
Preparation and photocatalytic activity of composite films containing clustered TiO2 particles and mineral tourmaline powders
LIANG Jin-sheng(梁金生), MENG Jun-ping(孟军平), LIANG Guang-chuan(梁广川), FENG Yan-wen(冯艳文), DING Yan(丁 燕)Institute of Power Source and Ecomaterials Science, Hebei University of Technology, Tianjin 300130, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
The novel composite films containing clustered TiO2 particles and fine tourmaline particles on the surface of copper webs were prepared by the sol-gel method. The microstructures of the composite films were investigated by scanning electron microscopy (SEM), and the photocatalytic activity of the films was evaluated by photocatalytic degradation of methyl orange, respectively. The results indicate that tourmaline particles can obviously influence the microstructures of TiO2 films and enhance the photocatalytic activity due to their spontaneous permanent polarity and high radiotechnology of far infrared. During preparing the composite films, the clustered TiO2 particles with lots of nano-sized ladder layers can grow on the surface of fine tourmaline particles, the thickness of ladder layer is 10 nm, and the average diameter of nano-sized TiO2 particles is 15 nm.
Key words:
TiO2; composite materials; sol-gel method; photocatalytic activity; tourmaline;
1 Introduction
Ever since the discovery of the photocatalytic property of titania by Fujishima and Honda in 1972[1], the photocatalytic technique has now become one of the popular research subjects in the fields of photochemistry and environment protection due to its lower cost, non-toxicity, stronger antioxidation and so on [2-4]. When TiO2 powders were applied as photocatalysts for water or air purification, they showed high photocatalytic activity due to their large surface area. However, to reuse the TiO2 powders photocatalysts and to achieve low-turbidity water, technical problems may arise from the undesirable particle agglomeration and necessary particle-fluid separation after UV light irradiation. To avoid the use of powders, many researchers have been developing various ways to apply TiO2 coatings on various substrates. The use of TiO2 thin films as photocatalysts has many advantages compared to that of TiO2 powders. However, the formation of TiO2 thin films on substrates significantly reduces the surface area of TiO2 photocatalysts, resulting in a decrease of the photocatalytic activity. If TiO2 particles could be uniformly formed on a substrate with a large surface area such as ceramics, stainless steel sheets, copper webs and so on, the prepared TiO2 photocatalysts would not only have a large surface area and keep high photocatalytic activity, but also avoid the disadvantages of TiO2 powders photocatalysts.
Unfortunately, their industrial application is still limited to date due mainly to the higher electron-hole recombination to lead to low photocatalytic efficiency. So far, numerous efforts have been made to reduce the recombination such as metal elements embedding, microwave-assisted or e1ectro-assisted photocatalysis [5-7], etc.
Tourmaline is a kind of complex borosilicate mineral belonging to the trigonal space group. The general chemical formula can be written as XY3Z6Si6- O18(BO3) 3 W4, where X is Na, Ca, K or vacancies; Y is Mg2+, Fe2+, Mn2+, Al, Fe3+, Mn3+, Li; Z is Al, Fe3+, Cr3+, Mg; W is OH, F, O. One of the most important features among the electric properties of tourmaline is the possession of spontaneous and permanent poles, which can produce an electric dipole, especially in a small granule with a diameter of several microns or less [8,9]. Therefore, a strong electric field exists on the
surface of a tourmaline granule[9,10]. The electric field effects of tourmaline particles can be applied in various fields. Also, there exists a high radiotechnology of far infrared, which can activate water molecules and increase the oxygen solved in the water [8,11,12]. Moreover, tourmaline has many other functions such as shielding of electromagnetic wave and releasing of negative ions. It has supplied a new effective approach to purify environment [9,13-15]. In this process, it is possible to form a novel titania with large surface area on the surface of tourmaline. Moreover, the prepared titania containing tourmaline is easily coated on various kinds of substrates such as copper webs. This way is very simple and does not require any special and expensive equipment. Besides, under the coordinating of far infrared and electric field, the enhanced electro-assisted photocatalysis would take place in the system including tourmaline and titania. Nevertheless, the effects of fine tourmaline particles on microstructure and photocatalytic activity of TiO2 composite films containing tourmaline particles haven’t been reported so far.
In the present work, the composite films containing clustered TiO2 particles and fine tourmaline particles (T/TiO2) were uniformly formed on the surface of the copper webs from an alkoxide solution. To the best of our knowledge, this is the first report on the preparation and photocatalytic activity of composite films containing clustered TiO2 particles and fine tourmaline particles formed on the copper webs. The preparing methods, forming mechanism, and surface morphologies of the composite films were discussed. The photocatalytic activity of the composite films was evaluated using methyl orange as the target pollutant. The influences of tourmaline on the forming of TiO2 particles, and the mechanism of enhancing photocatalytic activity of the TiO2 composite films were also studied.
2 Experimental
2.1 Preparation of composite sol
The chemical composition of the starting alkoxide solution was Ti (OC4H9) 4∶C2H5OH∶CH3COOH=10.2∶ 48∶7.2 in mass ratio. Ti (OC4H9) 4 and CH3COOH were dissolved in ethanol. After stirring for 1 h at the speed of 100 r/min, the blending solution, including the black tourmaline particles, deionizing water and ethanol, was added dropwise into the above solution in 30 min. After stirring for 3 h, HCl was mixed with the solution. The resultant alkoxide solution was kept standing at room temperature for hydrolysis reaction for 2 h, resulting in the saffron and semitransparent T/TiO2 composite sol, whose pH was 2.0. The tourmaline particles used here were schorl ores from Xinjiang Uygur Autonomous Region, China. The particle size of the schorl ores (D50) was 0.94 μm. The main chemical compositions of tourmaline were as follow (%, by dry mass): SiO2 34.6, Al2O3 34.98, B2O3 10.94, Fe2O3 15.8, Na2O 0.91, K2O 0.036, MgO 0.2.
2.2 Preparation of TiO2 composite films
The copper webs were used as substrates for thin films. Before preparation, the copper webs (5 cm×5 cm) were subjected to pre-treatment, which was as follows. The copper webs were washed by acid in order to remove any oxide or impurity. Then the copper webs were rinsed by deionizing water and ethanol, respectively. Finally, the webs were well prepared after drying. The composite films on the substrates were prepared from the above T/TiO2 composite sol solution by dipping-withdrawing in ambient atmosphere. The webs were firstly dipped into the sol for 5 min. The withdrawal speed was 1 cm/min. The substrates coated with T/TiO2 composite gel films were dried naturally, and subsequently heat-treated at 250 ℃ for 3 h in air using an electric oven. The following T/TiO2 composite films were thus prepared.
2.3 Microstructures and photocatalytic activity of TiO2 composite films
The microstructures of T/TiO2 composite films were determined by SEM (Philips XL30-TMP). Furthermore, the element analysis of T/TiO2 composite films was performed by EPA (EDAX-PHENIX).
The photocatalytic activity of T/TiO2 composite films was evaluated by methyl orange degradation. A UV lamp (25 W, 253.7 nm) was used as a light source. The averaged intensity of UV irradiance was 49 mW/cm2 by measuring with a UV irradiance meter (UVZDZ-1). The concentration of methyl orange was determined by a 756MC U-V spectrometer.
3 Results and discussion3.1 Films SEM characterization
Fig.1 shows the microstructure of T/TiO2 composite films heat-treated at 250 ℃ for 3 h. It can be seen that there are lots of clustered particles distributing uniformly on the copper whose average particle size is about 14 ?m, in which the clustered particles are piled up by particles themselves. From the element analysis of T/TiO2 composite films, clustered particles contain Fe, Mg, Si, Ti, O, Cl elements, from which it is affirmed that the inner of the clustered particles includes tourmaline particles, that is, the clustered particles are composite composing of many TiO2 particles developed using tourmaline particles as core. Around the clustered
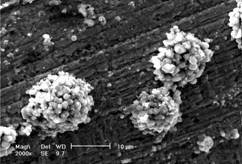
Fig.1 SEM images of TiO2 composite films containing tourmaline particles on surface of copper
particles, there exists a TiO2 film including Ti, O and Cl elements and so on.
Fig.2 shows the elemental line scanning analysis of the composite films. It is clearly seen that the line distribution of oxygen element is consistent with the around area, that is, both areas have same oxygen content, whereas the content of Ti and Cl on the surface of the clustered particles is higher than around area, especially for the Cl. The positive Ti groups and Cl were adsorbed due to the polarity of tourmaline particles, resulting in the enrichment of the two elements on the surface of tourmaline.
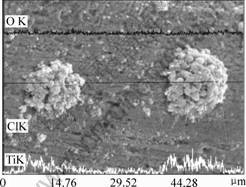
Fig.3 shows the microstructure of TiO2 particles on the surface of tourmaline. It can be seen that there exists clear ladder layers structure of TiO2 particles formed on the surface of tourmaline. The nano-sized heaves are distributed on the surface of the particles, thereinto the average diameter of the clustered particles and the TiO2 particles are 14 and 2 μm, respectively; the thickness of ladder layer is 10 nm, and the average diameter of nano-sized TiO2 particles is 15 nm. There exists evident particles spaces between TiO2 particles.
Fig.4 shows the electrical field strength around tourmaline particles. Tourmaline is a unique polar mineral. There brings into being the polar electric charge

Fig.3 SEM images of microstructure of clustered TiO2 particles on surface of tourmaline
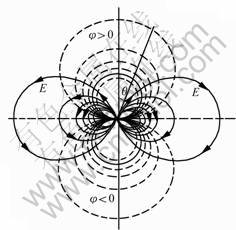
Fig.4 Electrical field strength around tourmaline particles (Tourmaline particle is located in coordinate origin; c axis is parallel with horizontal axis)
because of the existence of the spontaneous polarityresulted from no inosculation of the center of positive and negative charge. The polar electric charges, like the free electric charges, are the source fields inducing electrical field strength, which behaves there exist some static charges on both sides of c axis of tourmaline. When a tourmaline particle is small enough, the tourmaline particle can act as a pair of electric dipole. The electrical field strength paralleling c axis is the highest. The electrical field strength turns on the inverse ratio with r3, consequently, when the r-value is increased, the electrical field strength of tourmaline decreases more quickly than the point charge. The tourmaline particles can be seen as many individual micro-electrical fields. When tourmaline is processed from crystal to particles, the specific surface area and the number of broken bonds will increase, thus the tourmaline has the tendency of spontaneously adsorbing external ions and atoms.
Fig.5 shows the forming mechanism of clustered particles in T/TiO2 composite films. In our study, under the action of anode electric field, the negative chlorine Cl- ions were preferentially adsorbed on the nucleus of the surface of anode of tourmaline, forming the first nucleus. The positive titanium groups [Ti (OR) 4(H3O)nn+] were adsorbed due to the negative nucleus of chlorine ion, thereby impelling the growing of crystal. However, there existed the reciprocity between the two Cl- nucleuses, resulting in the weaker adsorption of Cl- among the clearance of the nucleus to the titanium ion. At the same time, the strong electric field of tourmaline excluded the adsorption of titanium ion. Hence, the crystal particles preferentially grew at the Cl- positive direction of stronger gravitation instead of the Cl- space area of weaker gravitation. As the crystal growing, titanium ions gradually gathered, the area of positive electricity was formed. Adding the anode electric field of tourmaline, the adsorption of titanium ions was weakened, which stopped the continuing growing, forming the first layer of titanium crystal. Meanwhile, the positive property was increased, accompanying with the strengthened adsorption of Cl-, forming the second layer of Cl- crystal. The titanium layer was grown more sites, and circling in turn; finally the mushroom titanium particles with ladder layers were formed.
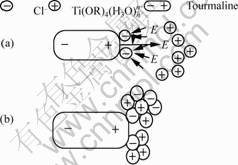
Fig.5 Schematic diagram showing forming mechanism of clustered parlicles in TiO2 composite film: (a) Electric field interaction between Cl- crystal nucleus and anode of tourmaline; (b) Structure of TiO2 particles on the anode of tourmaline
Furthermore, when the solvent in the titanium sol was volatilized thoroughly, also the crystal growing was finished, the remanent positive titanium ions were lack of forming the whole titanium crystal layer, preferentially growing in the nucleus, consequently forming the little heaves at the center of nucleus, which was significant for the increasing of the active sites and improving the photocatalytic efficiency.
3.2 Photocatalytic activity
Fig.6 show the photocatalytic activity of T/TiO2 composite films by one layer (0.1 micron) and two layers (0.2 micron), respectively. Since all the curves can be fitted roughly to a straight line, the photocatalytic degradation reaction can be assumed to follow a pseudo-first-order expression as
![]() (1)
(1)
where c0 and c are the initial concentration and the reaction concentration of methyl orange, respectively, k is the apparent reaction rate in term of min-1. For Fig.6(a), the k-value (4.7×10-3) of curve (1) is obviously higher than that of curve (2) (3.1×10-3), which is well known to have a high photocatalytic activity. Meanwhile, for Fig.6(b), k-value (7.1×10-3) of curve (1) is higher than that of curve (2) (3.3×10-3) photocatalytic activity.

Fig.6 Effects of tourmaline particles on photocatalytic degradation methylorange of TiO2 films: (a) One layer; (b) Two layers
The main reasons why tourmaline can improve the photocatalytic activity of TiO2 are as follows. 1) The clustered TiO2 particles using tourmaline as core had bigger specific surface area than that of pure TiO2 films, and the particles space of TiO2 had stronger adsorption to the methyl orange, also the nano-sized heaves on the surface of TiO2 particles made into bigger surface roughness which supplied more photocatalytic active sites. Hence, the ladder layers structure and the clustered particles made of nano-heaves TiO2 supplied better photocatalytic condition for the adsorption and degradation of the methyl orange. 2) Tourmaline has permanent pole, whose electric field strength is 107 -104 V/cm3 in rang of tens of micron on tourmaline surface. Under the irradiation of ultraviolet, the photocatalytic reaction took place between tourmaline and TiO2 coated on the surface of tourmaline, and the photoexcited electrons could be transferred the surface of tourmaline. Thus, the recombination of e- and h+ was reduced. Consequently, the utilization of h+ and the efficiency of photocatalytic reaction were enhanced. 3) The far infrared irradiated by tourmaline could activate the water molecules and decrease the association of the water molecules[16], in favor of the process of photocatalysis. When the inhere frequency of basic particles of substance irradiated by the far infrared matches the outside far infrared, the substance can preferably adsorb radiant energy, produce resonance and heat the substance quickly. A lot of inorganic compound and most organic macromolecular compound both have stronger adsorption ranges, which can accelerate the degradation reaction. Moreover, when the T/TiO2 composite films were prepared by coating a fewer layers, there formed more clustered particles of TiO2 using tourmaline as core, which could quite enhance the photocatalytic activity of the composite films. Also, the article supplies a way that one crystal (titania) gets to grow on the surface of another mineral crystal with spontaneous polarity such as tourmaline.
4 Conclusions
The novel composite films containing clustered TiO2 particles and fine tourmaline particles on the surface of copper webs could be prepared by the sol-gel method. The tourmaline particles obviously influenced the microstructures of TiO2 films and enhanced the photocatalytic activity due to their spontaneous permanent polarity and a high radiotechnology of far infrared. During preparing the T/TiO2 composite films, the clustered TiO2 particles with lots of nano-sized ladder layers could grow on the surface of fine tourmaline particles. The average diameter of the clustered particles and the TiO2 particles are 14 and 2 μm, respectively. The thickness of ladder layer is 10 nm, and the average diameter of nano-sized TiO2 particles is 15 nm. It is promising that the materials can be made into multifunctional photocatalytic materials for purifying environment and improving health.
References
[1] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 328(7): 37-38.
[2] HOFFMAN M R, MARTIN S T, CHOI W, et al. Environmental applications of semiconductor photocatalysis[J]. Chem Rev, 1995, 95(1): 69-96.
[3] LINCEBIGLER A L, LU G Q, YATES J T. Photocatalysis on TiO2 surfaces: principle, mechanisms, and selected results[J]. Chem Rev, 1995, 95(3): 735-58.
[4] FUJISHIMA A, RAO T N, TRYK D A. Titanium dioxide photocatalysis [J]. J Photochem Photobio C: Photochem Rev, 2000(1): 1-21.
[5] TAKAMI K, SAGAWA T, UEHARA H, et al. Photocatalytic De-NOx-ing building materials[J]. Catalysts & Catalysis, 1999, 41(4): 295.
[6] JONES A P, WATTS R J. Dry phases titanium dioxide-mediated photocatalysis: basis for in situ surface destruction of hazardous chemicals[J]. JEnviron Engin, 1997, 10: 974-980.
[7] BUTTERFIELD I M, CHRISTENSEN P A, CURTIS T P, et al. Water disinfection using an immobilised titanium dioxide film in a photochemical reactor with electric film enhancement[J]. Water Res, 1997, 31(3): 675.
[8] NAKAMURA T, KUBO T. The tourmaline group crystals reaction with water[J]. Ferroelectrics, 1992, 137: 13-31
[9] JI Z J, JIN Z Z, LIANG J S, et al. Observation of electric dipole on polar crystalline tourmaline granules[J]. Synth Cryst, 2002, 31(5): 503-508.
[10] YAMAGUCHI S. Surface electric fields of tourmaline[J]. J Appl Phys A, 1983, 31: 183-185
[11] BARTON R J. Refinement of the crystal structure of buergerite and the absolute orientation of tourmalines[J]. Acta Crystallographica, 1969, B25: 1524-1533.
[12] YOSHINORI K, HIKOHIRO S. Tourmaline Composite Grains and Apparatus Using Them[P]. USP6034013, 2000.
[13] JI Z J, JIN Z Z, LIANG J S, et al. Influence of tourmaline on pH value of water[J]. Chin Environ Sci, 2002, 22(6): 515-519.
[14] YANG R Z, LIAO Z T, CHEN X D. Thermoelectric character of natural blank tourmaline[J]. J Gems Gemmology, 2000, 2(1): 34-38.
[15] TANG Y H, WU R H, ZHANG X H. The mechanism of applying tourmaline to purifying Cu2 +-doped waste water[J]. Acta Petrolo-gica Et Mineralogoca, 2002, 21(2): 192-196.
[16] TAO X, MA W H, ZHANG T Y, ZHAO J C. Efficient photooxidative degradation of organic compounds in the presence of iron tetrasulfophthalocyanine under visible light irradiation[J]. Angew Chem Inted, 2001, 40(16): 3014-3016.
Foundation item: Project(E2004000033) supported by the Natural Science Foundation of Hebei Province, China
Corresponding author: LIANG Jin-sheng; Tel: +86-22-26582575; E-mail: liang_jinsheng@sina.com


