
J. Cent. South Univ. (2020) 27: 1074-1103
DOI: https://doi.org/10.1007/s11771-020-4352-8

Hydrogen generation from methanol reforming for fuel cell applications: A review
SUN Zhao(孙朝), SUN Zhi-qiang(孙志强)
School of Energy Science and Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Methanol is regarded as an important liquid fuel for hydrogen storage, transportation, and in-situ generation due to its convenient conveyance, high energy density, and low conversion temperature. In this work, an overview of state-of-the-art investigations on methanol reforming is critically summarized, including the detailed introduction of methanol conversion pathways from the perspective of fuel cell applications, various advanced materials design for catalytic methanol conversion, as well as the development of steam methanol reformers. For the section of utilization pathways, reactions such as steam reforming of methanol, partial oxidation of methanol, oxidative steam reforming of methanol, and sorption-enhanced steam methanol reforming were elaborated; For the catalyst section, the strategies to enhance the catalytic activity and other comprehensive performances were summarized; For the reactor section, the newly designed steam methanol reformers were thoroughly described. This review will benefit researchers from both fundamental research and fuel cell applications in the field of catalyzing methanol to hydrogen.
Key words:
methanol reforming; hydrogen generation; fuel cell; catalyst; reformer;
Cite this article as:
SUN Zhao, SUN Zhi-qiang. Hydrogen generation from methanol reforming for fuel cell applications: A review [J]. Journal of Central South University, 2020, 27(4): 1074-1103.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4352-81 Introduction
Energy is the driving force and lifeline of the national economy. Energy development, as well as rational and effective utilization is the headspring of the entire social development. At present, the political and economic structure of the world has undergone profound adjustments, and the relationship between energy supply and demand has gone through deep changes. The energy and resources are increasingly constrained, ecological and environmental issues are prominent, and the pressure to adjust the economic structure, improve energy efficiency, and ensure energy security is further increased. Energy development faces a series of new problems and challenges [1].
Fuel cells are electrochemical energy devices that directly convert the chemical energy of a fuel into electrical energy, also known as an electrochemical generator. It belongs to the fourth generation technology after hydroelectric power generation, thermal power generation, and atomic power generation. The fuel cells can convert part of the chemicals’ Gibbs free energy into electrical energy by an electrochemical reaction, which is not restricted by the Carnot cycle effect, resulting in a higher efficiency compared to the conventional power generation technology. Thus, the fuel cells are seen as one of the most promising energy power plants from the perspective of energy conservation and ecological protection.
According to the operation temperature, the fuel cells can be divided into low-temperature fuel cells and high-temperature fuel cells [2]. Common low-temperature fuel cells include alkaline fuel cells (AFCs), phosphoric acid fuel cells (PAFCs), and polymer electrolyte membrane fuel cells (PEMFCs), etc. Fuel cells such as molten carbon fuel cells (MCFCs) and solid oxide fuel cells (SOFCs) are operated at high temperatures [3, 4]. Power generation with applied PEMFCs is a new power generation technology and its broad application can be comparable with computer technology, especially in the transportation applications due to its high power output, fast response time, high energy-conversion efficiency, and continuous power supply mode. The unit cell is composed of an anode, a cathode, and a proton exchange membrane (PEM). Taking hydrogen as the fuel, H2 is oxidized at the anode, the protons are transported through the PEM to the cathode and the electrons are transported via the external circuit to the cathode. At the cathode, the protons from the anode are combined with the electrons, which reduces the oxygen with water generation. The typical operation procedures of PEMFC are presented in Figure 1 and the involved reactions are as follows:
 (1)
(1)
 (2)
(2)

Figure 1 Operating principle of polymer electrolyte membrane fuel cells
With the development of PEMFCs, different types of membrane materials can be operated at different temperatures. For instance, the PEMFCs loaded with perfluorosulfonic acid (PFSA) membranes are generally operated at 80 °C or lower ones. Such low operating temperatures result in a very high requirement on the carbon monoxide concentration because the Pt-based catalysts perform very low resistance to CO, generally no more than 50×10-6 in the case of the deactivation. The high-temperature proton exchange membrane fuel cells (HT-PEMFCs) can be operated at 120-200 °C with phosphoric acid doped polybenzimidazole membranes being utilized as the FEMS which can enhance the CO tolerance up to 3×10-2.
Hydrogen energy is considered to be the next generation of secondary clean energy to solve the increasingly serious energy crisis and environment pollution due to its high energy density and zero greenhouse gas emission [5]. More importantly, hydrogen has been proved to be the most suitable energy carrier for PEMFCs. However, hydrogen is different from fossil fuels, which cannot be directly obtained or exploited from the natural environment. Conventionally, hydrogen is generated from methane steam reforming; the process is much complicated which generally goes through methane desulfurization, reforming, water-gas shift, and CO2 removal/PSA, etc. Besides, the safe storage and transportation of hydrogen have also been the obstacles to the wide deployment of hydrogen energy [6]. Methanol can be also directly utilized in PEMFCs, nevertheless, performing limited oxidation kinetics, leading to its crossover from the anode to the cathode.
Compared with hydrogen generation from fossil fuels at high temperatures, in situ hydrogen production from oxygenated fuels such as methanol and ethanol at relatively low temperatures is mostly preferred. Moreover, utilizing liquid fuels as the hydrogen carrier could be an efficient choice for hydrogen transportation and hydrogen storage [7]. Methanol is seen as a promising hydrogen carrier among the liquid fuels to produce hydrogen due to the absence of C—C bonds, leading to a lower temperature for methanol conversion. Hydrogen production from methanol conversion performs the advantages of low sulfur content, high-volume energy density, and high H/C mole ratio, making it attractive for in-situ hydrogen production [8-10]. Methanol is generated mostly from fossil fuels such as natural gas or syngas from coal. It is to be noted that methanol can be also considered a renewable feedstock because it can be obtained from biomass-derived syngas.
This work summarizes 1) the recent five years’ developments on the technologies from methanol to hydrogen; 2) the latest progress on the catalysts for methanol conversion; 3) the newly developed methanol steam reformers for efficient methanol conversion. Methanol decomposition (MD) tends to occur at relatively high temperatures, which dissociates methanol into hydrogen and carbon monoxide. High-concentration carbon monoxide is not conductive to PEMFCs and thus it is not further discussed in this study.
2 Utilization pathways
In this part, several approaches for converting methanol to hydrogen were described which mainly include steam reforming of methanol (SRM), partial oxidation of methanol (POM), oxidative steam reforming of methanol (OSRM), and sorption- enhanced steam methanol reforming as presented in Figure 2 [11-14]. The SRM can be classified into thermo-catalytic methanol steam reforming, photo- catalytic methanol steam reforming, high- temperature methanol steam reforming, and aqueous-phase reforming of methanol in detail. The methanol conversion can be judged by H2/CO2 mole ratios: 1) As for SRM, the n(H2)/n(CO2) mole ratio should be equal to 3; 2) For POM, the oxygen participates the partial oxidation of methanol, resulting in the mole ratio of n(H2)/n(CO2)=2; 3) The OSRM reaction meets 2<>2)/n(CO2)<3 because both steam and oxygen involve the methanol conversion; 4) The n(H2)/n(CO2) mole ratio of SE-SMR is higher than 3 because the produced CO2 from methanol can be absorbed by the specific adsorbent such as MgO-based CO2 sorbent or MgO-hydrotalcite.
2.1 Steam reforming of methanol
Of the methanol conversion technologies, SRM reaction is an endothermic reaction, which produces the highest yield of hydrogen and the highest H2 to CO2 mole ratio. Based on the existing research, the SRM processes can be mainly classified into thermos-catalytic methanol steam reforming, photo-catalytic methanol steam reforming, high-temperature methanol steam reforming, and aqueous-phase reforming of methanol. The main reaction of SRM is shown below:
Steam reforming of methanol:
 (3)
(3)
2.1.1 Thermo-catalytic methanol steam reforming
Thermo-catalytic methanol steam reforming is the most industrial kind of mature technology [15-17]. It is also the most commonly employed process to produce hydrogen from methanol. Besides the overall reaction as shown in Reaction (3), two side reactions are commonly considered, which are methanol decomposition and water-gas shift reaction seen below:
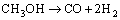 (4)
(4)
 (5)
(5)
Side reactions are prone to occur especially under a low steam-to-methanol mole ratio, which accompanied by the formation of dimethyl ether, methane, and methyl-formate, etc. [18-20]. The reactions involved are presented below:
 (6)
(6)
 (7)
(7)
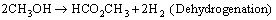 (8)
(8)
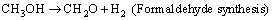 (9)
(9)
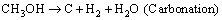 (10)
(10)
Thermodynamic calculation of SRM related processes was conducted for parameter optimization and thermodynamic performance analysis. OZCAN et al [21] performed an optimization study on the SRM process integrated with HT-PEMFC by using ASPEN Plus. Parameters such as temperature, pressure, and n(H2O)/n(CH3OH) mole ratio on the product distribution were thoroughly discussed. Simulation results indicate that the optimized temperature, pressure, and n(H2O)/n(CH3OH) for the production of HT-PEMFC-grade hydrogen were identified to be 246 °C, 1.01×105 Pa, and n(H2O)/n(CH3OH)=5.6. Further, the carbon monoxide concentration is ranged from 30×10-6 to 1700×10-6 within the optimized conditions, which can be used at the anode of HT-PEMFC.
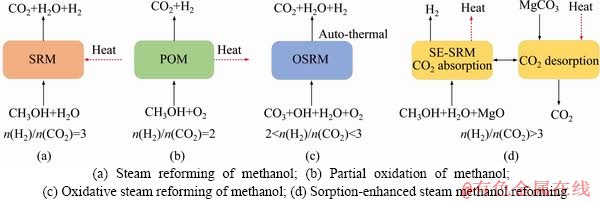
Figure 2 Methanol conversion pathways:
Considering that the SRM reaction is endothermic, some researchers proposed the integrated system for heat recovery of SRM as well as achieving poly-generation. WU et al [22] utilized a heat recovery system to heat the reforming gas in a methanol steam reformer, integrating with a diesel engine and a cooled exhaust gas recirculation device for smoke, PM2.5, and NOx emissions reduction. The flowchart of the related experimental setup is shown in Figure 3. Their setup mainly composed of a single-cylinder diesel engine, an eddy-current dynamometer, operation panel, methanol steam reforming system, flame trapper, exhaust gas recirculation unit, and detection system. Results show that the maximum heat recovery efficiency can increase by 17.5% and the integrated system achieves the energy saving and pollutants decreasing.
WANG et al [23] studied the thermodynamic performance of a fuel cell tri-generation system as displayed in Figure 4, which consisted of a solar heat collection system, a methanol steam reforming system, a fuel cell power system, and a recovered heat utilization system. The thermodynamic simulation results indicate that the system efficiencies under the summer and winter designing conditions are 73.7% and 51.7%, and the systematic exergy efficiencies are 18.8% and 26.1%, respectively. Therefore, the heat required by SRM reaction can be compensated by a poly-generation system. HOSSEINI et al [24] came up with a multi-generation system, which comprised of a MCFC system coupled with SRM, methanol synthesis process with the distillation process, as well as combined heat and power cycle as displayed in Figure 5. The entire system can simultaneously produce electricity, pure methanol, and hot water under the overall exergy destruction, exergy efficiency, and energy efficiency at 116353 kW, 58.4%, and 83.7%, respectively.

Figure 3 Experimental setup of combustion engine equipped with waste heat recovery system for reforming gas heating and smoke, PM2.5, and NOx emissions reduction (1-Diesel engine; 2, 3, 4, 5-Fuel-injection devices; 6-Flame trapper; 7, 8-Signal processors; 9-Operation panel; 10-Exhaust gas chamber; 11, 12, 13, 14-Monitoring devices; 15, 16-Eddy-current dynamometer; 17-Preheating pipe; 18, 31-Flowmeters; 19-Temperature sensor; 20-Reforming body; 21-Pump; 22-Methanol-aqueous storage device; 23, 24, 25-Devices for transporting/storing hydrogen-rich gas; 26-Inlet chamber; 27, 29-Valves; 28-Exhaust gas cooler; 30-Carbon absorber; 32, 33-Electric heating device) [22]
Three reaction pathways for the thermo- catalytic methanol steam reforming have been proposed by the researchers. The first one is the methanol decomposition followed by water-gas shift, which is doubtful based on the experimental and DFT calculation results. SANTACESARIA et al [25] explored the SRM reaction using Cu-Zn catalysts. Results indicate that the CO concentration is much lower than the equilibrium concentration of water-gas shift reaction. Pathway II and pathway III pointed out that methyl-formate and formaldehyde are the main intermediates, respectively. YI et al [26] verified the generation of methyl-formate on the Au/CeO2 surface via in-situ temperature program surface reaction (TPSR). Other researchers proposed that formaldehyde (HCHO) is the important intermediate during SRM over copper and group VIII (Rh, Pd, and Pt, etc.) metal-based catalysts [27, 28]. It is identified that methanol is dehydrogenated to formaldehyde followed by a nucleophilic attack of H2O to form formic acid, further decomposing to H2 and CO2. The above-mentioned investigations reveal that the reaction pathways of SRM seem to be diverse under different catalysts or conditions, which deserves further explorations.

Figure 4 Flowchart of electricity, cooling, and heating multi-generation system integrated with solar methanol reforming (1, 2, 3, 4-Heattransferoil; 5, 6, 7-Fuel mixed with methanol and desalinated water; 8-Vaporized methanol; 9, 10, 11-Synthesis gas with unreacted vaporized methanol; 12-Synthesis gas; 13-Liquid methanol and water; 14, 16-Hydrogen; 15-CO/CO2; 17-Air; 18, 19-Waste heat form PAFC; 20, 21-Steam; 22, 23-Hot/chilled water; 24-Electricity) [23]

Figure 5 Flowchart of integrated MCFC, SRM, and methanol synthesis process, and combined heat and power cycle [24]
2.1.2 Photo-catalytic methanol steam reforming
Photo-catalytic SRM technology is a newly developed method for methanol utilization. The conventional SRM meets these challenges: Copper- based catalyst is efficient at the temperature higher than 250 °C. Nevertheless, such temperature would result in the occurrence of side reactions such as methanol decomposition with higher carbon monoxide being obtained. Moreover, relatively high temperatures will cause the incensement of energy consumption as well as catalyst sintering and agglomeration. Based on the above-mentioned problems, SUN et al [29] utilized a photo-assisted method for methanol steam reforming, in which photon energy was introduced to decrease the reaction temperature of SRM. Photo-assisted SRM is carried out to see if the photon energy could reduce energy consumption and enhance the SRM efficiency. The experimental setup of photo-assisted SRM is mainly comprised of an argon cylinder, a pump for transporting aqueous methanol, a preheater, a fixed bed reactor equipped with a Xe lamp, a condenser, and a gas chromatography as presented in Figure 6. By comparing the in situ DRIFT results with and without light, it is found that the introduction of light can promote the dissociation of formate acid to CO2 and H2. Moreover, the solar-assisted Cu-Zn-Ti oxide can achieve the activation of Cu species at relatively low temperature (200 °C). The hydrogen production rate reaches 50.6 and 76.2 mmol/(g·h) at 200 and 210 °C, respectively with no CO detected. CHIARELLO et al [30] conducted the photocatalytic steam reforming of methanol on Pt/TiO2 using in situ attenuated total reflection infrared spectroscopy. Results indicate that the adsorption, desorption, and reactivity properties of the TiO2 surface are strongly influenced in the presence of Pt nanoparticles. It is also proved that the photo-promoted electrons can be trapped by the Pt particles with the increase of electron-hole separation based on the variation of the IR spectrum background upon UV-vis irradiation.
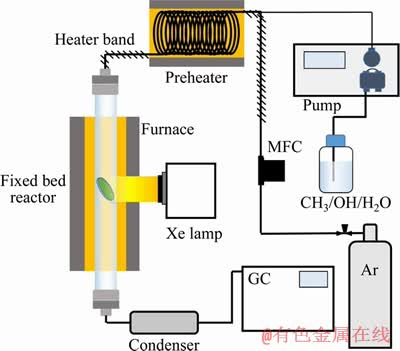
Figure 6 Experimental setup for solar-assisted SRM [29]
2.1.3 High-temperature methanol steam reforming
High-temperature SRM would result in the methanol decomposition with high-concentration carbon monoxide generation and thus the SRM reaction is generally conducted at no more than 300 °C. However, SHARMA et al [31] conducted a very interesting work, investigating various bimetallic catalysts supported on Al2O3-Zn-ZrO2 at the temperature as high as 500 °C with CO-free hydrogen production. It is found that the bimetallic catalyst (Fe:Cu=1:1) supported on the Al2O3- Zn-ZrO2 was observed with an interesting synergy, resulting in the particle size reduction and reducibility enhancement. Furthermore, it is found that the Cu-Fe catalyst with Cu content more than 75% can reduce the CO selectivity to zero. The related reaction mechanism was proposed according to the diffuse reflectance infrared Fourier transform spectroscopy (DRIFTs) results: the presence of Fe2O3 as the surface species converts the produced CO to CO2, thus no carbon monoxide can be detected in the final product stream.
2.1.4 Aqueous-phase reforming of methanol
Aqueous-phase reforming (APR) is a promising technology that converts organic compounds in aqueous solutions preferably to hydrogen and carbon dioxide. APR has also been proved to be an energy-efficient pathway due to no requirement of evaporating feedstock to the gas phase [32]. Some researchers conducted the aqueous-phase steam reforming of methanol (AP-SRM) and it is found that the main challenge for AP-SRM is the development of high- performance catalysts. Furthermore, the catalyst should not be in favor of the cleavage of C—O bonds as well as the hydrogenation of CO and/or CO2. The hydrogenation of CO and CO2 would result in the generation of methane as shown below:
 (11)
(11)
 (12)
(12)
Pt-based catalyst supported by Al2O3 achieves the highest activity and hydrogen selectivity in AP-SRM [33, 34]. The results from SHABAKER et al [33] indicate that the aqueous-phase reforming of oxygenated hydrocarbons (methanol and ethylene glycol) over Pt/Al2O3 catalyst reaches nearly 100% selectivity for the formation of H2. CORTRIGHT et al [35] demonstrated that hydrogen can be produced from alcohols at near 500 K through an aqueous-phase reforming process by using a platinum-based catalyst, with ethylene glycol and methanol being almost completely converted into hydrogen and carbon dioxide. STEKROVA et al [36] conducted the aqueous- phase reforming of methanol experiments over nickel modified Ce, Zr, and La oxides. Methanol conversion >50% and hydrogen production efficiency >40% can be achieved with the Ni/25%CeO2-ZrO2 catalyst. Cyano-carboxylic modified carbon nitride was conducted by TAN et al [37] for enhancing photocatalytic hydrogen generation from aqueous-phase methanol reforming, by the synthesis procedure shown in Figure 7. It is found that the cyano-carboxylic improved carbon nitride about 205 times more active than unmodified carbon nitride, exhibiting enhanced methanol reforming performance for hydrogen production.
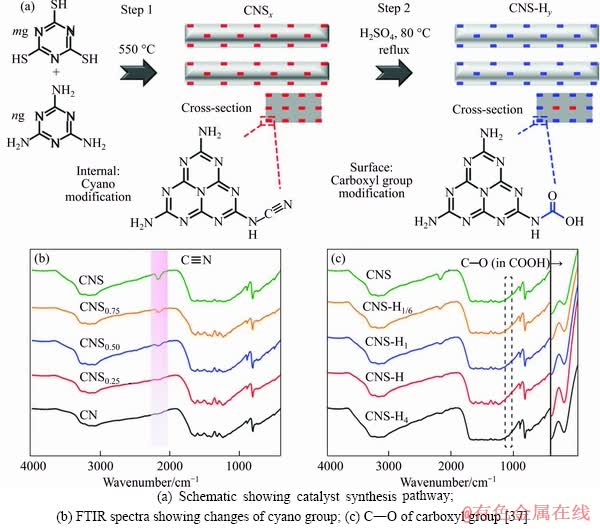
Figure 7 Identification of cyano and carboxyl groups on carbon nitride:
2.2 Partial oxidation of methanol
Compared with SRM, POM is an exothermic reaction with the advantages of high reaction kinetics, no additional heat required when the reaction reaches a steady-state, and the produced hydrogen-rich gas can be also utilized as the supply source of the fuel cell [38-41]. Recent studies from KIM et al [42] indicate that POM is a combination of combustion of methanol (COM) and SRM reaction. In this case, POM could be a complicated reaction, which was affected by the partial pressure of oxygen and the reaction temperatures. At the beginning, high O2 partial pressure leads to the occurrence of COM; the overall reaction will gradually transform from COM to POM due to the generation of H2O from COM. Therefore, the displayed POM reaction shown below can be regarded as an intermediate state between COM and SRM.
Partial oxidation of methanol:
 (13)
(13)
On one hand, the POM produces a lower concentration of hydrogen compared with SRM which is attributed to the oxidation of methanol to CO2. Moreover, the oxygen is required to complete the oxidation of methanol, which significantly increase the energy consumption of the system and increases the cost of transportation; On the other hand, the POM reaction is necessary when the startup process of the hydrogen-driven vehicle is considered. Some researchers propose the utilization of waste heat from PEMFCs to maintain the auto-thermal operation. This idea can be realized after the stable operation of hydrogen- driven vehicles. Regarding the startup process, the exothermic POM reaction could be a feasible method for supplying sufficient heat and driving the normal operation of the fuel cell.
It is reported that copper-based catalyst supported by zinc oxide performed high methanol conversion and H2 selectivity in POM reaction [43-46]. LEE et al [38] proposed a simple method for modulating the oxygen vacancies on metallic Cu/ZnO catalysts for partial oxidation of methanol with low CO generation. For the catalytic test, the mole ratio of oxygen to methanol was controlled at 0.5. The gas hourly space velocity (GHSV) and weight hourly space velocity (WHSV) were controlled at 60000 h-1 and 9.48 h-1, respectively. It is found that the efficiency of both oxygen vacancies contained Cu/ZnO samples prepared by co-precipitation and deposition precipitation is excellent with 100% methanol conversion and 95% of hydrogen in methanol being converted to hydrogen at 250 °C. When the experiments were carried out at 150 °C, 70% methanol can be transformed and 75% of hydrogen in methanol is converted to hydrogen. It is concluded that the oxygen vacancies on the surface can promote an affinity for adsorbing the oxygen atoms of reactants, induce the decomposition of intermediates species, and catalyze the carbon monoxide oxidation at a relatively low temperature.
CARRAZAN et al [47] conducted an interesting work by adding the reaction products (CO2, H2, and CO2+H2) to the feed during partial oxidation of methanol using a Cu/ZnO/Al2O3 catalyst. The addition of reaction products changes the performance of the catalyst compared with the standard condition with no product gas feeding. Importantly, by modulating the composition of reaction feeds, CO-free hydrogen can be generated which was essential for the re-utilization of gaseous products and heat from the outlet of PEMFC. It is inferred that the existence of reaction products significantly influences the oxidation state of Cu. Moreover, the suppression of CO generation could be attributed to the high content of CuO. Thus, it is suggested that by utilizing the reaction products can promote the CuO generation and inhibits the formation of CO, which could be a potential pathway for the suppression of CO generation during POM and the utilization of waste heat from PEMFC.
2.3 Oxidative steam reforming of methanol
The OSRM process combines the SRM and POM reactions. The OSRM process can directly produce a relatively high concentration of hydrogen at the places where it is needed, obtaining a mixture of H2 and CO2. The mole ratio of H2/CO2 is between 2 and 3. By combining a suitable proportion of endothermic SRM reaction and exothermic POM reaction, no external heat is required [48]. It is reported that the combustion of methanol occurs first followed by SRM, in which the SRM starts when substantial of O2 is converted [42, 49]. Thus, the heat released by complete oxidation of methanol can be used to provide the energy required for SRM. The overall OSRM reaction is shown below:
 (14)
(14)
On one hand, the auto-thermal conversion of methanol makes OSRM reaction more flexible, in which the OSRM can even be applied in mobile phones, laptops, meteorological stations, as well as some other low-power devices. It also performs the properties of quick startup and shutdown. On the other hand, the requirement of oxygen supplement would enhance the system energy consumption and it involves the problem of oxygen carrying and transportation.
Carbon nanotubes were widely explored as the support material for OSRM. SHTYKA et al [50] conducted the carbon nanotube supported Ru, Au, and Pt for OSRM. The surface of carbon nanotubes was functionalized by an acidified potassium permanganate solution. MIERCZYNSKI et al [11] studied the catalytic performance of bimetallic Au-Cu and Au-Ni catalysts supported by multi-walled carbon nanotubes (MWCNTs) in oxy-steam reforming of methanol. The metal phases of Cu and Ni were introduced on the MWCNTs by wet impregnation using aqueous solutions of Cu(NO3)2 and Ni(NO3)2. The gold was introduced on the surface of copper or nickel catalyst by using the deposition-precipitation method [51]. Characterizations such as X-ray diffraction (XRD), scanning electron microscopy with X-ray microanalysis (SEM-EDS), thermos-gravimetric analysis, NH3 temperature-programmed desorption (NH3-TPD) were carried out to analyze the performance of MWCNT supported catalysts.The formations of MWCNTs supported Au-Cu and Au-Ni alloys are proved after their reduction under the conditions of 5 vol.% H2 in Ar at 300 °C and 500 °C, respectively. Compared to the monometallic system, the OSRM reaction at 300 °C with applied Au-Cu performs significant improvements in the catalytic activity and H2 selectivity, which may be attributed to the formation of alloy compounds. Furthermore, bimetallic Au-Ni/MWCNTs catalyst performs the best property from the perspective of CO selectivity. It is to be noted that the Au-Cu/MWCNTs catalyst exhibited almost 100% methanol conversion and CO was not detected, which is expected to be utilized in fuel cell if the cyclic stability can be further verified.
Cu-Zn-loaded anodic aluminum oxide plate catalyst has been proposed for OSRM reaction. KIM et al [52] conducted the OSRM reaction over an anodic aluminum oxide-supported Cu-Zn catalyst. A strongly bonded porous alumina layer is grown on an alumina metal surface by anodic oxidation, which serves as good support for loading active component [53-55]. They verified that the high thermal conductivity of the catalyst is conducive to suppress the hot spot formation, making the temperature profile smooth along the reactor. EAIMSUMANG et al [56] investigated the dependence of CeO2 morphology in the copper oxide on CeO2 supports for OSRM reaction. Different morphologies of CeO2, which includes rod-shaped, cube-shaped, and mixed shapes, are prepared by changing the hydrothermal temperature from 100 °C to 220 °C. It is concluded that the morphological structure of CeO2 shows a great impact on its catalytic activity in the OSRM reaction. The rod-shaped CeO2 performed the highest performance for methanol conversion and CO suppression which could be attributed to the well dispersion of CuO nanoparticles, the existence of Cu+ species as the active site and oxygen vacancies, as well as the stronger interaction between CuO and CeO2.
More recently, SUN et al [57] reported chemical looping-based oxidative steam reforming of methanol, which is seen as a potential pathway for auto-thermal hydrogen generation. The catalytic oxygen carrier, Cu2O-Ca2Fe2O5, was designed for the chemical looping oxidative steam reforming of methanol experiments, in which the lattice oxygen in Cu2O-Ca2Fe2O5 participates the CH3OH-to-H2 conversion (see Figure 8). Results indicate that the 40 mol.% Cu-loaded Cu2O-Ca2Fe2O5 shows the highest catalytic activity of the synthesized catalytic oxygen carriers, and the presence of Ca2Fe2O5 tunes the redox activity and mobility of the lattice oxygen, obtaining a H2 production rate of 37.6 μmol H2/(g COC·s) under a temperature of 240 °C.
2.4 Sorption-enhanced SRM
According to the main reactions of SRM as shown in Reaction 3, CO2 and H2 are the main products with no carbon monoxide production. Nevertheless, SRM is generally accompanied by side reaction with the generation of methane and carbon monoxide. The sorption-enhanced methanol steam reforming (SE-SRM) could be a potential approach for significantly suppressing the occurrence of side reactions with high-purity hydrogen production. The medium-temperature adsorbent is required for the SE-SRM process, in which the carbon dioxide produced from methanol conversion can be absorbed by the MgO or MgO-based hydrotalcite, promoting the equilibrium of water-gas shift reaction to move forward. Thus, a few researchers explored the sorption-enhanced SRM to obtain high-purity hydrogen with a lower concentration of CO production [58-60]. LI et al [59] conducted a sorption-enhanced methanol reforming process in conjunction with PEMFCs by using the software Honeywell UniSim Design. The flowchart of the SE-SMR integrated with PEMFC was illustrated in Figure 9. A time-dependent model based on detailed reaction kinetics is developed to describe the reactor dynamics of methanol reforming (CO2 adsorption) and regeneration stage (CO2 desorption). Results reveal that the SE-SMR reduces the concentrations of both CO2 and CO from methanol conversion, thereby producing high-purity H2 in a single unit.
Design. The flowchart of the SE-SMR integrated with PEMFC was illustrated in Figure 9. A time-dependent model based on detailed reaction kinetics is developed to describe the reactor dynamics of methanol reforming (CO2 adsorption) and regeneration stage (CO2 desorption). Results reveal that the SE-SMR reduces the concentrations of both CO2 and CO from methanol conversion, thereby producing high-purity H2 in a single unit.
WU et al [58] conducted both thermodynamic calculation and experimental investigation of the sorption-enhanced steam methanol reforming process. The results from the thermodynamic calculation applying the software Matlab showed that a CO2 adsorption ratio of 95%, hydrogen concentration of 98.36 vol.%, CO concentration of 32.8×10-6 could be obtained under a steam/methanol mole ratio of 2. For the experimental exploration, it was performed in a fixed-bed reactor loaded with commercial CuO/ZnO/Al2O3 catalyst and 22%K2CO3-promoted hydrotalcite as the moderate-temperature CO2 sorbent. More importantly, the reaction temperature was found to decrease by 50 °C and the hydrogen concentration was increased by 20% of SE-SMR under a same methanol conversion compared with the SRM reaction. QI et al [61] presented a similar work by combining copper-based catalysts with hydrotalcite for SE-SMR. Two kinds of packed modes are studied: as for case I, the reactor was packed with the mixture of K-hydrotalcite particles and CuO/ZnO/Al2O3 particles; for case II, a composite sorbent-catalyst was fed into the reactor. Results revealed that both the packing modes could significantly promote the SRM reaction. However, by combining the CuO/ZnO/Al2O3 catalyst with K-hdyrotalcite to be a composite material, the methanol conversion performed a decreasing tendency due to the loss of catalytic activity under the alkaline atmosphere under K-hydrotalcite.

Figure 8 Schemic of chemical looping oxidative stream reforming of methanol [57]

Figure 9 Simulation flowchart of absorption-enhanced reforming (AER) of methanol with integration of PEMF [59]
3 Catalysts
Catalyst is the core of the methanol conversion process, in which all the above-mentioned reactions cannot achieve the efficient conversion of methanol without the utilization of catalysts. The most commonly utilized catalytic system can be classified into copper-based catalysts and noble- metal catalysts. With the development of the catalysis, some advanced materials such as bi-functional alloys, metal-organic frameworks (MOFs), and single-atom catalysts (SACs) were also gradually developed for efficient methanol conversion. Thus, this part summarizes the latest developments on catalysts as shown in Table 1 for Cu-based catalysts and Table 2 for noble metal catalysts and other activity-improved catalysts.
3.1 Cu-based catalysts
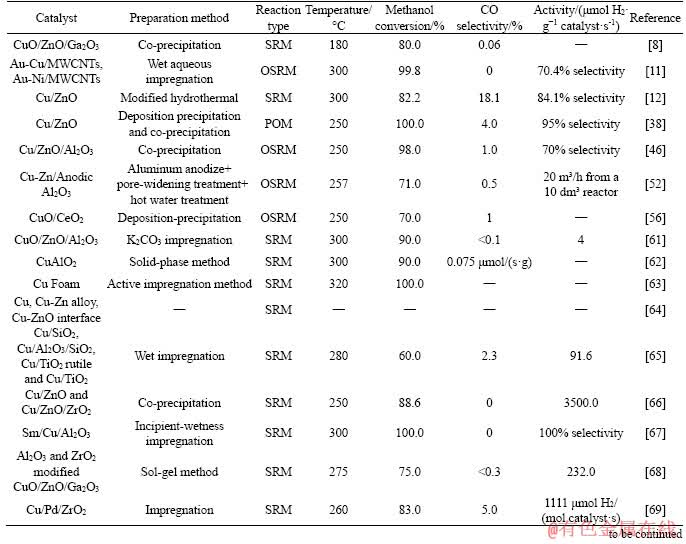
MA et al [93] reported a nickel-phyllosilicate supported Cu-Ni bimetallic catalysts for the steam reforming of dimethyl ether (DME). The reforming of DME mainly concludes the hydrolysis of DME with methanol generated as the intermediate and methanol steam reforming with the production of carbon dioxide and hydrogen. By designing the formation of Cu-Ni alloy, the selectivity of CO and CH4 can be suppressed with high-concentration hydrogen and ultra-low-concentration CO (<1000×10-6) production. The morphologies of the synthesized samples were presented in Figure 10. The d-spacing of 0.207 nm in CuNi-PS-400 sample indicates the formation of Cu0.81Ni0.19(111) alloy. Their mechanistic investigations illustrate that the Cu-Ni alloy promotes the adsorption of CO at relatively high temperatures, simultaneously suppressing the CO dissociation to CH4. MAYR et al [121] designed the Cu-Zr bimetallic material Cu51Zr14, which was characterized by high- resolution electron microscopy and energy- dispersive X-ray spectroscopy. The microstructural evolution and DTA/TG/MS analysis were investigated to analyze the surface and bulk structure, as well as the composition of the CO2-selective state in SRM with the structural steering effects identification. They concluded that the newly modulated Cu-ZrO2 interface could be a significant descriptor steering the CO2 selectivity.
Table 1 Summary of methanol reforming works for copper-based catalysts
Continued

Table 2 Summarize of methanol reforming works for non-copper-based catalysts

3.2 Noble metal catalysts
LI et al [102] proposed the modulation of the ZnPd intermetallic catalyst which was used for SRM reaction. It is observed that the reactivity of Pd/ZnO changes with the formation of ZnPd intermetallic compounds and the ZnPd/ZnO interface. Moreover, the addition of Pd decreases the energy gap by increasing the adsorption heat of methanol to 73 kJ/mol, while decreasing the adsorption energy of water to 96 kJ/mol, making the SRM reaction proceed at a lower temperature. Of the tested three catalysts (ZnO, Pd/ZnO, and ZnPd/ZnO), ZnPd/ZnO performs the best catalytic activity because of the competitive adsorption and subsequent reaction between the reactants.
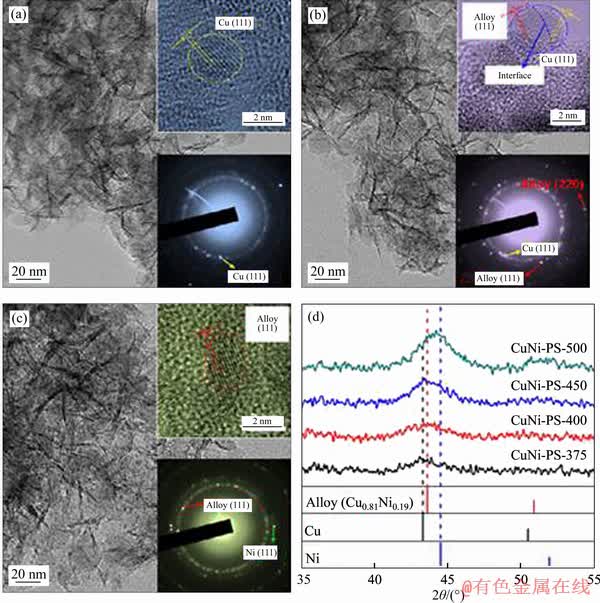
Figure 10 TEM images of nickel-phyllosilicate supported Cu-Ni bimetallic reduced at 375 °C (CuNi-PS-375) (a), nickel-phyllosilicate supported Cu-Ni bimetallic reduced at 400 °C (CuNi-PS-400) (b), nickel-phyllosilicate supported Cu-Ni bimetallic reduced at 500 °C (CuNi-PS-500) (c) and XRD patterns of CuNi-PS-T samples (d) [93]
3.3 Plasma-assisted catalyst
Researchers have reported that plasma treatment can substantially change the performance of the catalysts. Glow discharge plasma belongs to a non-thermal plasma, which can be used for the surficial treatment of the catalysts before the calcination process [122-124]. In this work of Bagherzadeh, CuO/ZnO/Al2O3 catalyst was prepared by using hydrothermal and co-precipitation methods followed by the treatment of glow discharge plasma [125]. The synthesized catalysts were exposed to glow discharge plasma for 45 min at 1000 V, and the plasma-assisted CuO/ZnO/Al2O3 catalyst was studied for SRM reaction. According to the characterizations (XRD and EDX mapping) of the plasma-assisted samples, it is found that the glow discharge plasma leads to more dispersion of CuO(111) crystallite plate, and furthermore, plasma-assisted and co-precipitation synthesized CuO/ZnO/Al2O3 performs the best uniform dispersion of the elements. The experimental setup for SRM using plasma-treated CuO/ZnO/Al2O3 catalyst is displayed in Figure 11. By applying a 1 kV voltage, generated from a high voltage DC generator, between the cathode and anode electrodes, non-thermal glow discharge plasma can be produced in the plasma chamber with samples treatment. Results revealed that the plasma-assisted precipitation synthesized CuO/ZnO/Al2O3 sample performs the highest methanol conversion and the lowest carbon monoxide selectivity. Moreover, the plasma- modified catalyst can maintain its original catalytic activity after long-term SRM reaction.
Similar treatments were conducted by SEYEDI et al [94]. A series of CuO/ZnO/Al2O3/ ZrO2 catalyst was prepared by using hybrid coprecipitation-plasma methods with hydrothermal, thermos-chemical, and coprecipitation being utilized for comparison. Results indicate that the plasma-assisted CuO/ZnO/Al2O3/ZrO2 sample achieves a methanol conversion of 100% at 240 °C and it remains a high catalytic performance after 24 h. LIAN et al [126] also performed an oxidative pyrolysis reforming of methanol in warm plasma for on-board hydrogen generation, in which energy efficiency of 74%, energy cost of 0.45 kW·h/(N·m3),and methanol conversion of 88% can be achieved under the conditions of η(O2)/η(C)=0.30, η(S)/η(C)=0.5, and specific energy input equals 24 kJ/mol.

Figure 11 Experimental setup for assessment of CuO/ZnO/Al2O3 catalyst for SRM under assistant of plasma [125]
3.4 Metal-organic frameworks
MOF related materials are deserved to be exploited to reduce the reaction temperature required for SRM while decreasing CO selectivity. Preparation methods like co-precipitation, carbon template, homogeneous precipitation, hydrothermal synthesis, wet impregnation are mostly used approaches, and it is found that the catalytic performance is strongly related to the preparation methods. Metal-organic-framework (MOF), highly porous crystalline materials constructed from metal templates and organic linkers, have been widely developed due to their promising applications such as catalysis and CO2 capture [127-129]. ZENG et al [119] reported a Pd/ZnO catalyst which was derived from zeolite imidazolate framework-8 (ZIF-8) as the precursor, in which NaHB4 reduced Pd ions were supported by the ZIF-8. The synthesis process of the MOF material is presented in Figure 12. By using the MOF precursor derived Pd@ZnO alloy, the methanol conversion reaches as high as 98% and CO2 selectivity at 86.3% under the conditions of 0.1 MPa, steam/CH3OH mole ratio at 1.2, and WHSV=43.2 L CH3OH/(g catalyst·h)with 0.1 g catalyst addition.

Figure 12 Synthesis process of Pd nanoparticles on MOF (a) and illustration of possible mechanism of SRM on PdZn alloy (b) [119]
3.5 Single-atom catalysts
In the recent five years, single-atom catalysts (SACs) are seen as a new frontier in heterogeneous catalysis and have been paid much attention due to their specific catalytic properties [130-133]. SACs have demonstrated their superior performances in many catalytic reactions including CO oxidation [134], water-gas shift reaction [135], selective hydrogenation/oxidation [136, 137]. GU et al [120] reported single Pt1 and Au1 atoms stabilized by the lattice oxygen on ZnO(1010) facet for SRM reaction. Their density function theory (DFT) results indicate that the catalysis with applied single precious metal atoms can lower the reaction barriers, change on the reaction pathways, and substantially improve the catalytic activity, owing to their strong binding to the intermediates. The most favorable reaction pathway for SRM on ZnO, SA-Au1/ZnO, and SA-Au1/ZnO is presented in Figure 13. The reaction pathways can be classified into two pathways: I, the association of HCHO from CH3OH with —OH from H2O; and II, decomposition of HCHO to CO accompanied by water-gas shift reaction. Experimental results show that the catalytic activity of single Pt1 sites was 1000 times higher than that of ZnO, which provides valuable insights for the supported single atoms utilization in methanol conversion. CAO et al [125] recently proposed single-atom gold oxo-clusters for catalyzing the heterogeneous methanol self- coupling reaction. The experimental results indicate that the single-atom gold oxo-clusters prepared in an alkaline solution can direct the heterogeneous coupling of two methanol molecules to methyl formate and hydrogen with a 100% selectivity below 180 °C. Catalytic methanol coupling provides a new approach for methanol conversion to hydrogen.
4 Steam methanol reformer
The design of a steam methanol reformer is essential for methanol reforming to hydrogen. To achieve its connection with fuel cells and application in hydrogen vehicles, the reactor should generally have a high surface-area-to-volume ratio,high heat transfer efficiency to make full use of the waste heat from the fuel cell [138]. The reactor should also be capable of maintaining isothermal conditions. In this part, the recently reported state-of-the-art micro steam methanol reformers were thoroughly summarized as shown in Table 3.
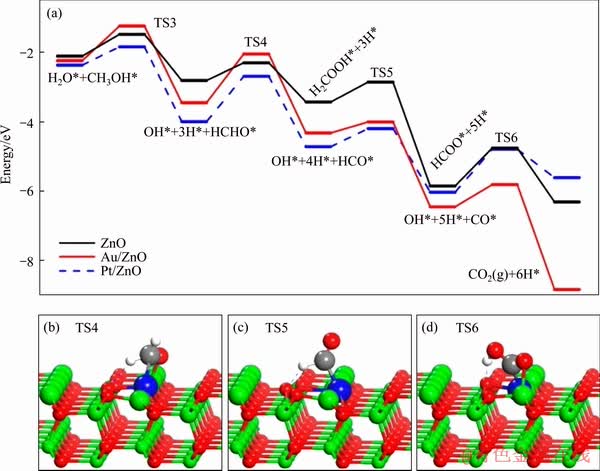
Figure 13 Most favorable reaction pathways for CH3OH*+H2O*→CO2+6H* on ZnO, Au1/ZnO, and Pt1/ZnO without showing TS1 and TS2 explicitly (a), schematic structures for transition state TS4 (b), TS5 (c) and TS6 (d) on Pt1/Au1/ZnO(1010) [120]
Table 3 Summary of research on design and development of reactors for methanol conversion
Continued
Continued
TIAN et al [169] designed a micro-reactor for SRM, with structured PdZnAl/Cu fiber catalyst filling in the micro reactor. The reaction conditions were studied to optimize the operation parameters. Their micro-reactor system is mainly composed of an inlet system, a micro-reactor, a condenser, and a product detector. The schematic diagram of the proposed micro-reactor structure is presented in Figure 14. It can be observed that the micro reforming is mainly composed of an inlet tube, heating cartridges, thermocouples, a reactor body, and the outlet tube. The reactor body includes an evaporation chamber and a reforming chamber loaded with foam metal for catalytic methanol reforming. The novel structured fiber catalyst with the incorporated micro-reactor shows great potential in the field of hydrogen production for fuel cell utilization.
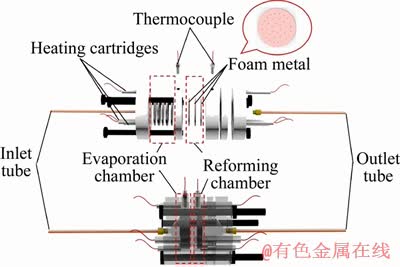
Figure 14 Schematic diagram of utilized micro steam methanol reformer [169]
ZHENG et al [139] conducted the performance optimization of SRM for hydrogen production with applying an error backpropagation and a genetic algorithm method via a cylindrical micro reactor. According to Figure 15, the micro reactor includes a main chamber, an inlet tube, an outlet tube, thermocouples, and some heating cartridges. The main chamber is formed by stacking the evaporation chamber and the reforming chamber. The porous copper fiber was put inside the reforming chamber, catalyzing the SRM at specific temperatures. LYTKINA et al [141] discussed the performance of SRM in a conventional flow reactor and a membrane reactor by using a Pd-Ru alloy membrane on the bimetallic catalyst (Pt-Ru, Pd-Ru, Rh-Ru) supported on detonation nanodiamond. The schematic illustration of the membrane reactor is displayed in Figure 16. In a typical experiment,0.3 g sample was mixed with granulated quartz followed by placing the solid mixture to the reaction zone of membrane reactor. The membrane reactor is mainly comprised of two stainless steel compartments in the form of parallelepipeds, which was separated by a dense Pd-Ru alloy membrane. The thickness of the membrane was 60 μm. Moreover, the reactor was hermetically sealed using two washers, which are copper washer and graphlex washer, respectively. Results indicate that stable hydrogen can be obtained from the outlet of the membrane reactor. No CO and other impurities were detected in the permeate zone, even at the temperature of 400 °C.
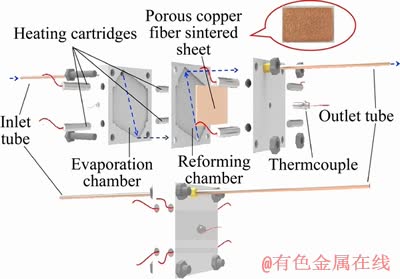
Figure 15 Plate micro reactor for SRM with hydrogen production [139]

Figure 16 Schematic illustration of membrane reactor for steam methanol reforming [140]
RIBEIRINHA et al [161] conducted the experimental and modeling research by integrating the HT-PEMFC with a cellular membrane reforming for SRM, which is a very interesting work. In their work, the combination of packed bed membrane reactor cell with a HT-PEMFC was proposed as displayed in Figure 17. The 4-μm thick self-supported Pd-Ag membrane is obtained by magnetron-sputtering. The prepared membranes showed a H2/N2 mole selectivity of ca. 5800 and permeability to the hydrogen of 2.94×10–6 mol·m·s–1·m–2·bar–0.8 at 473 K without activation treatment. In addition, the device allowed efficient heat integration, where the heat produced in the electrochemical process was used to perform the SRM reaction.
TAJRISHI et al [151] utilized Zn, Ce, and Zr modified mesoporous Cu/SBA-15 nanocatalyst for SRM in a microchannel reactor. The plates of microreactor, catalyst-coated microchannel plate, and assembled fuel processor with cartridge heaters and K-type thermocouples are shown in Figure 18. The size of the designed parallel-type microchannel reactor is 12 cm×8 cm×1 cm with 20 parallel microchannels being utilized. In a typical experiment, a homogeneous suspension comprised of catalyst, hydroxypropyl cellulose, and distilled water was obtained by constantly stirring at 60 °C followed by the injection of the slurry into the microchannels. SRM process with applied microchannel was conducted under the conditions of 300 °C, WHSV at 43.68 h-1, and the S/C molar ratio of 2. The methanol conversion and hydrogen selectivity achieved 95.2% and 94.6%, respectively.

Figure 17 Assembly scheme of integrated HT-PEMFC and steam methanol reformer:

Figure 18 Plates of microreactor (a), catalyst-coated microchannel plate (b) and assembled fuel processor with cartridge heaters and K-type thermocouples (c) [151]
5 Conclusions
In-situ methanol conversion to hydrogen is considered a potential pathway for solving the challenges of hydrogen storage and transportation. In this work, the superiority and the limitation of methanol reforming including the utilization pathways, catalysts, as well as reformers were discussed deeply, which aims at providing the implications for methanol derived hydrogen utilization in the PEMFCs.
In the conversion section, the detailed methanol conversion pathways for hydrogen production are thoroughly reviewed, which includes the steam reforming of methanol, partial oxidation of methanol, oxidative steam reforming of methanol, and sorption-enhanced steam methanol reforming. The OSRM and POM reactions are recommended for providing hydrogen during the startup of PEMFCs due to the auto-thermal or exothermic operation. SMR and SE-SMR reactions are more suitable for hydrogen supply during the stable operation of PEMFCs to obtain a higher quality of the hydrogen. Notably, the methanol coupling reaction is a satisfying approach for high-purity hydrogen and methyl format co-generation except for the methanol reforming reactions. In the catalyst section, recently developed catalysts for methanol- to-hydrogen conversion have also been intensively elaborated. In addition to the widely studied copper- based catalysts and noble metal catalysts, single- atom catalysts, metal-organic frameworks, as well as plasma-assisted catalysts also make a great breakthrough in catalytic methanol conversion, which further expanded the catalytic system for hydrogen production from methanol reforming. In the reactor section, different types of reformers used for methanol reforming were introduced, which includes membrane reactors, microchannel reformers, laminated SRM microreactors, etc. The development of steam methanol reformers should completely consider the issues of heat transfer, flow characteristics, catalyst loading, and matching with fuel cells, etc.
In response to the above-discussed methanol utilization pathways, methanol conversion catalysts, and methanol steam reformers, the research orientations to be solved and/or optimized in the near future are proposed as follows:
1) The temperature required for methanol conversion can be further reduced under the premise of ensuring a relatively high methanol conversion;
2) The carbon monoxide concentration can be controlled at a lower level to prevent the deactivation of the electrode materials in fuel cells;
3) The catalysts that perform a high catalytic activity and long-term stability are highly desired;
4) The design of the reformer should enhance the heat transfer from PEMFC to micro reformer, maximizing the energy efficiency of the integrated fuel cell system.
References
[1] MU Z L, BU S C, XUE B. Environmental legislation in china: Achievements, challenges and trends [J]. Sustainability, 2014, 6(12): 8967-8979. DOI: 10.3390/su6128967.
[2] ZHANG J, XIANG Y, LU S F, JIANG S P. High temperature polymer electrolyte membrane fuel cells for integrated fuel cell-methanol reformer power systems: A critical review [J]. Advanced Sustainable Systems, 2018, 2: 8-9. DOI: 10.1002/adsu.201700184.
[3] HOSSEINI S S, MEHRPOOYA M, ALSAGRI A S, ALROBAIAN A A. Introducing, evaluation and exergetic performance assessment of a novel hybrid system composed of MCFC, methanol synthesis process, and a combined power cycle [J]. Energy Conversion and Management, 2019, 197: 111878. DOI: 10.1016/j.enconman.2019.111878.
[4] MEHRPOOYA M, SADEGHZADEH M, RAHIMI A, POURIMAN M. Technical performance analysis of a combined cooling heating and power (CCHP) system based on solid oxide fuel cell (SOFC) technology—A building application [J]. Energy Conversion and Management, 2019, 198: 111767. DOI: 10.1016/j.enconman.2019.06.078.
[5] WANG J L, WANG H F, HU P. Theoretical insight into methanol steam reforming on indium oxide with different coordination environments [J]. Science China: Chemistry, 2018, 61(3): 336-343. DOI: 10.1007/s11426-017-9139-x.
[6] KAFTAN A, KUSCHE M, LAURIN M, WASSERSCHEID P, LIBUDA J. KOH-promoted Pt/Al2O3 catalysts for water gas shift and methanol steam reforming: An operando DRIFTS-MS study [J]. Applied Catalysis B: Environmental, 2017, 201: 169-181. DOI: 10.1016/j.apcatb.2016.08.016.
[7] MCNICOL B D, RAND D A J, WILLIAMS K R. Fuel cells for road transportation purposes—Yes or no [J]? Journal of Power Sources, 2001, 100(1): 47-59. DOI: 10.1016/S0378- 7753(01)00882-5.
[8] RIBEIRINHA P, MATEOS P C, BOAVENTURA M, SOUSA J, MENDES A. CuO/ZnO/Ga2O3 catalyst for low temperature MSR reaction: Synthesis, characterization and kinetic model [J]. Applied Catalysis B: Environmental, 2018, 221: 371-379. DOI: 10.1016/j.apcatb.2017.09.040.
[9] SILVA H, MATEOS P C, RIBEIRINHA P, BOAVENTURA M, MENDES A. Low-temperature methanol steam reforming kinetics over a novel CuZrDyAl catalyst [J]. Reaction Kinetics, Mechanisms and Catalysis, 2015, 115(1): 321-339. DOI: 10.1007/s11144-015-0846-z.
[10] SA S, SILVA H, BRANDAO L, SOUSA J M, MENDES A. Catalysts for methanol steam reforming-A review [J]. Applied Catalysis B: Environmental, 2010, 99(1, 2): 43-57. DOI: 10.1016/j.apcatb.2010.06.015.
[11] MIERCZYNSKI P, VASILEV K, MIERCZYNSKA A, MANIUKIEWICZ W, SZYNKOWSKA M I, MANIECKI T P. Bimetallic Au-Cu, Au-Ni catalysts supported on MWCNTs for oxy-steam reforming of methanol [J]. Applied Catalysis B: Environmental, 2016, 185: 281-294. DOI: 10.1016/j.apcatb.2015.11.047.
[12] MATEOS P C, SILVA H, TANAKA D A P, LIGUORI S, LULIANELLI A, BASILE A, MENDES A. CuO/ZnO catalysts for methanol steam reforming: The role of the support polarity ratio and surface area [J]. Applied Catalysis B: Environmental, 2015, 174: 67-76. DOI: 10.1016/ j.apcatb.2015.02.039.
[13] WANG C Y, BOUCHER M, YANG M, SALTSBURG H, FLYTZANI S M. ZnO-modified zirconia as gold catalyst support for the low-temperature methanol steam reforming reaction [J]. Applied Catalysis B: Environmental, 2014, 154: 142-152. DOI: 10.1016/j.apcatb.2014.02.008.
[14] ZHANG H, SUN J M, DAGLE V L, HALEVI B, DATYE A K, WANG Y. Influence of ZnO facets on Pd/ZnO catalysts for methanol steam reforming [J]. ACS Catalysis, 2014, 4(7): 2379-2386. DOI: 10.1021/ cs500590t.
[15] XU X H, SHUAI K P, XU B. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen [J]. Catalysts, 2017, 7(6): 183. DOI: 10.3390/catal7060183.
[16] KUBACKA A, FERNANDEZ G M, MARTINEZ A A. Catalytic hydrogen production through WGS or steam reforming of alcohols over Cu, Ni and Co catalysts [J]. Applied Catalysis A: General, 2016, 518: 2-17. DOI: 10.1016/j.apcata.2016.01.027.
[17] SCHULLER G, VAZQUEZ F V, WAIBLINGER W, AUVINEN S, RIBEIRINHA P. Heat and fuel coupled operation of a high temperature polymer electrolyte fuel cell with a heat exchanger methanol steam reformer [J]. Journal of Power Sources, 2017, 347: 47-56. DOI: 10.1016/ j.jpowsour.2017.02.021.
[18] RIBEIRINHA P, SCHULLER G, BOAUENTURA M, MENDES A. Synergetic integration of a methanol steam reforming cell with a high temperature polymer electrolyte fuel cell [J]. International Journal of Hydrogen Energy, 2017, 42(19): 13902-13912. DOI: 10.1016/j.ijhydene.2017.01.172.
[19] THATTARATHODY R, ARTOUL M, DIGILOV R M, SHEINTUCH M. Pressure, diffusion, and S/M ratio effects in methanol steam reforming kinetics [J]. Industrial & Engineering Chemistry Research, 2018, 57(9): 3175-3186. DOI: 10.1021/acs.iecr.7b05033.
[20] THATTARATHODY R, SHEINTUCH M. Kinetics and dynamics of methanol steam reforming on CuO/ZnO/ alumina catalyst [J]. Applied Catalysis A: General, 2017, 540: 47-56. DOI: 10.1016/j.apcata.2017.04.012.
[21] OZCAN O, AKIN A N. Thermodynamic analysis of methanol steam reforming to produce hydrogen for HT-PEMFC: An optimization study [J]. International Journal of Hydrogen Energy, 2019, 44(27): 14117-14126. DOI: 10.1016/j.ijhydene.2018.12.211.
[22] WU H W, HSU T T, FAN C M, HE P H. Reduction of smoke, PM2.5, and NOX of a diesel engine integrated with methanol steam reformer recovering waste heat and cooled EGR [J]. Energy Conversion and Management, 2018, 172: 567-578. DOI: 10.1016/j.enconman.2018.07.050.
[23] WANG J J, WU J, XU Z L, LI M. Thermodynamic performance analysis of a fuel cell trigeneration system integrated with solar-assisted methanol reforming [J]. Energy Conversion and Management, 2017, 150: 81-89. DOI: 10.1016/j.enconman.2017.08.012.
[24] HOSSEINI S S, MEHRPOOYA M, ALSAGRI A S, ALROBAIAN A A. Introducing, evaluation and exergetic performance assessment of a novel hybrid system composed of MCFC, methanol synthesis process, and a combined power cycle [J]. Energy Conversion and Management, 2019, 197: 111878. DOI: 10.1016/j.enconman.2019.111878.
[25] SANTACESARIA E, CARRA S. Kinetics of catalytic steam reforming of methanol in a CSTR reactor [J]. Applied Catalysis B: Environmental, 1983, 5: 345-358.
[26] YI N, SI R, SALTSBURG H, FLYTZANI- STEPHANOPOULOS M. Steam reforming of methanol over ceria and gold-ceria nanoshapes [J]. Applied catalysis B: Environmental, 2010, 95: 87-92. DOI: 10.1016/j.apcatb. 2009.12.012.
[27] BREEN J P, ROSS J R. Methanol reforming for fuel-cell applications: Development of zirconia-containing Cu-Zn-Al catalysts [J]. Catalysis Today, 1999, 51: 521-533. DOI: 10.1016/S0920-5861(99)00038-3.
[28] SHISHIDO T, YAMAMOTO Y, MORIOKA H, TAKEHIRE K. Production of hydrogen from methanol over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation: Steam reforming and oxidative steam reforming [J]. Journal of Molecular Catalysis A: Chemical, 2007, 268: 185-194. DOI: 10.1016/j.molcata.2006.12.018.
[29] SUN Z X, FANG S Y, LIN Y, HU Y H. Photo-assisted methanol steam reforming on solid solution of Cu-Zn-Ti oxide [J]. Chemical Engineering Journal, 2019, 375: 121909. DOI: 10.1016/j.cej.2019.121909.
[30] CHIARELLO G L, FERRI D, SELLI E. In situ attenuated total reflection infrared spectroscopy study of the photocatalytic steam reforming of methanol on Pt/TiO2 [J]. Applied Surface Science, 2018, 450: 146-154. DOI: 10.1016/j.apsusc.2018.04.167.
[31] SHARMA R, KUMAR A, UPADHYAY R K. Bimetallic Fe-promoted catalyst for CO-free hydrogen production in high-temperature-methanol steam reforming [J]. Chemcatchem, 2019, 11(18): 4568-4580. DOI: 10.1002/cctc. 201901062.
[32] HUBER G W, DUMESIC J A. An overview of aqueous-phase catalytic processes for production of hydrogen and alkanes in a biorefinery [J]. Catalysis Today, 2006, 111(1): 119-132. DOI: 10.1016/j.cattod.2005.10.010.
[33] SHABAKER J W, DAVDA R R, HUBER G W, CORTRIGHT R D, DUMESIC J A. Aqueous-phase reforming of methanol and ethylene glycol over alumina- supported platinum catalysts [J]. Journal of Catalysis, 2003, 215(2): 344-352. DOI: 10.1016/S0021-9517(03)00032-0.
[34] DAVDA R R, SHABAKER J W, HUBER G W, CORTRIGHT R D, DUMESIC J A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts [J]. Applied Catalysis B: Environmental, 2005, 56(1, 2): 171-186. DOI: 10.1016/j.apcatb.2004.04.027.
[35] CORTRIGHT R D, DAVDA R R, DUMESIC J A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water [J]. Nature, 2002, 418(6901): 964-967. DOI: 10.1038/nature01009.
[36] STEKROVA M, RINTA P A, KARINEN R. Hydrogen production via aqueous-phase reforming of methanol over nickel modified Ce, Zr and La oxide supports [J]. Catalysis Today, 2018, 304: 143-152. DOI: 10.1016/j.cattod.2017. 08.030.
[37] TAN H, KONG P, LIU M X, GU X M, ZHENG Z F. Enhanced photocatalytic hydrogen production from aqueous-phase methanol reforming over cyano-carboxylic bifunctionally-modified carbon nitride [J]. Chemical Communication, 2019, 55(83): 12503-12506. DOI: 10.1039/c9cc06600d.
[38] LEE K Y, HUANG Y J. Low CO generation on tunable oxygen vacancies of non-precious metallic Cu/ZnO catalysts for partial oxidation of methanol reaction [J]. Applied Catalysis B: Environmental, 2014, 150: 506-514. DOI: 10.1016/j.apcatb.2013.12.044.
[39] WANG Z, XI J, WANG W, LU G. Selective production of hydrogen by partial oxidation of methanol over Cu/Cr catalysts [J]. Journal of Molecular Catalysis A: Chemical, 2003, 191(1): 123-134. DOI: 10.1016/S1381-1169(02) 00352-7.
[40] CUBEIRO M L, FIERRO J L G. Selective production of hydrogen by partial oxidation of methanol over ZnO-supported palladium catalysts [J]. Journal of Catalysis, 1998, 179(1): 150-162.
[41] MO L, ZHENG X, YEH C T. Selective production of hydrogen from partial oxidation of methanol over silver catalysts at low temperatures [J]. Chemical Communication, 2004, 4(12): 1426-1427. DOI: 10.1039/b401463d.
[42] KIM J H, JANG Y S, KIM D H. Multiple steady states in the oxidative steam reforming of methanol [J]. Chemical Engineering Journal, 2018, 338: 752-763. DOI: 10.1016/ j.cej.2018.01.075.
[43] ESPINOSA L A, LAGO R M, PENA M A, FIERRO J L G. Mechanistic aspects of hydrogen production by partial oxidation of methanol over Cu/ZnO catalysts [J]. Topics in Catalysis, 2003, 22(3, 4): 245-251. DOI: 10.1023/ A:1023663604190.
[44] AGRELL J, BIRGERSSON H, BOUTONNET M, MELIAN-CABRERA I, NAVARRO R M, FRERRO J L G. Production of hydrogen from methanol over Cu/ZnO catalysts promoted by ZrO2 and Al2O3 [J]. Journal of Catalysis, 2003, 219(2): 389-403. DOI: 10.1016/s0021-9517 (03)00221-5.
[45] AGRELL J, BOUTONNET M, FIERRO J L G. Production of hydrogen from methanol over binary Cu/ZnO catalysts: Part II. Catalytic activity and reaction pathways [J]. Applied Catalysis A: General, 2003, 253(1): 213-223. DOI: 10.1016/S0926-860X(03)00521-0.
[46] AGRELL J, HASSELBO K, JANSSON K, JARAS S G, Boutonnet M. Production of hydrogen by partial oxidation of methanol over Cu/ZnO catalysts prepared by microemulsion technique [J]. Applied Catalysis A: General, 2001, 211(2): 239-250. DOI: 10.1016/S0926-860X(00)00876-0.
[47] CARRAZAN S R G, WOJCIESZAK R, BLANCO R M, MATEOS P C, RUIZ P. Modulation of the selectivity in partial oxidation of methanol over CuZnAl catalysts by adding CO2 and/or H2 into the reaction feed [J]. Applied Catalysis B: Environmental, 2015, 168: 14-24. DOI: 10.1016/j.apcatb.2014.12.019.
[48] TURCO M, BAGNASCO G, CAMMARANO C, SENESE P, COSTANTINO U, SISANI M. Cu/ZnO/Al2O3 catalysts for oxidative steam reforming of methanol: The role of Cu and the dispersing oxide matrix [J]. Applied Catalysis B: Environmental, 2007, 77(1, 2): 46-57. DOI: 10.1016/j.apcatb.2007.07.006.
[49] REITZ T L, CZAPLEWSKI K F, LANG J C, POPP K E, KUNG H H. Time-resolved XANES investigation of CuO/ZnO in the oxidative methanol reforming reaction [J]. Journal of Catalysis, 2001, 199(2): 193-201. DOI: 10.1006/jcat.2000.3141.
[50] SHTYKA O, HIGASHINO Y, KEDZIORA A, DUBKOV S, GROMOV D, MANIECKI T P. Monometallic Ru, Au, and Pt catalysts deposited on carbon nanotubes for oxidative steam reforming of methanol [J]. Fibre Chemistry, 2018, 50(4): 301-305. DOI: 10.1007/ s10692-019-09980-9.
[51] SANGEETHA P, CHANG L H, CHEN Y W. Gold catalysts on TiO2 support for preferential oxidation of CO in H2 stream: Effect of base agent [J]. Materials Chemistry and Physics, 2009, 118(1): 181-186. DOI: 10.1016/ j.matchemphys.2009.07.022.
[52] KIM D H, KIM J H, JANG Y S, KIM J C. Hydrogen production by oxidative steam reforming of methanol over anodic aluminum oxide-supported Cu-Zn catalyst [J]. International Journal of Hydrogen Energy, 2019, 44(20): 9873-9882. DOI: 10.1016/j.ijhydene.2018.11.009.
[53] GANLEY J C, RIECHMANN K L, SEEBAUER E G, MASEL R I. Porous anodic alumina optimized as a catalyst support for microreactors [J]. Journal of Catalysis, 2004, 227(1): 26-32. DOI: 10.1016/j.jcat.2004.06.016.
[54] POINERN G E J, ALIAND N, FAWCETT D. Progress in nano-engineered anodic aluminum oxide membrane development [J]. Materials, 2011, 4: 487-526. DOI: 10.3390/ma4030487.
[55] LINGA R E, KARUPPIAH J, LEE H C, KIM D H. Steam reforming of methanol over copper loaded anodized aluminum oxide (AAO) prepared through electrodeposition [J]. Journal of Power Sources, 2014, 268: 88-95. DOI: 10.1016/j.jpowsour.2014.05.082.
[56] EAIMSUMANG S, PETCHAKAN S, LUENGNARUEMICHAI A. Dependence of the CeO2 morphology in CuO/CeO2 catalysts for the oxidative steam reforming of methanol [J]. Reaction Kinetics Mechanisms and Catalysis, 2019, 127(2): 669-690. DOI: 10.1007/s11144- 019-01570-4.
[57] SUN Z, ZHANG X H, LI H F, LIU T, SANG S E, CHEN S Y, DUAN L B, ZENG L, XIANG W G, GONG J L. Chemical looping oxidative steam reforming of methanol: A new pathway for auto-thermal conversion [J]. Applied Catalysis B: Environmental, 2020, 269: 118758. DOI: 10.1016/ j.apcatb.2020.118758.
[58] WU X, WU S. Production of high-purity hydrogen by sorption-enhanced steam reforming process of methanol [J]. Journal of Energy Chemistry, 2015, 24(3): 315-321. DOI: 10.1016/S2095-4956(15)60317-5.
[59] LI M, DURAISWAMY K, KNOBBE M. Adsorption enhanced steam reforming of methanol for hydrogen generation in conjunction with fuel cell: Process design and reactor dynamics [J]. Chemical Engineering Science, 2012, 67(1): 26-33. DOI: 10.1016/j.ces.2011.07.024.
[60] IRURETAGOYENA D, HELLGARDT K, CHADWICK D. Towards autothermal hydrogen production by sorption- enhanced water gas shift and methanol reforming: A thermodynamic analysis [J]. International Journal of Hydrogen Energy, 2018, 43(9): 4211-4222. DOI: 10.1016/j.ijhydene.2018.01.043.
[61] QI T Y C, YANG Y, WU Y J, WANG J, LI P, YU J G. Sorption-enhanced methanol steam reforming for hydrogen production by combined copper-based catalysts with hydrotalcites [J]. Chemical Engineering and Processing-Process Intensification, 2018, 127: 72-82. DOI: 10.1016/j.cep.2018. 03.022.
[62] QING S J, HOU X N, LIU Y J, LI L D, WANG X, GAO Z X, FAN W B. Strategic use of CuAlO2 as a sustained release catalyst for production of hydrogen from methanol steam reforming [J]. Chemical Communication, 2018, 54(86): 12242-12245. DOI: 10.1039/ c8cc06600k.
[63] ZHENG T Q, ZHOU W, GAO Y, YU W, LIU Y X, ZHANG C Y, ZHENG C C, WAN S L, LIN J D, XIANG J H. Active impregnation method for copper foam as catalyst support for methanol steam reforming for hydrogen production [J]. Industrial & Engineering Chemistry Research, 2019, 58(11): 4387-4395. DOI: 10.1021/acs.iecr.8b05241.
[64] WANG S S, SU H Y, GU X K, LI W X. Differentiating intrinsic reactivity of copper, copper-zinc alloy, and copper/zinc oxide interface for methanol steam reforming by first-principles theory [J]. Journal of Physical Chemistry C, 2017, 121(39): 21553-21559. DOI: 10.1021/acs.jpcc. 7b07703.
[65] DIAZ P M A, MOYA J, SERRANO R J C, FARIA J. Interplay of support chemistry and reaction conditions on copper catalyzed methanol steam reforming [J]. Industrial & Engineering Chemistry Research, 2018, 57(45): 15268-15279. DOI: 10.1021/acs.iecr.8b02488.
[66] SANCHES S G, FLORES J H, DA SILVA M I P. Cu/ZnO and Cu/ZnO/ZrO2 catalysts used for methanol steam reforming [J]. Molecular Catalysis, 2018, 454: 55-62. DOI: 10.1016/j.mcat.2018.05.012.
[67] LEI Y Q, LUO Y M, LI X F, LU J C, MEI Z Q, PENG W, CHEN R, CHEN K Z, CHEN K Z, CHEN D K, HE D D. The role of samarium on Cu/Al2O3 catalyst in the methanol steam reforming for hydrogen production [J]. Catalysis Today, 2018, 307: 162-168. DOI: 10.1016/j.cattod.2017.05.072.
[68] LIU X Y, TOYIR J, de la PISCINA P R, HOMS N. Hydrogen production from methanol steam reforming over Al2O3- and ZrO2-modified CuOZnOGa2O3 catalysts [J]. International Journal of Hydrogen Energy, 2017, 42(19): 13704-13711. DOI: 10.1016/j.ijhydene.2016.12.133.
[69] AZENHA C S R, MATEOS PEDRERO C, QUEIROS S, CONCEPCION P, MENDES A. Innovative ZrO2-supported CuPd catalysts for the selective production of hydrogen from methanol steam reforming [J]. Applied Catalysis B: Environmental, 2017, 203: 400-407. DOI: 10.1016/ j.apcatb.2016.10.041.
[70] SAIDI M. Performance assessment and evaluation of catalytic membrane reactor for pure hydrogen production via steam reforming of methanol [J]. International Journal of Hydrogen Energy, 2017, 42(25): 16170-16185. DOI: 10.1016/j.ijhydene.2017.05.130.
[71] PU Y C, LI S R, YAN S, HUANG X, WANG D, YE Y Y, LIU Y Q. An improved Cu/ZnO catalyst promoted by Sc2O3 for hydrogen production from methanol reforming [J]. Fuel, 2019, 241: 607-615. DOI: 10.1016/ j.fuel.2018.12.067.
[72] KHZOUZ M, GKANAS E I, DU S F, WOOD J. Catalytic performance of Ni-Cu/Al2O3 for effective syngas production by methanol steam reforming [J]. Fuel, 2018, 232: 672-683. DOI: 10.1016/j.fuel.2018.06.025.
[73] AJAMEIN H, HAGHIGHI M, ALAEI S. Influence of propylene glycol/nitrates ratio on microwave-assisted combustion synthesis of CuO-ZnO-Al2O3 nanocatalyst: Structural and catalytic properties toward hydrogen production from methanol [J]. Materials Research Bulletin, 2018, 102: 142-152. DOI: 10.1016/j.materresbull.2018. 02.026.
[74] MOHTASHAMI Y, TAGHIZADEH M. Performance of the ZrO2 promoted CuZnO catalyst supported on acetic acid-treated MCM-41 in methanol steam reforming [J]. International Journal of Hydrogen Energy, 2019, 44(12): 5725-5738. DOI: 10.1016/j.ijhydene.2019.01.029.
[75] AJAMEIN H, HAGHIGHI M, ALAEI S. The role of various fuels on microwave-enhanced combustion synthesis of CuO/ZnO/Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming [J]. Energy Conversion and Management, 2017, 137: 61-73. DOI: 10.1016/j.enconman. 2017.01.044.
[76] BOSSOLA F, SCOTTI N, SOMODI F, CODURI M, EVANGELISTI C, DAL SANTO V. Electron-poor copper nanoparticles over amorphous zirconia-silica as all-in-one catalytic sites for the methanol steam reforming [J]. Applied Catalysis B: Environmental, 2019, 258: 118016. DOI: 10.1016/j.apcatb.2019.118016.
[77] JAMPA S, JAMIESON A M, CHAISUWAN T, LUENGNARUEMITCHAI A, WONGKASEMJIT S. Achievement of hydrogen production from autothermal steam reforming of methanol over Cu-loaded mesoporous CeO2 and Cu-loaded mesoporous CeO2-ZrO2 catalysts [J]. International Journal of Hydrogen Energy, 2017, 42(22): 15073-15084. DOI: 10.1016/j.ijhydene.2017.05.022.
[78] MAYR L, SHI X R, KOPFLE N, KLOTZER B, ZEMLYANOV D Y, PENNER S. Tuning of the copper-zirconia phase boundary for selectivity control of methanol conversion [J]. Journal of Catalysis, 2016, 339: 111-122. DOI: 10.1016/j.jcat.2016.03.029.
[79] GAC W, ZAWADZKI W, GRELUK M, SLOWIK G, MACHOCKI A, PAPAVASILIOU J, AVGOUROPOULOS G. Investigation of the inhibiting role of hydrogen in the steam reforming of methanol [J]. Chemcatchem, 2019, 11(14): 3264-3278. DOI: 10.1002/cctc.201900738.
[80] KHANI Y, BAHADORAN F, SOLTANALI S, AHARI J S. Hydrogen production by methanol steam reforming on a cordierite monolith reactor coated with Cu-Ni/LaZnAlO4 and Cu-Ni/gamma-Al2O3 catalysts [J]. Research on Chemical Intermediates, 2018, 44(2): 925-942. DOI: 10.1007/s11164- 017-3144-8.
[81] LIU Y J, QING S J, HOU X N, QIN F J, WANG X, GAO Z X, XIANG H W. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming [J]. Catalyst Science & Technology, 2017, 7(21): 5069-5078. DOI: 10.1039/c7cy01236e.
[82] MAITI S, DAS D, PAL K,LLORCA J, SOLER L, COLUSSI S, TROVARELLI A, PRIOLKAR K R, SARODE P R, ASAKURA K. Methanol steam reforming behavior of sol-gel synthesized nanodimensional CuxFe1-xAl2O4 hercynites [J]. Applied Catalysis A: General, 2019, 570: 73-83. DOI: 10.1016/j.apcata.2018.11.011.
[83] YANG S, ZHOU F, LIU Y, ZHANG L, CHEN Y, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming [J]. International Journal of Hydrogen Energy, 2019, 44(14): 7252-7261. DOI: 10.1016/j.ijhydene.2019.01.254.
[84] TONG W Y, WEST A, CHEUNG K, YU K M, TSANG S C E. Dramatic effects of gallium promotion on methanol steam reforming Cu-ZnO catalyst for hydrogen production: Formation of 5 angstrom copper clusters from Cu-ZnGaOx [J]. ACS Catalysis, 2013, 3(6): 1231-1244. DOI: 10.1021/ cs400011m.
[85] RUANO D, CORED J, AZENHA C, PEREZ-DIESTE V, MENDES A, MATEOS-PEDRERO C, CONCEPCION P. Dynamic structure and subsurface oxygen formation of a working copper catalyst under methanol steam reforming conditions: An in situ time-resolved spectroscopic study [J]. ACS Catalysis, 2019, 9(4): 2922-2930. DOI: 10.1021/acscatal.8b05042.
[86] HE X H, WANG Y, ZHANG X, DONG M, WANG G F, ZHANG B S, NIU Y M, YAO S Y, HE X, LIU H C. Controllable in situ surface restructuring of cu catalysts and remarkable enhancement of their catalytic activity [J]. ACS Catalysis, 2019, 9(3): 2213-2221. DOI: 10.1021/acscatal.8b04812.
[87] CAO L, LU M H, LI G, ZHANG S Y. Hydrogen production from methanol steam reforming catalyzed by Fe modified Cu supported on attapulgite clay [J]. Reaction Kinetics Mechanisms and Catalysis, 2019, 126(1): 137-152. DOI: 10.1007/s11144-018-1493-y.
[88] LIU Y J, QING S J, HOU X N, QIN F J, WANG X, GAO Z X, XIANG H W. Cu-Ni-Al spinel oxide as an efficient durable catalyst for methanol steam reforming [J]. Chemcatchem, 2018, 10(24): 5698-5706. DOI: 10.1002/ cctc.201801472.
[89] FASANYA O A, ALHAJRI R, AHMED O U, MYINT M T Z, ATTA A Y, JIBRIL B Y, DUTTA J. Copper zinc oxide nanocatalysts grown on cordierite substrate for hydrogen production using methanol steam reforming [J]. International Journal of Hydrogen Energy, 2019, 44(41): 22936-22946. DOI: 10.1016/j.ijhydene.2019.06.185.
[90] KUO M T, CHEN Y Y, HUNG W Y, LIN S F, LIN H P, HSU C H, SHIH H Y, XIE W A, LI S N. Synthesis of mesoporous Cu-Fe/silicates catalyst for methanol steam reforming [J]. International Journal of Hydrogen Energy, 2019, 44(28): 14416-14423. DOI: 10.1016/j.ijhydene.2019. 03.014.
[91] KIM J H, JANG Y S, KIM J C, KIM D H. Anodic aluminum oxide supported Cu-Zn catalyst for oxidative steam reforming of methanol [J]. Korean Journal of Chemical Engineering, 2019, 36(3): 368-376. DOI: 10.1007/s11814- 018-0211-9.
[92] MAYR L, KOPFLE N, KLOTZER B, GOTSCH T, BERNARDI J, SCHWARZ S, KEILHAUER T, ARMBRUSTER M, PENNER S. Microstructural and chemical evolution and analysis of a self-activating CO2-Selective Cu-Zr bimetallic methanol steam reforming catalyst [J]. Journal of Physical Chemistry C, 2016, 120(44): 25395-25404. DOI: 10.1021/acs.jpcc.6b07824.
[93] MA K, CUI Z H, ZHANG Z T, HUANG J J, SUN Z R, TIAN Y, DING T, LI X G. Alloy-mediated ultra-low CO selectivity for steam reforming over Cu-Ni bimetallic catalysts [J]. ChemCatChem, 2018, 10(18): 4010-4017. DOI: 10.1002/cctc.201800684.
[94] SEYEDI A M, HAGIGHI M, RAHEMI N. Significant influence of cutting-edge plasma technology on catalytic properties and performance of CuO-ZnO-Al2O3-ZrO2 nanocatalyst used in methanol steam reforming for fuel cell grade hydrogen production [J]. Ceramics International, 2017, 43(8): 6201-6213. DOI: 10.1016/j.ceramint.2017.02.018.
[95] BAGHERZADEH S B, HAGHIGHI M. Plasma-enhanced comparative hydrothermal and coprecipitation preparation of CuO/ZnO/Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming [J]. Energy Conversion and Management, 2017, 142: 452-465. DOI: 10.1016/ j.enconman.2017.03.069.
[96] ZHANG Y D, ZHAO Y W, HAO Y. A study on steam reforming of methanol over a novel nanocatalyst of compound metal oxides [J]. Cleaner Energy for Cleaner Cities, 2018, 152: 192-197. DOI: 10.1016/j.egypro. 2018.09.087.
[97] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on gamma-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming [J]. International Journal of Hydrogen Energy, 2017, 42(15): 9930-9937. DOI: 10.1016/j.ijhydene.2017.01.229.
[98] CAO J, MA Y F, GUAN G Q, HAO X G, MA X L, WANG Z D, KUSAKABE K, ABUDULA A. Reaction intermediate species during the steam reforming of methanol over metal modified molybdenum carbide catalysts [J]. Applied Catalysis B: Environmental, 2016, 189: 12-18. DOI: 10.1016/j.apcatb.2016.02.021.
[99] ZHANG R B, HUANG C Q, ZONG L J, LU K, WANG X W, CAI J X. Hydrogen production from methanol steam reforming over TiO2 and CeO2 pillared clay supported Au catalysts [J]. Applied Sciences Basel, 2018, 8(2): 167. DOI: 10.3390/app8020176.
[100] SHI J, MAHR C, MURSHED M M, GESING T M, ROSENAUER A, BAUMER M, WITTSTOC A. Steam reforming of methanol over oxide decorated nanoporous gold catalysts: A combined in situ FTIR and flow reactor study [J]. Physical Chemistry Chemical Physics, 2017, 19(13): 8880-8888. DOI: 10.1039/c6cp08849j.
[101] NOWICKA E, ALTHAHBAN S M, LUO Y, KRIEGEL R, SHAW G, MORGAN D J, HE Q, WATANABE M, ARMBRUSTER M, KIELY C J. Highly selective PdZn/ZnO catalysts for the methanol steam reforming reaction [J]. Catalysis Science & Technology, 2018, 8(22): 5848-5857. DOI: 10.1039/c8cy01100a.
[102] LI X Y, LI L, LIN J, QIAO B T, YANG X F, WANG A Q, WANG X D. Reactivity of methanol steam reforming on ZnPd intermetallic catalyst: Understanding from microcalorimetric and FT-IR studies [J]. Journal of Physical Chemistry C, 2018, 122(23): 12395-12403. DOI: 10.1021/ acs.jpcc.8b03933.
[103] PLONER K, GOTSCH T, KOGLER G, THALINGER P, BERNARDI J, ZHAO Q, ZHOU C, KLOTZER B, PENNER S. Structural and catalytic properties of Ag- and Co3O4-impregnated strontium titanium ferrite SrTi0.7Fe0.3O3- delta in methanol steam reforming [J]. Industrial & Engineering Chemistry Research, 2017, 56(46): 13654- 13662. DOI: 10.1021/acs.iecr.7b03778.
[104] EAIMSUMANG S, WONGKASEMJIT S, PONGSTABODEE S, SMITH SM, RATANAWILAI S, CHOLLACOOP N, LUENGNARUEMITCHAI A. Effect of synthesis time on morphology of CeO2 nanoparticles and Au/CeO2 and their activity in oxidative steam reforming of methanol [J]. Journal of Rare Earths, 2019, 37(8): 819-828. DOI: 10.1016/j.jre.2018.11.010.
[105] LIU Z Y, YAO S Y, JOHNSTON P A, XU W Q, RODRIGUEZ J A, SENANAYAKE S D. Methanol steam reforming over Ni-CeO2 model and powder catalysts: Pathways to high stability and selectivity for H2/CO2 production [J]. Catalysis Today, 2018, 311: 74-80. DOI: 10.1016/j.cattod.2017.08.041.
[106] LYTKINA A A, OREKHOVA N V, ERMILOVA M M, YAROSLAVTSEV A B. The influence of the support composition and structure (MXZr1-XO2-delta) of bimetallic catalysts on the activity in methanol steam reforming [J]. International Journal of Hydrogen Energy, 2018, 43(1): 198-207. DOI: 10.1016/j.ijhydene.2017.10.182.
[107] MIERCZYNSKI P, MIERCZYNSKA A, CIESIELSKI R, MANIUKIEWICZ W, ROGOWSKI J, MANIECKI T P, DUBKOV S, SYSA A, GROMOV, SZYNKOWSKA M I. Modern Ni and Pd-Ni catalysts supported on Sn-Al binary oxide for oxy-steam reforming of methanol [J]. Energy Technology, 2018, 6(9): 1687-1699. DOI: 10.1002/ente. 201700840.
[108] KAMYAR N, KHANI Y, AMINI M M, BAHADORAN F, SAFARI N. Embedding Pt-SnO manoparticles into MIL-101(Cr) pores: Hydrogen production with low carbon monoxide content from a new methanol steam reforming catalyst [J]. ChemistrySelect, 2019, 4(20): 6113-6122. DOI: 10.1002/slct.201901071.
[109] KRIEGEL R, IVARRSSON D C A, ARMBRUSTER M. Formic acid decomposition over ZnPd-Implications for methanol steam reforming [J]. ChemCatChem, 2018, 10(12): 2664-2672. DOI: 10.1002/cctc.201800194.
[110] BARRIOUS C E, BALTANAS M A, BOSCO M V, BONIVARDI A L. On the surface nature of bimetallic PdZn particles supported on a ZnO-CeO2 nanocomposite for the methanol steam reforming reaction [J]. Catalysis Letters, 2018, 148(8): 2233-2246. DOI: 10.1007/s10562-018- 2441-1.
[111] RAMESHAN C, LORENZ H, ARMBRUSTER M, KASATHIN I, KLOTZER B, GOTSCH T, PLONER K, PENNER S. Impregnated and co-precipitated Pd-Ga2O3, Pd-In2O3 and Pd-Ga2O3-In2O3 catalysts: Influence of the microstructure on the CO2 selectivity in methanol steam reforming [J]. Catalysis Letters, 2018, 148(10): 3062-3071. DOI: 10.1007/ s10562-018-2491-4.
[112] KIM G J, KIM M S, BYUN J Y, HONG S C. Effects of Ru addition to Pd/Al2O3 catalysts on methanol steam reforming reaction: A mechanistic study [J]. Applied Catalysis A: General, 2019, 572: 115-123. DOI: 10.1016/j.apcata.2018. 12.035.
[113] WANG C Y, OUYANG M Y, LI M W, LEE S, FLYTZANI- STEPHANOPOULOS M. Low-coordinated Pd catalysts supported on Zn1Zr1Ox composite oxides for selective methanol steam reforming [J]. Applied Catalysis A: Genernal, 2019, 580: 81-92. DOI: 10.1016/j.apcata.2019. 05.006.
[114] BARRIOS C E, BOSCO M V, BALTANAS M A, BONIVARDI A L. Hydrogen production by methanol steam reforming: Catalytic performance of supported-Pd on zinc-cerium oxides' nanocomposites [J]. Applied Catalysis B: Environment, 2015, 179: 262-275. DOI: 10.1016/j.apcatb. 2015.05.030.
[115] LIU X, MEN Y, WANG J G, HE R, WANG Y Q. Remarkable support effect on the reactivity of Pt/In2O3/MOx catalysts for methanol steam reforming [J]. Journal of Power Sources, 2017, 364: 341-350. DOI: 10.1016/j.jpowsour.2017.08.043.
[116] TAGHIZADEH M, AKHOUNDZADEH H, REZAYAN A, SADEGHIAN M. Excellent catalytic performance of 3D-mesoporous KIT-6 supported Cu and Ce nanoparticles in methanol steam reforming [J]. International Journal of Hydrogen Energy, 2018, 43(24): 10926-10937. DOI: 10.1016/j.ijhydene.2018.05.034.
[117] CAI F F, LU P J, IBRAHIM J J, FU Y, ZHANG J, SUN Y H. Investigation of the role of Nb on Pd-Zr-Zn catalyst in methanol steam reforming for hydrogen [J]. International Journal of Hydrogen Energy, 2019, 44(23): 11717-11733. DOI: 10.1016/j.ijhydene.2019. 03.125.
[118] MIERCZYNSKI P, MIERCZYNSKA A, CIESIELSKI R, MOSINSKA M, NOWOSIELSKA M, CZYLKOWSKA A, MANIUKIEWICZ W, SZYNKOWSKA M I. High active and selective Ni/CeO2-Al2O3 and Pd-Ni/CeO2-Al2O3 catalysts for oxy-steam reforming of methanol [J]. Catalysts, 2018, 8(9): 380. DOI: 10.3390/ catal8090380.
[119] ZENG Z, LIU G, GENG J F, JING D W, HONG X L, GUO L J. A high-performance PdZn alloy catalyst obtained from metal-organic framework for methanol steam reforming hydrogen production [J]. International Journal of Hydrogen Energy, 2019, 44(45): 24387-24397. DOI: 10.1016/ j.ijhydene.2019.07.195.
[120] GU X K, QIAO B T, HUANG C Q, DING W C, SUN K J, ZHAN E S, ZHANG T, LIU J Y, LI W X. Supported single Pt1/Au1 atoms for methanol steam reforming [J]. ACS Catalysis, 2014, 4(11): 3886-3890. DOI: 10.1021/ cs500740u.
[121] MAYR L, KLOTZER B, SCHMIDMAIR D, KOPFLE N, BERNARDI J, SCHWARZ S, ARMBRUSTER M, PENNER S. Boosting hydrogen production from methanol and water by in situ activation of bimetallic Cu-Zr species [J]. Chemcatchem, 2016, 8(10): 1778-1781. DOI: 10.1002/cctc. 201600361.
[122] RAHEMI N, HAGHIGHI M, BABALUO A A, JAFARI M F, ESTIFAEE P. Plasma assisted synthesis and physicochemical characterizations of Ni-Co/Al2O3 nanocatalyst used in dry reforming of methane [J]. Plasma Chemistry and Plasma Processing, 2013, 33(4): 663-680. DOI: 10.1007/s11090- 013-9460-x.
[123] CHENG D G, ZHU X, BEN Y H, HE F, CUI L, LIU C J. Carbon dioxide reforming of methane over Ni/Al2O3 treated with glow discharge plasma [J]. Catalysis Today, 2006, 115(1-4): 205-210. DOI: 10.1016/j.cattod.2006.02.063.
[124] RAHEMI N, HAGHIGHI M, BABALUO A A, JAFARI M F, ALLAHYARI S. CO2 reforming of methane over Ni-Cu/Al2O3-ZrO2 nanocatalyst: The influence of plasma treatment and process conditions on catalytic properties and performance [J]. Korean Journal of Chemical Engineering, 2014, 31(9): 1553-1563. DOI: 10.1007/s11814-014-0123-2.
[125] CAO S, YANG M, ELNABAWY A O, TRIMPALIS A, LI S, WANG C, GOLTL F, CHEN Z H Y, LIU J L, SHAN J J. Single-atom gold oxo-clusters prepared in alkaline solutions catalyse the heterogeneous methanol self-coupling reactions [J]. Nature Chemistry, 2019, 11(12): 1098-1105. DOI: 10.1038/s41557- 019-0345-3.
[126] LIAN H Y, LI X S, LIU J L, ZHU X B, ZHU A M. Oxidative pyrolysis reforming of methanol in warm plasma for an on-board hydrogen production [J]. International Journal of Hydrogen Energy, 2017, 42(19): 13617-13624. DOI: 10.1016/j.ijhydene.2016.10.166.
[127] WANG Y, GAO X, LIN C H, SHI L Y, LI X H, WU G L. Metal organic frameworks-derived Fe-Co nanoporous carbon/graphene composite as a high-performance electromagnetic wave absorber [J]. Journal of Alloys and Compounds, 2019, 785: 765-773. DOI: 10.1016/j.jallcom.2019.01.271.
[128] WANG C C, YI X H, WANG P. Powerful combination of MOFs and C3N4 for enhanced photocatalytic performance [J]. Applied Catalysis B: Environmental, 2019, 247: 24-48. DOI: 10.1016/j.apcatb.2019.01.091.
[129] GARCIA-GARCIA P, MULLER M, CORMA A. MOF catalysis in relation to their homogeneous counterparts and conventional solid catalysts [J]. Chemical Science, 2014, 5(8): 2979-3007. DOI: 10.1039/c4sc00265b.
[130] HAN B, LANG R, TANG H L, XU J, GU X K, QIAO B T, LIU J. Superior activity of Rh-1/ZnO single-atom catalyst for CO oxidation [J]. Chinese Journal of Catalysis, 2019, 40(12): 1847-1853. DOI: 10.1016/S1872-2067(19)63411-X.
[131] GUO X, FANG G, LI G, MA H, FAN H J, YU L, MA C, WU X, DENG D H, WEI M M. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen [J]. Science, 2014, 344(6184): 616-619. DOI: 10.1126/ science.1253150.
[132] YANG M, LI S, WANG Y, HERRON J A, XU Y, ALLARD L F, LEE S, HUANG J, MAVRIKAKIS M, FLYTZANI- STEPHANOPOULOS M. Catalytically active Au-O(OH)x- species stabilized by alkali ions on zeolites and mesoporous oxides [J]. Science, 2014, 346(6216): 1498-1501. DOI: 10.1126/science.1260526.
[133] WANG A, LI J, ZHANG T. Heterogeneous single-atom catalysis [J]. Nature Reviews Chemistry, 2018, 2(6): 65-81. DOI: 10.1038/s41570-018-0010-1.
[134] NIE L, MEI D, XIONG H, PENG B, REN Z, HERNANDEZ X I P, DELARIVA A, WANG M, ENGELHARD M H, KOVARIK L. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation [J]. Science, 2017, 358(6369): 1419-1423. DOI: 10.1126/science. aao2109.
[135] YANG M, LIU J, LEE S, ZUGIC B, HUANG J, ALLARD L F, FLYTZANI-STEPHANOPOUPOS M. A common single-site Pt(II)-O(OH)x-species stabilized by sodium on “active” and “inert” supports catalyzes the water-gas shift reaction [J]. Journal of the American Chemical Society, 2015, 137(10): 3470-3473.
[136] YAN H, CHENG H, YI H, LIN Y, YAO T, WANG C L, LI J J, WEI S Q, LU J L. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene [J]. Journal of the American Chemical Society, 2015, 137(33): 10484-10487. DOI: 10.1021/jacs.5b06485.
[137] LI X M, LI X D. Interrogating interactions and modifications of histones in live cells [J]. Cell Chemical Biology, 2018, 25(1): 1-3. DOI: 10.1016/j.chembiol.2018.01.003.
[138] JAHNNISCH K, HESSEL V, LOWE H, BAERNS M. Chemistry in microstructured reactors [J]. Angewandte Chemie-International Edition, 2004, 43(4): 406-446. DOI: 10.1002/anie.200300577.
[139] ZHENG T, ZHOU W, YU W, KE Y Z, LIU Y X, LIU R L, HUI K S. Methanol steam reforming performance optimisation of cylindrical microreactor for hydrogen production utilising error backpropagation and genetic algorithm [J]. Chemical Engineering Journal, 2019, 357: 641-654. DOI: 10.1016/ j.cej.2018.09.129.
[140] LYTKINA A A, MIRONOVA E Y, OREKHOVA N V, ERMILOVA M M, YAROSLAVTSEV A B. Ru-containing catalysts for methanol and ethanol steam reforming in conventional and membrane reactors [J]. Inorganic Materials, 2019, 55(6): 547-555. DOI: 10.1134/S0020168519060104.
[141] LIU Y X, ZHOU W, CHEN L, LIN Y, CHU X Y, ZHENG T Q, WAN S L. Optimal design and fabrication of surface microchannels on copper foam catalyst support in a methanol steam reforming microreactor [J]. Fuel, 2019, 253: 1545-1555. DOI: 10.1016/j.fuel.2019.05.099.
[142] LIAN H Y, LIU J L, LI X S, ZHU X B, WEBER A Z, ZHU A M. Plasma chain catalytic reforming of methanol for on-board hydrogen production [J]. Chemical Engineering Journal, 2019, 369: 245-252. DOI: 10.1016/j.cej.2019.03. 069.
[143] HERDEM M S, MUNDHWA M, FARHAD S, HAMDULLAHPUR F. Multiphysics modeling and heat distribution study in a catalytic microchannel methanol steam reformer [J]. Energy & Fuels, 2018, 32(6): 7220-7234. DOI: 10.1021/acs.energyfuels.8b01280.
[144] PERNG S W, HORNG R F, WU H W. Influence of necking configuration of a methanol steam reformer on catalyst amount and reforming performance [J]. Energy Sources Part A-Recovery Utilizaton and Environmental Effects, 2019. DOI: 10.1080/15567036.2019.1651795.
[145] HIRAMATSU H, SAKURAI M, MAKI T, KAMEYAMA H. Stacked etched aluminum flow-through membranes for methanol steam reforming [J]. International Journal of Hydrogen Energy, 2017, 42(15): 9922-9929. DOI: 10.1016/j.ijhydene.2017.01.106.
[146] WANG F, LI LJ, LIU Y Y. Effects of flow and operation parameters on methanol steam reforming in tube reactor heated by simulated waste heat [J]. International Journal of Hydrogen Energy, 2017, 42(42): 26270-26276. DOI: 10.1016/j.ijhydene.2017.09.002.
[147] ZHOU W, YU W, KE Y Z, LIU Y X, WAN S L, LIN J D. Size effect and series-parallel integration design of laminated methanol steam reforming microreactor for hydrogen production [J]. International Journal of Hydrogen Energy, 2018, 43(42): 19396-19404. DOI: 10.1016/j.ijhydene.2018. 08.199.
[148] SARI A, SABZIANI J. Modeling and 3D-simulation of hydrogen production via methanol steam reforming in copper-coated channels of a mini reformer [J]. Journal of Power Sources, 2017, 352: 64-76. DOI: 10.1016/ j.jpowsour.2017.03.120.
[149] YAO L, WANG F, WANG L, WANG G Q. Transport enhancement study on small-scale methanol steam reforming reactor with waste heat recovery for hydrogen production [J]. Energy, 2019, 175: 986-997. DOI: 10.1016/j.energy.2019. 03.157.
[150] ZHENG B, SUN P, ZHAO Q, LIU YQ, ZHANG Z L. Effects of particle sizes on methanol steam reforming for hydrogen production in a reactor heated by waste heat [J]. International Journal of Hydrogen Energy, 2019, 44(11): 5615-5622. DOI: 10.1016/j.ijhydene.2018.07.163.
[151] TAJRISHI O Z, TAGHIZADEH M, KIADEHI A D. Methanol steam reforming in a microchannel reactor by Zn-, Ce- and Zr-modified mesoporous Cu/SBA-15 nanocatalyst [J]. International Journal of Hydrogen Energy, 2018, 43(31): 14103-14120. DOI: 10.1016/j.ijhydene.2018.06.035.
[152] XU Z J, YANG S, HU G H, WANG Q H, LI J R. Numerical study of flow distribution uniformity for the optimization of gradient porosity configuration of porous copper fiber sintered felt for hydrogen production through methanol steam reforming micro-reactor [J]. International Journal of Hydrogen Energy, 2018, 43(9): 4355-4370. DOI: 10.1016/ j.ijhydene.2018.01.083.
[153] SARAFRAZ M M, SAFAEI M R, GOODARZI M, ARRJOMANDI M. Reforming of methanol with steam in a micro-reactor with Cu-SiO2 porous catalyst [J]. International Journal of Hydrogen Energy, 2019, 44(36): 19628-19639. DOI: 10.1016/j.ijhydene.2019.05.215.
[154] LYTKINA A A, OREKHOVA N V, ERMILOVA M M, PETRIEV I S, BARYSHEV M G, YAROSLAVTSEV A B. Ru-Rh based catalysts for hydrogen production via methanol steam reforming in conventional and membrane reactors [J]. International Journal of Hydrogen Energy, 2019, 44(26): 13310-13322. DOI: 10.1016/j.ijhydene.2019.03.205.
[155] KHANI Y, BAHADORAN F, SAFARI N, SOLTANALI S, TAHERI S A. Hydrogen production from steam reforming of methanol over Cu-based catalysts: The behavior of ZnxLaxAl1-xO4 and ZnO/La2O3/Al2O3 lined on cordierite monolith reactors [J]. International Journal of Hydrogen Energy, 2019, 44(23): 11824-11837. DOI: 10.1016/ j.ijhydene.2019.03.031.
[156] LEI H Y, LI J R, WANG Q H, XU Z J, ZHOU W, YU C L, ZHENG T Q. Feasibility of preparing additive manufactured porous stainless steel felts with mathematical micro pore structure as novel catalyst support for hydrogen production via methanol steam reforming [J]. International Journal of Hydrogen Energy, 2019, 44(45): 24782-24791. DOI: 10.1016/j.ijhydene.2019.07.187.
[157] LI J R, YU C L, XU Z J, WANG Q H, ZHOU W, ZHENG T Q. Preparing a novel gradient porous metal fiber sintered felt with better manufacturability for hydrogen production via methanol steam reforming [J]. International Journal of Hydrogen Energy, 2019, 44(43): 23983-23995. DOI: 10.1016/j.ijhydene.2019.07.142.
[158] KE Y, ZHOU W, CHU X Y, YUAN D, WAN S L, YU W, LIU Y X. Porous copper fiber sintered felts with surface microchannels for methanol steam reforming microreactor for hydrogen production [J]. International Journal of Hydrogen Energy, 2019, 44(12): 5755-5765. DOI: 10.1016/j.ijhydene.2019.01.141.
[159] PERNG S W, HORNG R F. Numerical analysis of performance enhancement and non-isothermal reactant transport of a cylindrical methanol reformer wrapped with a porous sheath under steam reforming [J]. International Journal of Hydrogen Energy, 2017, 42(38): 24372-24392. DOI: 10.1016/j.ijhydene.2017.07.170.
[160] ZHOU W, KE Y Z, PEI P C, YU W, CHU X Y, LI S L, YANG K. Hydrogen production from cylindrical methanol steam reforming microreactorwith porous Cu-Al fiber sintered felt [J]. International Journal of Hydrogen Energy, 2018, 43(7): 3643-3654. DOI: 10.1016/j.ijhydene.2017.12. 118.
[161] RIBEIRINHA P, ABDOLLAHZADEH M, PEREIRA A, RELVAS F, BOAVENTURA M, MENDES A. High temperature PEM fuel cell integrated with a cellular membrane methanol steam reformer: Experimental and modelling [J]. Applied Energy, 2018, 215: 659-669. DOI: 10.1016/j.apenergy.2018.02.029.
[162] RIBEIRINHA P, ABDOLLAHZADEH M, SOUSA JM, BOAVENTURA M, MENDES A. Modelling of a high-temperature polymer electrolyte membrane fuel cell integrated with a methanol steam reformer cell [J]. Applied Energy, 2017, 202: 6-19. DOI: 10.1016/j.apenergy.2017. 05.120.
[163] PERNG S W, HORNG R F, WU H W. Effect of a diffuser on performance enhancement of a cylindrical methanol steam reformer by computational fluid dynamic analysis [J]. Applied Energy, 2017, 206: 312-328. DOI: 10.1016/ j.apenergy.2017.08.194.
[164] WANG Q H, YANG S, ZHOU W, LI J R, XU Z J, KE Y Z, YU W, HU G H. Optimizing the porosity configuration of porous copper fiber sintered felt for methanol steam reforming micro-reactor based on flow distribution [J]. Applied Energy, 2018, 216: 243-261. DOI: 10.1016/ j.apenergy.2018.02.102.
[165] TAHAY P, KHANI Y, JABARI M, BAHADORAN F, SAFARI N. Highly porous monolith/TiO2 supported Cu, Cu-Ni, Ru, and Pt catalysts in methanol steam reforming process for H2 generation [J]. Applied Catalysis A: General, 2018, 554: 44-53. DOI: 10.1016/j.apcata.2018.01.022.
[166] HEIDARZADEH M, TAGHIZADEH M. Methanol steam reforming in a spiral-shaped microchannel reactor over Cu/ZnO/Al2O3 catalyst: A computational fluid dynamics simulation study [J]. International Journal of Chemical Reactor Engineering, 2017, 15(4): 11. DOI: 10.1515/ijcre- 2016-0205.
[167] CHENG Z D, MEN J J, ZHAO X R, HE Y L, TAO Y B. A comprehensive study on parabolic trough solar receiver- reactors of methanol-steam reforming reaction for hydrogen production [J]. Energy Conversion and Management, 2019, 186: 278-292. DOI: 10.1016/j.enconman.2019.02.068.
[168] HERDEM M S, MUNDHWA M, FARHAD S, HAMDULLAHPUR F. Catalyst layer design and arrangement to improve the performance of a microchannel methanol steam reformer [J]. Energy Conversion and Management, 2019, 180: 149-161. DOI: 10.1016/ j.enconman.2018.10.094.
[169] TIAN J, KE Y, KONG G G, TAN M W, WANG Y, LIN J D, ZHOU W, WAN S L. A novel structured PdZnAl/Cu fiber catalyst for methanol steam reforming in microreactor [J]. Renewable Energy, 2017, 113: 30-42. DOI: 10.1016/j.renene.2017.04.070.
(Edited by HE Yun-bin)
中文导读
应用于燃料电池的甲醇重整制氢研究综述
摘要:甲醇液体燃料具有运输方便、能量密度高、转化温度低等优势,可作为氢能载体实时产氢并供给燃料电池。论文围绕最新甲醇重整制氢技术展开了详细的介绍,具体内容包括甲醇转化途径概述、甲醇转化催化剂研究进展与开发思路总结以及甲醇蒸汽重整微型反应器发展概述。甲醇转化途径部分对甲醇蒸汽重整、甲醇部分氧化、甲醇氧化重整以及吸附增强式甲醇重整反应进行了详细阐述;催化剂部分总结了近年来的催化剂发展趋势并给出了提高催化剂活性的方法与设计思路;甲醇重整微反应器部分,对新开发的蒸汽甲醇重整器进行了全面介绍。本篇综述对近5年来的甲醇重整制氢技术进行了总结梳理并给出了未来发展趋势,为甲醇氢载体供给燃料电池的理论研究与燃料电池应该提供参考依据。
关键词:甲醇重整;制氢;燃料电池;催化剂;重整反应器
Foundation item: Project(51876224) supported by the National Natural Science Foundation of China; Project(2020CX008) supported by the Innovation-Driven Project of Central South University, China
Received date: 2019-12-13; Accepted date: 2020-03-10
Corresponding author: SUN Zhi-qiang, PhD, Professor; Tel: +86-731-88879863; E-mail: zqsun@csu.edu.cn; ORCID: 0000-0003- 0518-3275
Abstract: Methanol is regarded as an important liquid fuel for hydrogen storage, transportation, and in-situ generation due to its convenient conveyance, high energy density, and low conversion temperature. In this work, an overview of state-of-the-art investigations on methanol reforming is critically summarized, including the detailed introduction of methanol conversion pathways from the perspective of fuel cell applications, various advanced materials design for catalytic methanol conversion, as well as the development of steam methanol reformers. For the section of utilization pathways, reactions such as steam reforming of methanol, partial oxidation of methanol, oxidative steam reforming of methanol, and sorption-enhanced steam methanol reforming were elaborated; For the catalyst section, the strategies to enhance the catalytic activity and other comprehensive performances were summarized; For the reactor section, the newly designed steam methanol reformers were thoroughly described. This review will benefit researchers from both fundamental research and fuel cell applications in the field of catalyzing methanol to hydrogen.


