/s。在充电过程中,可以明显地看到6个氧化峰,其中,4.31和4.41 V处的氧化峰对应LiVPO4F中锂离子的脱出电位,其余4个峰对应Li3V2(PO4)3中锂离子的脱出电位。在放电过程中,4.07V处较宽的还原峰对应LiVPO4F中锂离子嵌入电位及Li3V2(PO4)3前两个锂离子的嵌入电位。3.55和3.65 V处是Li3V2(PO4)3中第三个锂离子嵌入时的还原峰,与纯相Li3V2(PO4)3和LiVPO4F的氧化还原峰相比较,样品C中的峰位有所偏差,说明两种正极材料的复合对其氧化还原反应有所影响。
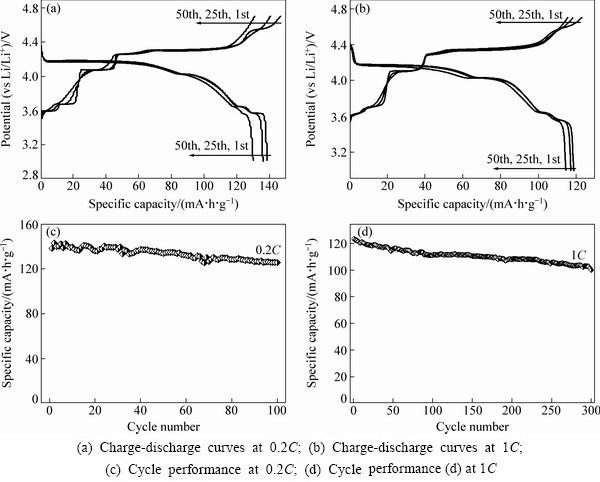
图7 xLi3V2(PO4)3·LiVPO4F/C复合材料在3~4.7 V电压范围内的充放电曲线和循环性能
Fig. 7 Charge-discharge curves and corresponding cycle performances of xLi3V2(PO4)3·LiVPO4F/C composite in voltage range of 3-4.7 V
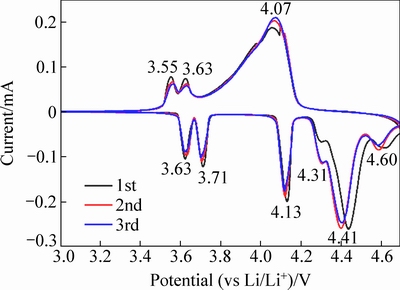
图8 xLi3V2(PO4)3·LiVPO4F/C复合材料的循环伏安图
Fig. 8 CV curves of xLi3V2(PO4)3·LiVPO4F/C composite
2.7 样品的倍率性能测试
图9(a)所示为750 ℃烧结3 h合成的样品C在不同倍率下的充放电曲线。在0.1C倍率充放电时,放电平台与0.2C时的一致,最高放电比容量可达152 mA·h/g。1C倍率时,最高放电比容量为124 mA·h/g,与图7中的结果接近。2C、4C倍率下的放电比容量分别在118和111 mA·h/g左右,在8C倍率下的放电比容量仍能保持较高的100 mA·h/g,表明此复合材料具有优异的倍率循环性能。当经过8C循环后再次恢复到0.1C倍率时,仍具有149 mA·h/g的放电比容量。说明材料的稳定性较好,在经过锂离子快速脱嵌后,其结构不会发生较大的变化。从图9(b)可以看出,在每个倍率下循环10圈的过程中,容量衰减较小,说明材料的循环稳定性较好。
样品C电化学性能的改善是Li3V2(PO4)3和LiVPO4F协同作用的结果。氟的掺杂对PO43-聚阴离子的诱导效应可能会扩展到Li3V2(PO4)3晶格,且改善了充放电过程中的电子转移性能。添加的氟可以保护电极材料免受电解液中出现的HF腐蚀[27-28],有助于保持良好的循环性能。其次,作为快离子导体的Li3V2(PO4)3拥有良好的锂离子脱嵌稳定性,所以复合材料拥有良好的倍率性能。此外,材料表面包覆的薄层无定形碳同样会提高材料的电子导电性,降低电荷扩散过程的阻力[29-30]。
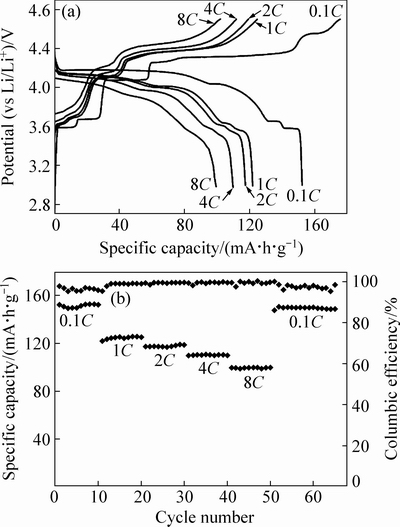
图9 xLi3V2(PO4)3·LiVPO4F/C/Li半电池在不同倍率下的充放电曲线和循环性能图
Fig. 9 Charge-discharge curves (a) and cycle performance (b) of xLi3V2(PO4)3·LiVPO4F/C/Li half cell at various rates
3 结论
1) 由于Li3V2(PO4)3和LiVPO4F存在明显的协同效应,xLi3V2(PO4)3·LiVPO4F/C复合正极材料(样品C)兼备了Li3V2(PO4)3的循环稳定性好、倍率性能佳的优点和LiVPO4F中4.2 V级放电电压平台带来的高能量密度的优势,此外还弥补了Li3V2(PO4)3在3~4.7 V 间充放电时放电电压平台缺失的缺陷。
2) xLi3V2(PO4)3·LiVPO4F/C复合正极材料(样品C)在3~4.7 V之间的循环稳定性较好,在1C倍率下最高放电比容量为119.7 mA·h/g,循环300圈后为97.5 mA·h/g。其倍率性能较好,在0.1C倍率下充放电可获得高达152 mA·h/g的放电比容量,倍率升高到8C时仍能保持100 mA·h/g的放电比容量。
3) 采用的机械活化辅助碳热还原法过程简单,设备要求低,原料利用率高,没有使用特殊的模板和原材料等,适合大规模生产应用。
REFERENCES
[1] ZHANG Hui-gang, YU Xin-di, BRAUN P V. Three-dimensional continuous ultrafast charge and discharge bulk battery electrodes[J]. Nature Nanotechnology, 2011, 6(5): 277-281.
[2] ZAGHIB K, MAUGER A, GROULT H, GOODENOUGH J B, JULIEN C M. Advanced electrodes for high power Li-ion batteries[J]. Materials, 2013, 6(3): 1028-1049.
[3] CHERNOVA N A, ROPPOLO M, DILLON A C, WHITTINGHAM M S. Layered vanadium and molybdenum oxides: batteries and electrochromics[J]. Journal of Materials Chemistry, 2009, 19(17): 2526-2552.
[4] THAPA A K. Development of cathode materials for Li-ion battery and megalo-capacitance capacitor[D]. Saga: Saga University, 2007.
[5] XU Bo, QIAN Dan-na, WANG Zi-ying, MENG Y S. Recent progress in cathode materials research for advanced lithium ion batteries[J]. Materials Science and Engineering, 2012, 73(5): 51-65.
[6] HE Ping, YU Hai-jun, LI De, ZHOU Hao-shen. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries[J]. Journal of Materials Chemistry, 2012, 22(9): 3680-3695.
[7] LIU W, OH P L, LIU X, LEE M J, CHO W G, CHAE S J, KIM Y S, CHO J P. Nickel-rich layered lithium transition-metal oxide for high-energy lithium‐ion batteries[J]. Angewandte Chemie International Edition, 2015, 54(15): 4440-4457.
[8] CHEN Jia-jun. Recent progress in advanced materials for lithium ion batteries[J]. Materials, 2013, 6(1): 156-183.
[9] PAN A Q, CHOI D, ZHANG J G, LIANG S Q, CAO G Z, NIE Z M, AREY B W, LIU J. High-rate cathodes based on Li3V2(PO4)3 nanobelts prepared via surfactant-assisted fabrication[J]. Journal of Power Sources, 2011, 196(7): 3646-3649.
[10] PAN An-qiang, LIU Jun, ZHANG Ji-gang, XU Wu, CAO Guo-zhong, NIE Zi-min, AREY B W, LIANG Shu-quan. Nano-structured Li3V2(PO4)3/carbon composite for high-rate lithium-ion batteries[J]. Electrochemistry Communications, 2010, 12(12): 1674-1677.
[11] YIN S C, STROBEL P S, GRONDEY H, NAZAR L F. Li2.5V2(PO4)3: A room-temperature analogue to the fast-ion conducting high-temperature γ-phase of Li3V2(PO4)3[J]. Chemistry of Materials, 2004, 16(8): 1456-1465.
[12] BARKER J, SAIDI M Y, SWOYER J L. Electrochemical insertion properties of the novel lithium vanadium fluorophosphate, LiVPO4F[J]. Journal of the Electrochemical Society, 2003, 150(10): A1394-A1398.
[13] GOVER R K B, BURNS P, BRYAN A, SAIDI S Y, SWOYER J L, BARKER J. LiVPO4F: A new active material for safe lithium-ion batteries[J]. Solid State Ionics, 2006, 177(26): 2635-2638.
[14] WANG Jie-xi, WANG Zhi-xing, Ll Xin-hai, GUO Hua-jun, XU Xian-wen, ZHANG Xiao-ping, XIAO Wei. xLi3V2(PO4)3·LiVPO4F/C composite cathode materials for lithium ion batteries[J]. Electrochimica Acta, 2013, 87: 224-229.
[15] WANG Ren-heng, XIAO Shun-hua LI Xin-hai, WANG Jie-xi, GUO Hua-jun, ZHONG Fu-xin. Structural and electrochemical performance of Na-doped Li3V2(PO4)3/C cathode materials for lithium-ion batteries via rheological phase reaction[J]. Journal of Alloys and Compounds, 2013, 575: 268-272.
[16] LIU Zhao-meng, PENG Wen-jie, SHIH Kai-min, WANG Jie-xi, WANG Zhi-xing, GUO Hua-jun, YAN Guo-chun, LI Xin-hai, SONG Liu-bin. A MoS2 coating strategy to improve the comprehensive electrochemical performance of LiVPO4F[J]. Journal of Power Sources, 2016, 315: 294-301.
[17] LIM H H, CHO A R, SIVAKUMAR N, KIM W S, YOON W S, LEE Y S. Improved rate capability of Li/Li3V2(PO4)3 cell for advanced lithium secondary battery[J]. Bulletin of Korean Chemical Society, 2011, 32: 1491-1494.
[18] BARKER J, GOVER R K B, BURNS P, BRYAN A, SAIDI M Y, SWOYER J L. Structural and electrochemical properties of lithium vanadium fluorophosphate, LiVPO4F[J]. Journal of Power Sources, 2005, 146: 516-520.
[19] WU Ling, LU Jia-jia, ZHONG Sheng-kui. Studies of xLiFePO4·yLi3V2(PO4)3/C composite cathode materials with high tap density and high performance prepared by sol spray drying method[J]. Journal of Solid State Electrochemistry, 2013, 17(8): 2235-2241.
[20] QIN Lai-fen, XIA Yong-gao, QIU Bao, CAO Hai-liang, LIU Yuan-zhuang, LIU Zhao-ping. Synthesis and electrochemical performances of (1-x)LiMnPO4·xLi3V2(PO4)3/C composite cathode materials for lithium ion batteries[J]. Journal of Power Sources, 2013, 239: 144-150.
[21] LIN Y C, FEY G T K, WU P J, CHANG J K, KAO H M. Synthesis and electrochemical properties of xLiFePO4·(1-x)LiVPO4F composites prepared by aqueous precipitation and carbothermal reduction[J]. Journal of Power Sources, 2013, 244: 63-71.
[22] ZHANG Bao, SHEN Chao, ZHENG Jun-chao, HAN Ya-dong, ZHANG Jia-feng, MING Lei, WANG Jian-long, QIN Sha-ne, LI Hui. Synthesis and characterization of a multi-layer core-shell composite cathode material LiVOPO4-Li3V2(PO4)3[J]. Journal of The Electrochemical Society, 2014, 161(5): A748-A752.
[23] HUANG Huan, YIN S C, KERR T, TAYLOR N, NAZAR L F. Nanostructured composites: A high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries[J]. Advanced Materials, 2002, 14(21): 1525-1528.
[24] FEY G T K, LU T L, WU F Y, LI W H. Carboxylic acid-assisted solid-state synthesis of LiFePO4/C composites and their electrochemical properties as cathode materials for lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2008, 12(7/8): 825-833.
[25] ATEBA MBA J M, MASQUELIER C, SUARD E, CROGUENNEC L. Synthesis and crystallographic study of homeotypic LiVPO4F and LiVPO4O[J]. Chemistry of Materials, 2012, 24(6): 1223-1234.
[26] WANG Jie-xi, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, ZHANG Yun-he, XIONG Xun-hui, HE Zheng-jiang. Synthesis and characterization of LiVPO4F/C using precursor obtained through a soft chemical route with mechanical activation assist[J]. Electrochimica Acta, 2013, 91: 75-81.
[27] KIM G H, KIM J H, MYUNG S T, YOON C S, SUN Y K. Improvement of high-voltage cycling behavior of surface-modified Li [Ni1∕3Co1∕3Mn1∕3]O2 cathodes by fluorine substitution for Li-ion batteries[J]. Journal of The Electrochemical Society, 2005, 152(9): A1707-A1713.
[28] 张培新, 张 利, 谭智博, 任祥忠. Si4+和F-掺杂对LiNi1/3Co1/3Mn1/3O2结构和电化学性能的影响[J]. 中国有色金属学报, 2011, 21(11): 2805-2811.
ZHANG Pei-xin, ZHANG Li, TAN Zhi-bo, REN Xiang-zhong. Effect of Si4+ and F- doping on structure and electrochemical properties of LiNi1/3Co1/3Mn1/3O2[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(11): 2805-2811.
[29] SU Li-wei, JING Yu, ZHOU Zhen. Li ion battery materials with core-shell nanostructures[J]. Nanoscale, 2011, 3(10): 3967-3983.
[30] 曹 亮, 王安安, 艾立华, 贾 明, 刘业翔. 石墨烯在锂离子电池材料性能优化中的应用[J]. 中国有色金属学报, 2016, 26(4): 807-820.
CAO Liang, WANG An-an, AI Li-hua, JIA Ming, LIU Ye-xiang. Application of grapheme in performance optimization of lithium ion battery materials[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 807-820.
Preparation and lithium storage properties of xLi3V2(PO4)3·LiVPO4F/C composite cathode material
LI Shi, LIANG Shu-quan, CAO Xin-xin, LUO Zhi-gao, ZHOU Jiang
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: A two-step mechanical activation and carbon thermal reduction method was used to synthesize xLi3V2(PO4)3·LiVPO4F/C composite cathode material, using actylene black as carbon source. The crystal structure and morphology were characterized by using XRD, SEM, and TEM techniques. In addition, the electrochemical performances of the prepared materials were characterized by cyclic voltammetry (CV) and galvanostatic charge/discharge tests. The results show that xLi3V2(PO4)3LiVPO4F/C composite cathode material has the advantages of good cyclic stability of Li3V2(PO4)3 and the high energy density of LiVPO4F. Meanwhile, it makes up for the shortcoming of the disappeared voltage platform of Li3V2(PO4)3 cathode within 3-4.7 V. The cyclic stability of the composite is good. The maximum value of discharge specific capacity is 119.7 mA·h/g under the rate of 1C which can retain 97.5 mA·h/g after 300 cycles. The rate performance is excellent. The composite electrode delivers a high capacity of 152 mA·h/g at 0.1C. When the current density increases to 8C, the electrode can still deliver a specific discharge capacity of 100 mA·h/g.
Key words: lithium-ion battery; cathode material; carbothermal reduction method; xLi3V2(PO4)3·LiVPO4F/C
Foundation item: Projects(51374255, 51302323, 51572299) supported by the National Natural Science Foundation of China
Received date: 2017-12-18; Accepted date: 2018-07-04
Corresponding author: LIANG Shu-quan; Tel: +86-731-88876691; E-mail: sqliang@mail.csu.edu.cn
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51374255, 51302323, 51572299)
收稿日期:2017-12-18;修订日期:2018-07-04
通信作者:梁叔全,教授,博士;电话:0731-88876691;E-mail: sqliang@mail.csu.edu.cn
摘 要:用乙炔碳作为碳源,采用机械活化辅助碳热还原两步法合成xLi3V2(PO4)3·LiVPO4F/C复合正极材料。采用XRD、SEM、TEM等技术对样品的晶体结构和微观形貌进行了表征,采用循环伏安法和恒流充放电等测试方法对合成样品的电化学性能进行分析研究。结果表明:xLi3V2(PO4)3·LiVPO4F/C复合正极材料兼备了Li3V2(PO4)3的循环稳定性好、倍率性能佳的优点和LiVPO4F能量密度高的优势,此外还弥补了Li3V2(PO4)3在3~4.7 V电压范围充放电时放电电压平台缺失的缺陷。该材料在3~4.7 V之间的循环稳定性较好,在1C倍率下最高放电比容量为119.7 mA·h/g,循环300圈后为97.5 mA·h/g。其倍率性能较好,在0.1C倍率下充放电可获得高达152 mA·h/g的放电比容量,倍率升高到8C时仍能保持100 mA·h/g的放电比容量。


