
Microstructure and properties of in situ A356/TiB2 composite
LE Yong-kang(乐永康)1, 2, CHEN Dong(陈 东)1 , MA Nai-heng(马乃恒)1,
ZHANG Wen-jing(张文静)2, MAO Jian-wei(毛健伟)2, WANG Hao-wei(王浩伟)1
1. State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University,
Shanghai 200030, China
2. Suzhou Institute for Nonferrous Metal Research, Suzhou 215026, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
In situ A356/TiB2 composites were successfully fabricated via in-melt reaction among aluminium alloy, K2TiF6 and KBF4 compounds. The composite was examined by using XRD, SEM and EDX techniques. The experimental results reveal that TiB2 are dispersed homogeneously into the aluminium alloy matrix. The mechanical properties of the composites increase significantly with the addition of reinforcement, and the tensile fractography of the composite exhibits to be ductile though the elongation of the composites decreases compared with the unreinforced matrix alloy.
Key words:
aluminium matrix composites; in situ; A356 alloy; TiB2; tensile properties;
1 Introduction
Problems are known to occur during ex situ processes with dispersoid distribution and settling out during casting, when particles are introduced deliberately and systematically, either in a powder blend or by additions to a melt. In situ synthesis is comparatively considered one of the innovative attractions as it can achieve an homogeneous dispersion of reinforcement and well bonding of the interfaces between particles and matrix metal, which are expected to be desirable for maximizing the mechanical properties, despite of the coupling with a small increase in materials cost, making it a competitive approach for fabrication of particle reinforced metal matrix composites (PRMMC).
It is essential for reinforcement produced to be thermodynamically stable with matrix. Among Al based PRMMCs, in situ Al-TiB2 composites have certain advantages such as without producing an embrittle layer resulting from the aggressive reaction between the reinforcement and molten aluminium alloys, together with its compatibility with the matrix interfacially and crystallographically [1], exhibiting higher strength, modulus, creep and wear resistance than conventional ex situ composites. These unique properties make them attractive for use as structural component materials in aerospace and automotive industries.
The earliest in situ work on Al-TiB2 composites was carried out by the LanxideTM technique [2], followed by the XDTM method [3]. Owing to the proprietary nature of these processes, few details are known apart from some proven work with the above techniques. Recently, a flux assisted in situ fabrication method for the production of TiB2 reinforced Al PRMMCs has emerged[4]. In this method, TiB2 particles are obtained via the metallothermic reduction of hexafluorotitanate (K2TiF6), tetrafluoroborate (KBF4) and liquid aluminium alloy according to the equation expressed as follows:
K2TiF6+2KBF4+10/3Al == TiB2+4/3(3KF·AlF3)+2AlF3 (1)
It is efficient for preparing the TiB2 reinforced aluminium matrix composites using this method. WANG et al [5] suggested that a single ceramic phase of TiB2 can be produced by controlling the [Ti]/[B] ratio. However, some other observations implied that Al3Ti was an unavoidable by-product when synthesizing the composites in the Al-Ti-B system [6].
Although the Al-Ti-B system has been adopted to fabricate in situ TiB2 particle reinforced Al-based composites by several researchers [1-9], the existing studies [4, 9] agreed that there is a lack of understanding of the reaction mechanisms, which hinders the optimization of the process and the application of Al-TiB2 products.
The aim of this paper is to investigate the morphology and dispersion of the in situ TiB2 particles within A356 alloy produced by mixing salts process. The microstructure and mechanical properties of the composites are concerned.
2 Experimental
Al/8%TiB2 (mass fraction) composites were fabricated using mixed salts reaction in the system of A356 alloy, K2TiF6 and KBF4 compounds at 850 ℃. The identical stoichiometric K2TiF6 and KBF4 were added into the molten alloy and kept stirring in the duration of reaction for 20 min. Then the composites were degassed in vacuum at 750℃ before pouring into a permanent mould.
A Rigaku D/max X-ray diffractometer with Co Kα radiation was used for phase identification. The microstructure of the materials was observed under the EPITHOT-200 optical microscope and JSM-6480 scanning electronic microscope (SEM). Then the specimens were steeped into HF acid for 3 h, followed by observation using SEM. The tensile specimens with a gauge diameter of 5 mm and length of 25 mm were machined and tested using INSPRON-8502 at a strain rate of 6×10-3 s-1. The fracture surfaces of the tensile tested samples were also observed by SEM.
3 Results and discussion
3.1 In situ formation of TiB2 particles
In Al-Ti-B system, several different reactions have been proposed among Al, Ti and B. The thermo- dynamic calculation [10] has consistently shown the presence of compound phases of AlB2, TiB2, Al3Ti and mixed boride phase of (Al,Ti)B2. The experimental result of XRD is shown in Fig.1. It is clear that the expected phase TiB2 is formed according to reaction (1) among stoichiometric K2TiF6, KBF4 compounds and aluminium alloy, with Al3Ti eliminated completely. The TiB2 particles are seen to disperse homogeneously in the matrix of the composite as illustrated in Fig.2. It is suggested that the relative proportion of Al3Ti phase increases with the increasing volume fraction of TiB2 [6]. However, the Al3Ti is considered to disappear with the temperature increasing because the Gibbs free energy of Al3Ti formation is significantly lower than that of AlB2 and TiB2 [11], resulting in a dominant reaction of in situ formation of TiB2 occurred in the system.
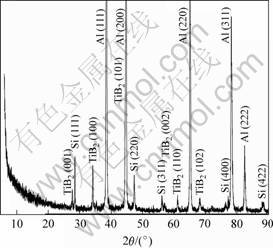
Fig.1 XRD pattern of as-fabricated A356/8%TiB2 composite
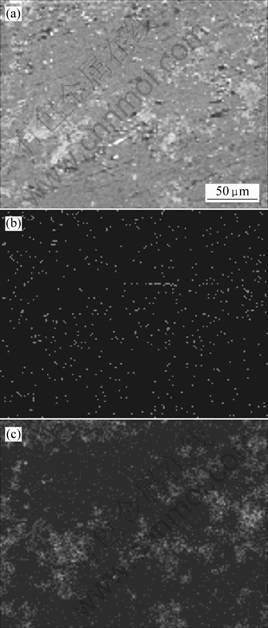
Fig.2 Elemental distributions of Al, Ti and B in as-fabricated A356/8%TiB2 composite: (a) SE image; (b) B; (c) Ti
3.2 Mechanical properties
Table 1 shows the tensile properties of the experimental materials. It can be found that the strength of the composites is enhanced significantly compared with the unreinforced A356 alloy. However, the ductility of the composites is decreased. The elastic modulus of the composites is substantially higher than that of A356 alloy, though the value is much lower than that of 110 GPa predicted by the application of the rule of mixture.
Table 1 Mechanical properties of experimental materials
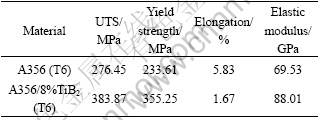
It is noted that the presence of unavoidable local agglomerations of the reinforcements can not undertake stress transmission efficiently when the material is employed under a given load. On the other hand, the stress concentration exists normally in the vicinity of the particles, resulting in the homogeneous formation in the matrix. Therefore, these particle agglomerations are believed to be actually harmful to the mechanical properties of the composites. The brittle phase Al3Ti is generally known to be the initiation of void and microcrack during the fracture of materials, resulting in the strength and ductility of the material decreasing.
3.3 Fractography
The presence of equiaxed and deep dimples in the composite as shown in Fig.3 indicates ductile fracture. The microvoids grow with increasing load until they are of sufficient size to coalesce, resulting in final fracture. The degree of plastic deformation and the ductility of the alloy would therefore depend upon the extent of growth of the voids. It can clearly be observed that there is a mixture of large and small dimples with a large degree of plastic sites containing inclusions and localized high stress.
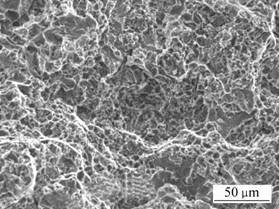
Fig.3 Tensile fracture surface of composite
The TiB2 particles are normally located at the bottom of the dimples, as shown in Fig.4. No large and deep dimples can be seen, implying that the bonding between TiB2 and matrix is generally good though debonding at some places can be detected. It is understood that the formation of crack normally undergoes three distinct phases, namely void initiation, void growth and void coalescence. Voids can grow in the direction transverse or parallel to the loading direction. Cracks form as voids coalesce with each other in the transverse direction. If they grow in a direction parallel to the loading direction, deep and large dimples and voids can be seen. Dimples are generally formed upon load in the experimental composites. However, the growth of the dimples ceases once they meet the hard in situ particles. This explains why some TiB2 particles have been observed at the bottom of dimples in the present investigation.
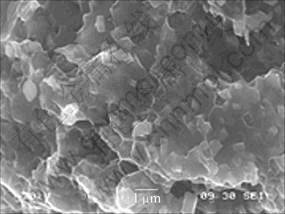
Fig.4 Morphology of in situ TiB2 particles on fracture surface of composite
4 Conclusions
1) The unexpected brittle phase Al3Ti is eliminated with the occurrence of TiB2 particles which disperse homogeneously into the aluminium matrix of the composite via the in-melt reaction in the system of A356 alloy, K2TiF6 and KBF4 compounds at 850 ℃.
2) The strength and modulus of the composites are improved remarkably with the presence of in situ TiB2 particles, whereas the ductility of the composites decreases owing to the particle agglomerations.
3) The primary fracture mechanisms of the composites are suggested to be a combined effect of the matrix cracks and debonding of the interfaces between particles and aluminium matrix.
References
[1] DOMETAKIS C, JHA A. Flux assisted dispersion of ceramic in molten aluminium alloys[A]. LIN R Y, et al. Design fundamental of high temperature composites, intermetallics and metal-ceramic system[C]. Warrendale, PA, TMS, 1995: 57-70.
[2] NEWKIRK M S, URQUHART A W, ZWICKER H R, BREVAL R. Formation of LanxideTM ceramic composites materials[J]. Journal of Materials Research, 1986, 1(1): 81-89.
[3] WOOD A R C. Materials for advanced studies and devices[J]. Metall Mater Trans A, 1988, 19(6): 749-758.
[4] WOOD J V, DAVIES P, KELLIE J L F. Properties of reactively cast aluminium-TiB2 alloys[J]. Materials Science and Technology, 1993, 9(10): 833-840.
[5] WANG L, ARSENAULT R J. Interfaces in XD processed TiB2/NiAl composites[J]. Metallurgical Transactions A, 1991, 22(12): 3013- 3018.
[6] TEE K L, LU L, LAI M O. In situ cast Al-TiB2 composite: processing and mechanical properties[J]. Materials Science and Technology, 2001, 17(2): 201-206.
[7] KRISHNARAO R V, SUBRAHMMANYAM J. Studies on the formation of TiB2 through carbothermal reduction of TiO2 and B2O3 [J]. Mater Sci Eng A, 2003, 362(1/2): 145-151.
[8] EMAMY M, MAHTA M, RASIZADEH J. Formation of TiB2 particles during dissolution of TiAl3 in Al-TiB2 metal matrix composite using an in situ technique[J]. Composite Science and Technology, 2006, 66: 1063-1066.
[9] LU L, LAI M O, SU Y, TEO H L, FENG C F. In situ TiB2 reinforced Al alloy composites[J]. Scripta Mater, 2001, 45(9): 1017-1023.
[10] BONDAR A. Ternary alloy system-phase diagrams, crystallographic and thermodynamic data critically evaluated by MSIT: Light metal system, Part 1[A]. EFFENBERG G, LLYENKO S. Landolt-bornstein, Group IV Physical Chemistry[M]. Heidelgerg: Springer Berlin, 2004: 94.
[11] YUE N L, LU L, LAI M O. Application of thermodynamic calculation in the in-situ process of Al/TiB2 [J]. Composite Structures, 1999, 47(5): 691-694.
(Edited by YUAN Sai-qian)
Corresponding author: LE Yong-kang; Fax: +86-512-62585618, E-mail: ykle@sjtu.edu.cn


