Effects of grafting cell penetrate peptide and RGD on endocytosis and biological effects of Mg-CaPNPs-CKIP-1 siRNA carrier system in vitro
来源期刊:中南大学学报(英文版)2021年第5期
论文作者:陈良建 易曼菲 贺惠莉 石蕾 邵春生 张博
文章页码:1291 - 1304
Key words:calcium phosphate nanoparticles; adhesion peptide; cell penetrate peptide; endocytosis; siRNA
Abstract: Calcium phosphate nanoparticles (CaPNPs) have good biocompatibility as gene carriers; however, CaPNPs typically exhibit a low transfection efficiency. Cell penetrate peptide (TAT) can increase the uptake of nanoparticles but is limited by its non-specificity. Grafting adhesion peptide adhesion peptide on carriers can enhance their targeting. The Plekho1 gene encodes casein kinase-2 interacting protein-1 (CKIP-1), which can negatively regulate osteogenic differentiation. Based on the above, we produced a Mg-CaPNPs-RGD-TAT-CKIP-1 siRNA carrier system via hydrothermal synthesis, silanization and adsorption. The effects of this carrier system on cell endocytosis and biological effects were evaluated by cell culture in vitro. The results demonstrate that CaPNPs with 7% Mg (60 nm particle size, short rod shape and good dispersion) were suitable for use as gene carriers. The carrier system boosted the endocytosis of MG63 cells and was helpful for promoting the differentiation of osteoblasts, and the dual-ligand system possessed a synergistic effect. The findings of this study show the tremendous potential of the Mg-CaPNPs-RGD-TAT-CKIP-1 siRNA carrier system for efficient delivery into cells and osteogenesis inducement.
Cite this article as: YI Man-fei, CHEN Liang-jian, HE Hui-li, SHI Lei, SHAO Chun-sheng, ZHANG Bo. Effects of grafting cell penetrate peptide and RGD on endocytosis and biological effects of Mg-CaPNPs-CKIP-1 siRNA carrier system in vitro [J]. Journal of Central South University, 2021, 28(5): 1291-1304. DOI: https://doi.org/10.1007/s11771-021-4697-7.

J. Cent. South Univ. (2021) 28: 1291-1304
DOI: https://doi.org/10.1007/s11771-021-4697-7
YI Man-fei(易曼菲)1, CHEN Liang-jian(陈良建)1, 2, HE Hui-li(贺惠莉)1,SHI Lei(石蕾)1, SHAO Chun-sheng(邵春生)1, ZHANG Bo(张博)1
1. Department of Stomatology, the Third Xiangya Hospital, Central South University,Changsha 410013, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: Calcium phosphate nanoparticles (CaPNPs) have good biocompatibility as gene carriers; however, CaPNPs typically exhibit a low transfection efficiency. Cell penetrate peptide (TAT) can increase the uptake of nanoparticles but is limited by its non-specificity. Grafting adhesion peptide adhesion peptide on carriers can enhance their targeting. The Plekho1 gene encodes casein kinase-2 interacting protein-1 (CKIP-1), which can negatively regulate osteogenic differentiation. Based on the above, we produced a Mg-CaPNPs-RGD-TAT-CKIP-1 siRNA carrier system via hydrothermal synthesis, silanization and adsorption. The effects of this carrier system on cell endocytosis and biological effects were evaluated by cell culture in vitro. The results demonstrate that CaPNPs with 7% Mg (60 nm particle size, short rod shape and good dispersion) were suitable for use as gene carriers. The carrier system boosted the endocytosis of MG63 cells and was helpful for promoting the differentiation of osteoblasts, and the dual-ligand system possessed a synergistic effect. The findings of this study show the tremendous potential of the Mg-CaPNPs-RGD-TAT-CKIP-1 siRNA carrier system for efficient delivery into cells and osteogenesis inducement.
Key words: calcium phosphate nanoparticles; adhesion peptide; cell penetrate peptide; endocytosis; siRNA
Cite this article as: YI Man-fei, CHEN Liang-jian, HE Hui-li, SHI Lei, SHAO Chun-sheng, ZHANG Bo. Effects of grafting cell penetrate peptide and RGD on endocytosis and biological effects of Mg-CaPNPs-CKIP-1 siRNA carrier system in vitro [J]. Journal of Central South University, 2021, 28(5): 1291-1304. DOI: https://doi.org/10.1007/s11771-021-4697-7.
1 Introduction
Gene therapy is a therapeutical method in which a target gene is efficiently and successfully transported to a target site through a gene vector. The target gene enters into and is expressed in the target cell, thus making full use of its specific therapeutic functions. Gene vectors are one of the key factors in gene therapy. Viral vectors possess outstanding transfection efficiencies, but there is a potential risk of immunogenicity and carcinogenicity [1]. Nonviral vectors include cationic polymers, liposomes and inorganic nanoparticles. Cationic polymers such as polyethylenimine (PEI) and polypropylenimine (PPI) can intrinsically alter the expressions of many endogenous genes, which can potentially lead to the exhibition of multiple biological effects in cells [2]. These off-target effects can affect the safety of the formulation. Liposomes are occasionally known to induce complement activation, which can lead to hypersensitivity reactions [3, 4]. A new type of inorganic nanoparticle, namely, calcium phosphate nanoparticles (CaPNPs), has received extensive attention. CaPNPs bind and protect DNA or small molecule interference RNA (siRNA); moreover, they show prominent biocompatibility, no immunogenicity and no tumorigenicity [5, 6]. CaPNPs can be easily degraded in the early lysosomes [7]. CaPNPs are convenient to prepare and easy to preserve, making them more economical than other options. However, as a gene vector, CaPNPs need to be improved with regards to their targeting and transfection efficiency. The endocytosis of CaPNPs by target cells is affected by the morphology, particle size and surface charge of the nanoparticles [8]. In the process of CaPNP preparation, Ca2+ is relatively active and easily replaced by other elements to form doped CaPNP compounds [9, 10]. Doping Mg increases the positive charge on the surface of CaPNPs, which facilitates the carrier system to carry the negatively charged gene [11]. The size of nanocarrier particles may be negatively correlated with the transfection efficiency, which means that the smaller the particles, the higher the transfection efficiency [12]. Additionally, it is usually difficult for large particles to pass through the cell membrane. And once it enters the cell, it may produce too much calcium making it impossible for the cell to survive [13]. Our research group found that the morphology of hydroxyapatite nanoparticles (HANPs) doped with 7.5% Mg were relatively uniform and slender with an average size of 30 nm [14]. A coculture of Mg-CaPNPs and Tb-CaPNPs with cells showed that the former easily migrated to the cell surface, which was beneficial for cell endocytosis [15]. However, the above transfection efficiency and targeting are still insufficient for the rapid and specific accumulation of vectors in target tissues.
TAT peptide, a well-known cell penetrating peptide, which is the basic region of the trans-activating transcriptional activator protein from human immunodeficiency virus type 1 (HIV-1), can enter cells through a variety of mechanisms and mediate efficient intracellular transport [16-18]. Through modeling, HO et al [19] analyzed the strong α helix characteristics of arginine residues with a positive charge on the surface of 48-57 amino acids in the TAT protein and found that this area could significantly increase the efficiency of penetrating cell membranes. However, TAT lacks cell specificity because it transports cargo through the electrostatic interaction of its positively charged amino acids with negatively charged cell surface glycoproteins [20]. Therefore, its targeted application is greatly limited. An adhesion peptide is a short peptide sequence composed of arginyl-glycyl-aspartic acid (RGD), which is a specific ligand of many types of integrin receptors on the cell membrane [21]. Some scholars have found that grafting RGD on the surface of biomaterials is beneficial to their adhesion, spread and proliferation on cells and enhances their targeting [22-24].
In recent years, it has been found that the Plekho1 gene encodes casein kinase-2 interacting protein-1 (CKIP-1), which can negatively regulate osteogenic differentiation. With increasing age, the bone mass advantage of CKIP-1-deficient mice was more significant than that of wild-type mice, indicating that the rates of new bone formation, bone mass, osteogenesis-related type 1 collagen, osteocalcin, and alkaline phosphatase all increased in CKIP-1-deficient mice. However, there was no change in the osteoprotegerin (OPG), RANK ligand and nuclear factor of activated T cells c1 (NFATc1) in osteoclasts [25]. The expression level of CKIP-1 is low in main organs and tissues and only high in terminal differentiated tissues, such as bone tissue, muscle and myocardium [26]. The specificity of CKIP-1 and its conservatism in high expression tissues suggest that CKIP-1 can be used as a target in gene regulation for bone regeneration therapy, while also demonstrating high biosafety [27]. If siRNA can be released slowly around the scaffold and transfected into bone-forming cells, CKIP-1 mRNA can be silenced, which will relieve the negative regulation of CKIP-1 on osteogenesis and initiate the osteogenic process; thus, new bone formation will be promoted.
In this study, CKIP-1 was used as the target to accurately design siRNA that acts on CKIP-1 mRNA. A silane coupling method is used to process Mg-CaPNPs to construct a siRNA-CaPNP carrier system grafted with RGD and TAT. Here, we investigated the effects of grafting RGD and TAT on the targeting and transfection efficiency of the siRNA-CaPNP carrier system. Furthermore, we studied the effect of siRNA transfection on CKIP-1 gene silencing and osteogenesis at the cellular and molecular levels.
2 Materials and methods
2.1 Materials
Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), diammonium hydrogen phosphate ((NH4)2HPO4), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), and polyethylene glycol 2000 (PEG2000) were obtained from the Yacoo Chemical Reagent Co., Ltd. (Suzhou, China); ammonium hydroxide (NH3·H2O) was supplied by the Ji’nan Hongyun Chemical Co., Ltd. (Jinan, China); 3-Aminopropyl triethoxysilane (APTES), 4-morpholineethanesulfonic acid (MES) and arginyl-glycyl-aspartic acid (RGD) were provided by the Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China); fetal bovine serum (FBS), RPMI medium 1640, trypsin-EDTA (0.25%), and penicillin-streptomycin liquid were purchased from GIBCO BRL (USA); CKIP-1 siRNA was provided by Shanghai GenePharma Co., Ltd. (Shanghai, China); phosphate-buffered saline (PBS) was obtained from HyClone(USA),1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) was supplied by the Beijing China-Ocean SciTec Co., Ltd. (Beijing, China); green fluorescent protein (GFP) was purchased from Bioss (Beijing, China). TAT peptide was obtained from the SBS Genetech Co., Ltd. (Beijing, China); the cell counting kit-8 (CCK-8) was obtained from Dojindo (Shanghai, China); the human bone alkaline phosphatase (BALP) enzyme-linked immunosorbent assay (ELISA) kit and human OC/BGP(Oosteocalcin) ELISA kit were purchased from the Elabscience Biotechnology Co., Ltd. (Wuhan, China). TRIzol reagent was provided by Invitrogen (USA); primer was supplied by the Sangon Biotech Co., Ltd. (Shanghai, China), and ReverTra Ace qPCR RT master mix with gDNA remover and KOD SYBR qPCR mix were obtained from TOYOBO(Shanghai, China).
2.2 Preparation of Mg-CaPNPs
Mg-CaPNPs with different amounts of doped Mg were prepared by hydrothermal synthesis, with a molar ratio of (Ca+Mg) to P of 1.67 and mass fractions of Mg to (Ca+Mg) of 0, 7%, 9%, 11%, 13% and 15%, respectively. Ca(NO3)2·4H2O (1 mol/L), Mg(NO3)2·6H2O (1 mol/L) and (NH4)2HPO4 (1 mol/L) solutions were prepared in double distilled water. Additionally, 2 wt% PEG2000 surfactant was added to a 1 mol/L Ca(NO3)2·4H2O solution. Mixed solutions of Ca(NO3)2·4H2O and Mg(NO3)2·6H2O were prepared, followed by the drop-wise addition of 1 mol/L (NH4)2HPO4 solution at a rate of 0.12 mL/min. The pH of the suspension was adjusted to 10 with NH3·H2O. The suspensions were sonicated in an ultrasonic oscillator (SB-5200DTDN, SCIENTZ Biotechnology Co., Ltd., Ningbo, China) for 1 h and then intermittently sonicated for 10 min in an ultrasonic cell disruption system (VCX130, Sonics, USA). Then, the suspensions were transferred to a hydrothermal synthesis reactor (Shanghai Guangying Instrument Co., Ltd., Shanghai, China) and hydrothermally synthesized at 170 °C for 3 h in an electric thermostatic blast drying oven (DHG-9243BS-III, ShangHai CIMO Medical Instrument Manufacturing Co., Ltd., Shanghai, China). After the reaction, the resulting suspensions were naturally cooled to room temperature, alternately washed with ethanol and double distilled water 3 times and centrifuged at 1000 r/min for 5 min. The precipitate nanoparticles were freeze-dried at -80 °C for 12 h under vacuum using a freeze-dryer (SCIENTZ-10N, SCIENTZ Biotechnology Co., Ltd., Ningbo, China). All of the chemicals used in the sample preparation were of analytical grade and used as received without further purification.
2.3 Characterization of Mg-CaPNPs
The prepared nanoparticles were morphologically characterized by transmission electron microscopy (TEM). Micrographs were taken on a Tecnai G2 F20 transmission electron microscope (FEI, USA) at 200 kV. The zeta potentials of the Mg-CaPNP solutions with different molar ratios were measured using a ZetaSizer Nano ZS instrument (Malvern Instruments, UK) at 25°C. The as-prepared particles were characterized using X-ray diffraction (XRD) for phase analysis. The X-ray analysis was carried out using an X-ray diffractometer (Advance D8, Bruker, Swit) with Cu Kα radiation (λ=1.5418  ) over a range of 20°<2θ<80°. The operational voltage and current were kept at 40 kV and 250 mA, respectively.
) over a range of 20°<2θ<80°. The operational voltage and current were kept at 40 kV and 250 mA, respectively.
2.4 Grafting RGD and TAT on surface of Mg-CaPNPs
RGD and TAT were grafted by silanization combined with carbodiimide. EDC-MES solution (5 (μg/mL), pH=5.5), RGD-PBS (500 μg/mL, pH=7.2) and TAT-PBS (500 μg/mL, pH=7.2) buffer solutions were prepared. The Mg-CaPNPs samples with 7% Mg content were sonicated in an APTES anhydrous ethanol solution with a volume ratio of 1:20 for 4 h and then alternatively washed with double distilled water and ethanol 3 times. The samples were ultrasonicated in EDC-MES buffer for 6 h and then alternately washed with double distilled water and MES buffer 3 times. The samples were immersed in RGD-PBS and TAT-PBS buffer solutions for 4 h, washed with double distilled water twice, and freeze-dried for 24 h to obtain Mg-CaPNPs-RGD, Mg-CaPNPs-TAT and Mg-CaPNPs-RGD-TAT powders.
2.5 Characterization of Mg-CaPNPs-RGD-TAT
The as-prepared particles were detected by X-ray photoelectron spectroscopy (XPS, ESCALAB 250 Xi1, ThermoFisher-VG Scientific, USA) and XRD.
2.6 Construction of Mg-CaPNPs-CKIP-1 siRNA carrier system
The sequence of CKIP-1 mRNA is as follows:
Sense:
5-GGACUUGGUAGCAAGGAAATT-3;
Antisense:
5-UUUCCUUGCUACCAAGUCCTT-3.
The CKIP-1 siRNA centrifuge tube was centrifuged at 12000 r/min at 4 °C for 1 min, and then 33 μL of diethylpyrocarbonate (DEPC) water was added so that the concentration of the siRNA solution was 0.5 μg/μL. The as-prepared samples were diluted with double distilled water to a concentration of 1 μg/μL and sterilized by a sterile syringe filter (0.22 μm, Millipore, Mass, USA) before 1 μg of siRNA was added. The suspensions were ultrasonically oscillated and mixed for 5 min and stored at room temperature for 20 min. Finally, four groups of samples were prepared, including Mg-CaPNPs-siRNA (MCCR), Mg-CaPNPs-RGD-siRNA (MCRCR), Mg-CaPNPs-TAT-siRNA (MCTCR) and Mg-CaPNPs-RGD-TAT-siRNA (MCRTCR), collectively referred to as the siRNA-CaPNPs carrier system.
2.7 Cell viability assay
To evaluate MG63 cell viability, a cell counting kit-8 (CCK-8; Beyotime Biotechnology) assay was applied. Human osteosarcoma cells (MG63, Cell Experimental Center of Xiangya Medical College of Central South University, Changsha, China) were seeded in a 96-well plate at a density of 5×103 cells per well in 100 μL of medium containing 10% FBS and incubated at 37°C under a 5% CO2 atmosphere for 24 h. Then, 10 μL of different liquids was added to the culture plate (the experimental group was 500 μg/mL MCCR, MCRCR, MCTCR and MCRTCR, the positive control group was a phenol solution, and the negative control group was a culture medium containing 10% FBS), and blank holes were set up. There were 7 groups with 4 compound holes in each group. After 24 and 72 h of culture, 10 μL of the CCK-8 solution was added into each well, and the cells were incubated for 2 h at 37°C. The absorbance (A) was measured by an ELISA microplate reader (ELx800, Bio-Tek, VT, USA) at 450 nm, and the cell viability (Vc) was calculated according to:
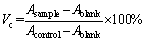 (1)
(1)
2.8 Transfection of siRNA-CaPNPs carrier system
The coculture experimental group was divided into four groups: MCCR, MCRCR, MCTCR and MCRTCR, all of which were labeled with 1 mg/mL GFP; the control group was the culture medium. An experimental group marked with GFP and the culture medium containing 10% FBS were prepared as a suspension with a concentration of 500 μg/mL. MG63 cells were cultured in 6-well plates at an initial seeding density of 5×104 cells per well in 1 mL of medium containing 10% FBS at 37°C and in a humidified atmosphere containing 5% CO2. The cells were cultured for 24 h until they completely adhered to the wall. The experimental group suspensions were used instead of the original culture medium, and they were placed in an incubator and cultured for an additional 1 h. After washing with PBS 3 times, images of white light and fluorescence were compared under a fluorescence inverted microscope (Olympus), and the endocytosis of MG63 cells was observed.
2.9 Alkaline phosphatase (ALP) activity assay
The ALP activity assay was performed to evaluate the effect of the siRNA-CaPNPs vector system on the early osteogenic differentiation of MG63 cells. The experimental groups were MCCR, MCRCR, MCTCR and MCRTCR, and the control group was the culture medium containing 10% FBS. The experimental groups and the culture medium containing 10% FBS were prepared as a suspension with a concentration of 500 μg/mL. Briefly, 2.5×104 cells per well were seeded in 24-well plates. After the cells were attached, the culture medium was replaced by experimental group suspensions. The cells were incubated at 37 °C and 5% CO2 for 1, 2, 3, 4 and 5 d. Each day the cell culture supernatant was taken, and the absorbance of each well at the 450 nm wavelength was measured by a human BALP ELISA kit.
2.10 Enzyme-linked immunosorbent assay (ELISA)
The experimental groups were the same as above, and the supernatant was collected from MG63 cells cocultured with the siRNA-CaPNPs carrier system for 1, 2, 3, 4 and 5 d. The released OC was quantified using an ELISA kit according to the manufacturer’s instructions. The concentration of osteocalcin (OC) was determined by correlation with the standard curve, and the result was expressed as the amount of OC (ng) per mL of supernatant.
2.11 Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
The effect of the siRNA-CaPNPs carrier system on the osteogenic differentiation of MG63 cells was assessed by real-time qRT-PCR to measure the mRNA expression of type I collagen (COL-I), osteocalcin (OC), and osteopontin (OPN) in all groups (the primer sequences used for the qRT-PCR study are shown in Table 1). The total RNA was extracted using TRIzol reagent, and the concentration of RNA was quantified using a nanodrop one microvolume UV-Vis spectrophotometer (Thermo Scientific, USA). Complementary DNA was synthesized from the total RNA using ReverTra Ace qPCR RT Master Mix with gDNA remover following the manufacturer’s protocol. Then, KOD SYBR qPCR mix was used for detection, and the target mRNA expressions were assayed on the real time constant fluorescence PCR instrument(Roche, Basel, Swit). Each sample was analyzed in triplicate. The expression of the relative target gene was calculated by the formula 2-delta cycle threshold (CT).
3 Results and discussion
3.1 Characterization of Mg-CaPNPs
3.1.1 Transmission electron microscopy analysis of Mg-CaPNPs
Figure 1 shows the TEM image of Mg-CaPNPs. When the doping amount of Mg2+ ranged from 0 to 7% (Figures 1(a) and (b)), the particles exhibited a short rod-shaped morphology, and the particle size of Mg-CaPNPs decreased from 100 to 60 nm; however, there was no significant difference in dispersibility. At doping amounts of 9%-15% (Figures 1(c)-(f)), the morphology changed with increasing Mg content. When the Mg contents were 11% and 13% (Figures 1(d) and (e)), the morphology appeared as irregular polygons, and the particle size increased. When the content of Mg was 15% (Figure 1(f)), the surface morphology of the particles was approximately round, and the particle size was greater than 100 nm. When the content of Mg was 9% (Figure 1(c)), the agglomeration was more obvious than that of other amounts of doped magnesium. It has been found that cations with an atomic radius of 0.09-0.13 nm can easily replace calcium ions (radius of 0.1 nm) in a solution. The ionic radius of Mg2+ is within this range, and thus, Mg2+ could inhibit the growth of CaPNP crystal particles, which was helpful for controlling the particle size of CaPNPs [28, 29]. Since the radius and properties of magnesium ions were different from those of the original calcium ion, the bond length of Mg-O is shorter than that of Ca-O, and with increasing Mg content, the ratio of the major axis to the minor axis became out of balance and resulted in lattice distortion, decreased crystallinity and grain size, and decreased thermal stability [30]. The results revealed that the doping of Mg affected the morphology, dispersion and average particle size of CaPNPs.
Table 1 Primer sequences used for qRT-PCR study
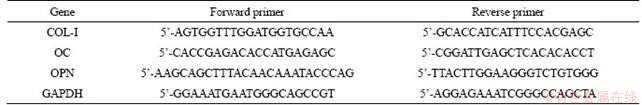
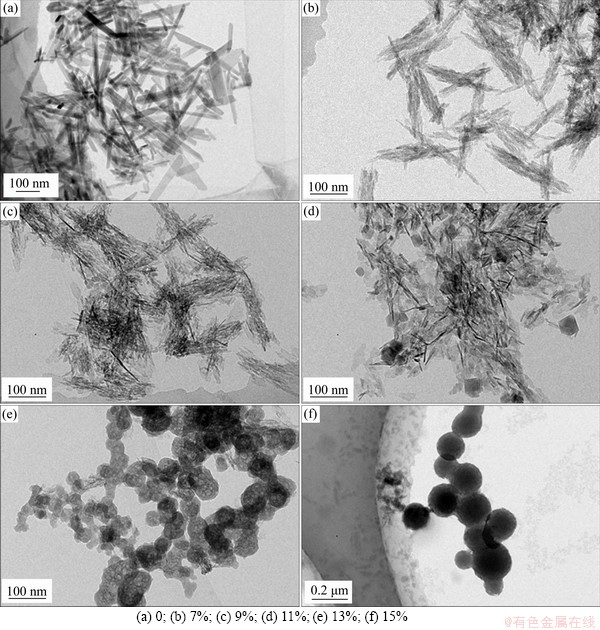
Figure 1 TEM images of undoped and doped CaPNPs with different Mg concentrations:
3.1.2 Zeta potentials of Mg-CaPNPs
The zeta potential magnitude is an important parameter to measure the stability of colloidal particles [31, 32]. The zeta potentials of CaPNPs in deionized water with different amounts of doped Mg are shown in Figure 2. When the doping amount of Mg2+ ranged from 0% to 9%, the absolute value of the zeta potential decreased with increasing Mg content. The zeta potentials with Mg content of 9% and 11% were smaller than those of the other Mg doping amounts. The absolute values of the zeta potential were larger when the Mg contents were 7%, 13% and 15%. The results showed that the doping amount of Mg could affect the zeta potential of the CaPNPs. Zeta potential is affected by factors such as colloidal size, morphology and surface charge [31, 32]. In this experiment, when the Mg contents were 7%, 13% and 15%, the absolute value of the zeta potential was larger, which indicated that the dispersion of nanoparticles was good. From the comparison of the morphology, particle size and dispersion of CaPNPs, it was found that the Mg contents of 7% and 13% were better than other Mg.
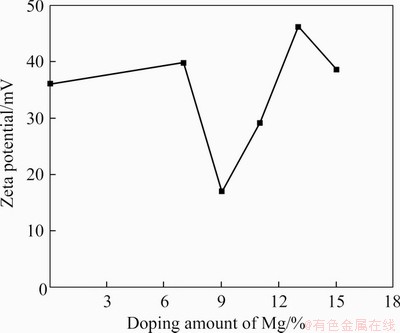
Figure 2 Zeta potentials of different Mg doping amounts of Mg-CaPNPs
3.1.3 XRD analysis of Mg-CaPNPs
Figure 3 shows the XRD spectrum of CaPNPs with different contents of Mg. These results showed that all the peaks of the nanoparticles doped with 7% and 13% Mg were basically the same as the diffraction peaks of hydroxyapatite (Ca10(PO4)6(OH)2) (PDF NO. 84-1998). With increasing Mg content, the main diffraction peak of the crystal weakened and broadened, which was attributed to the existence of Mg2+ ions in the CaPNP crystal and a certain degree of lattice distortion of the crystals. When the content of Mg was 13%, a small amount of white calcium phosphate (whitlockite) phase appeared in the sample. When the content of Mg reached 15%, an obvious whitlockite phase (PDF70-0682) appeared. At this time, the sample was a spherical aggregate because with too much Mg2+ it can no longer enter the CaPNP lattice, thus precipitating into a second phase.
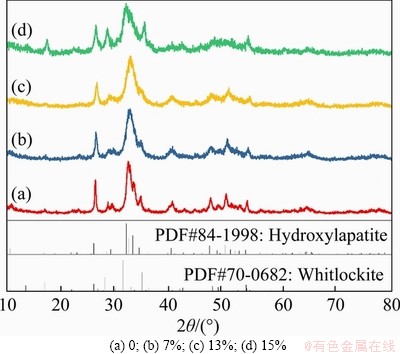
Figure 3 XRD patterns of CaPNPs with different Mg concentrations:
3.2 Characteristics of Mg-CaPNPs-RGD-TAT carrier system
Figure 4 shows the XRD spectra of Mg-CaPNPs with grafted RGD and TAT. The diffraction patterns of Mg-CaPNPs-RGD, Mg-CaPNPs-TAT and Mg-CaPNPs-RGD-TAT were basically the same as that of the ungrafted Mg-CaPNPs, and the peak position of the nanoparticles was basically unchanged after grafting RGD and TAT; furthermore, no new diffraction peak appeared, indicating that grafting RGD and TAT did not change the crystal structure of the nanoparticles.
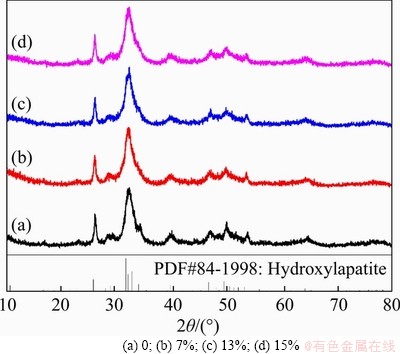
Figure 4 XRD patterns of Mg-CaPNPs-RGD-TAT carrier system:
Four groups of Mg-CaPNPs carriers were detected by photoelectron spectroscopy (XPS). Table 2 shows that Mg-CaPNPs did not contain N, while the Mg-CaPNPs grafted with RGD and TAT contained N, indicating that RGD and TAT were successfully grafted on the surface of the Mg-CaPNPs. Figure 5 reveals that the C of the Mg-CaPNPs group existed only in the form of C—C bonds, C—H bonds and C—N bonds. The C of the Mg-CaPNPs-RGD, Mg-CaPNPs-TAT and Mg-CaPNPs-RGD-TAT grafted groups existing in the form of C—H, C—N and N—C=O bonds (binding energy of (288.4±0.2) eV. The N—C=O bond was the chemical bond formed by the peptide reaction of silanized Mg-CaPNPs with RGD and TAT [22]. The results showed that RGD and TAT were chemically bound to the surface of the Mg-CaPNPs nanoparticles.
Table 2 Chemical composition of Mg-CaPNPs-RGD-TAT carriers (at%)
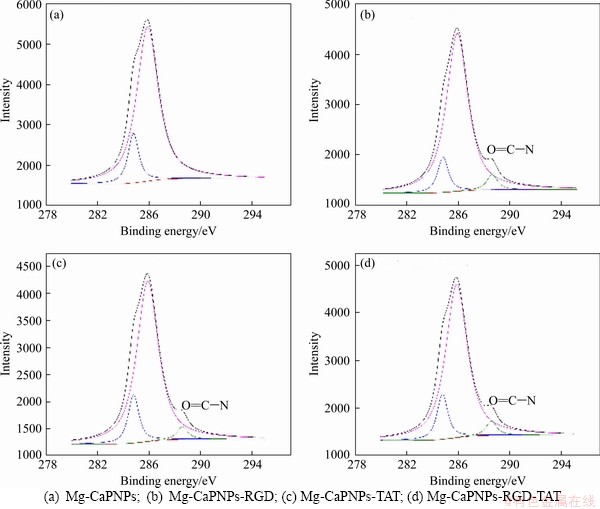
Figure 5 C spectra and fitting results of Mg-CaPNPs-RGD-TAT carriers:
3.3 Assessment of cell proliferation
As shown in Figure 6, the OD in the four experimental groups was not significantly different from that in the control group, nor was it observably different among the four experimental groups. The cell viability of each experimental group was greater than 78.8%, and the cytotoxicity grade was 1, meaning that it was nontoxic. The experimental results showed that the four carrier systems of MCCR, MCRCR, MCTCR and MCRTCR had no effect on the proliferation of MG63 cells and met the cytotoxicity requirements of biomaterials in the National Standard for Biological Evaluation of Medical Devices (GB/T16886.5.).
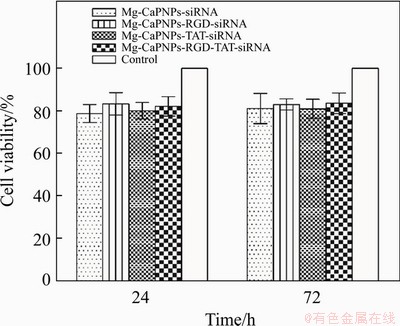
Figure 6 Cell viability of MG63 cells incubated with siRNA-CaPNPs carrier system
3.4 Cellular uptake
To study the effects of grafted RGD and/or grafted TAT on the endocytosis of siRNA-CaPNPs in MG63 cells, we cocultured the MCCR, MCRCR, MCTCR and MCRTCR carrier systems with MG63 cells for 1 h, and the intracellular fluorescence intensity was observed (Figure 7). The MG63 cells in the 4 experimental groups grew well (Figures 7(a)-(d)). The ratio of fluorescent cells to the total cells and the intracellular fluorescence intensity in the grafted group were higher than those in the ungrafted group (Figures 7(a′)-(d′)). Among the four experimental groups, the MCRTCR group had the highest ratio of fluorescent cells and the strongest intracellular fluorescence intensity (Figure 7(d′)). Studies have found that receptor-ligand mediation can improve the ability of target cells to take in nanoparticles and improve the ability of intraparticle biochemical treatment in target cells [33]. RGD is a specific ligand of many integrin receptors on the cell membrane, and it can bind to integrin receptors (α1β1 and αvβ1) along with other integrin receptors on the cell membrane of osteoblasts [34-37]. TAT is a type of polypeptide that can efficiently penetrate the cell membrane. TAT can bind to most substances in the form of noncovalent bonds and carry substances into cells; moreover, its transmembrane penetration is not specific [38]. TAT-modified inorganic nanoparticles show enhanced endocytosis ability [38]. In this study, the Mg-CaPNPs-siRNA with grafted RGD was cocultured with MG63. The RGD on the surface of the carrier specifically bound to integrin receptors such as α1β1 and αvβ1 on the MG63 cell membrane, mediated the adhesion of MCRCR to MG63, and promoted the endocytosis of the Mg-CaPNPs-siRNA carrier system in MG63 cells. The Mg-CaPNPs-siRNA with grafted TAT was cocultured with MG63, and it was found that TAT carried the Mg-CaPNPs-siRNA carrier system across the cell membrane into the cell. When the Mg-CaPNPs-siRNA carrier system was comodified with RGD and TAT and cocultured with MG63 cells, we considered that RGD could specifically bind to integrin receptors such as α1β1 and αvβ1 on the membrane of MG63 cells to promote the adhesion of the carrier system on the cell surface; meanwhile, TAT could efficiently carry the carrier system into the cell [39]. The results showed that grafting RGD and TAT could synergistically promote the endocytosis of the Mg-CaPNPs-siRNA carrier system in MG63 cells.
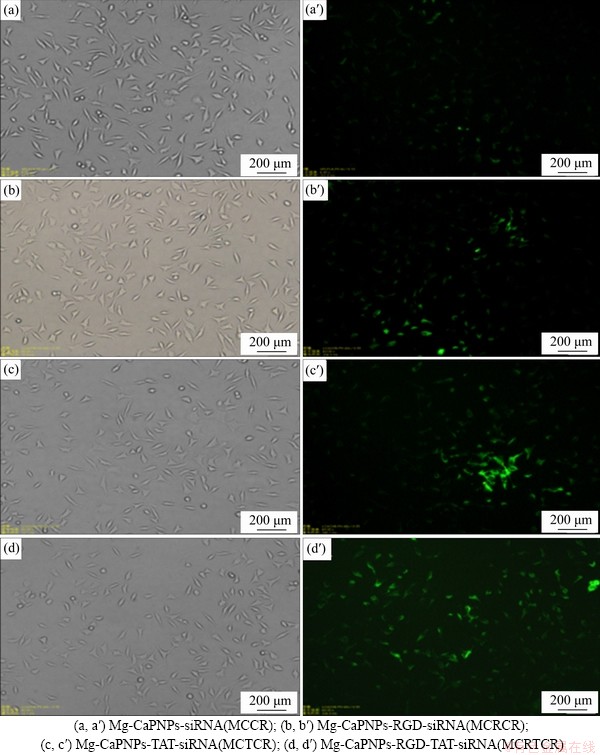
Figure 7 Microstructures of siRNA-CaPNPs carrier system with GFP co-cultured with MG63 for 1 h under visible light (a, b, c) and ultraviolet light (a′, b′, c′):
3.5 ALP activity
As shown in Figure 8, the alkaline phosphatase (ALP) activity of MG63 in the grafting group increased significantly after the 3rd day. There was no distinct difference in the ALP activity between the MCRCR group and the MCTCR group, but the ALP activity in the MCRTCR group was significantly higher than those in the other groups.
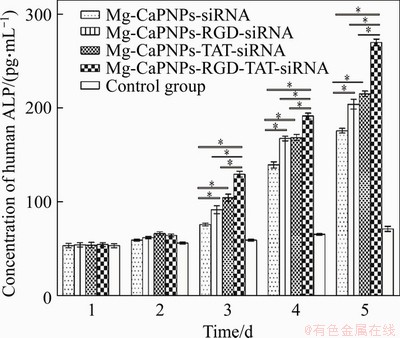
Figure 8 Effect of MCRTCR system on alkaline phosphatase (ALP) activity of MG63(*: p<0.05)
3.6 OC activity
Figure 9 shows that after the 4th day, the OC activity of MG63 was significantly enhanced in the grafted group (Figure 9). The OC secretion of MG63 showed no distinct difference between the MCRCR and MCTCR groups. However, the secretion of OC from MG63 cells cultured with MCRTCR was obviously higher than those from other groups. From the secretion of ALP and OC, it could be concluded that the grafting RGD and TAT onto the Mg-CaPNPs-siRNA carrier systems could promote their insertion in MG63 cells thus increasing CKIP-1 siRNA in the cells and promoting osteogenesis. Therefore, grafting RGD and TAT had a synergistic promotion effect.
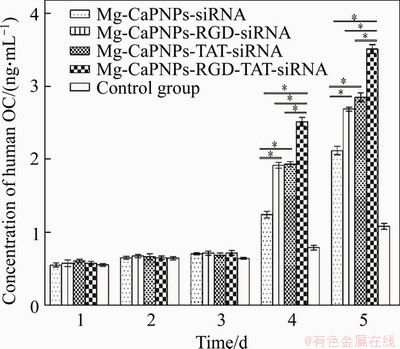
Figure 9 ELISA analysis showing the OC secretion of MG63 cultured with MCRTCR system from day 1 to day 5 (*: p<0.05)
3.7 Evaluation of bone-related gene expression
Real-time qRT-PCR analysis shows that the Mg-CaPNPs-siRNA carrier system with grafted RGD and TAT has enhanced the osteogenic differentiation of MG63 cells (Figure 10). As shown in Figure 10(a), from the 3rd day, the expression of the COL-1 gene was markedly enhanced in the grafted groups. In addition, the relative expression of COL-1 in MG63 cells cocultured with MCRTCR was obviously higher than that in cells cultured with MCTCR or MCRTCR. COL-1 gene expression is a prerequisite for bone tissue formation. COL-1 promotes bone tissue mineralization and plays an important role in the integrity of bone tissue structure and its biomechanical properties. It participates in bone construction and is the earliest sign of osteoblast differentiation [40]. The results show that the Mg-CaPNPs-siRNA carrier system with grafted RGD and TAT could promote the expression of COL-1 mRNA, which indicated that the system could promote the osteogenic differentiation of MG63 cells at the early stage of osteogenesis; furthermore, simultaneously grafting RGD and TAT was better than grafting only one.
The grafted groups promoted the upregulation of OC mRNA expression after the 5th day. Moreover, the expression of the OC gene in the MCRTCR group was obviously upregulated, which was better than that in the MCRCR group or MCTCR group (Figure 10(b)). OC is a noncollagen protein specifically synthesized and secreted by osteoblasts. OC mRNA is induced and expressed only after the beginning of the mineralization stage of MG63 cells, which is one of the main indicators for cells to enter the mineralization stage. It plays an important regulatory role in maintaining the normal rate of bone mineralization and inhibiting the formation of abnormal hydroxyapatite [41]. The results indicated that the Mg-CaPNPs-siRNA carrier system with grafted RGD and TAT promoted the mineralization function of osteoblasts, and the simultaneous grafting was better than the grafting only one.
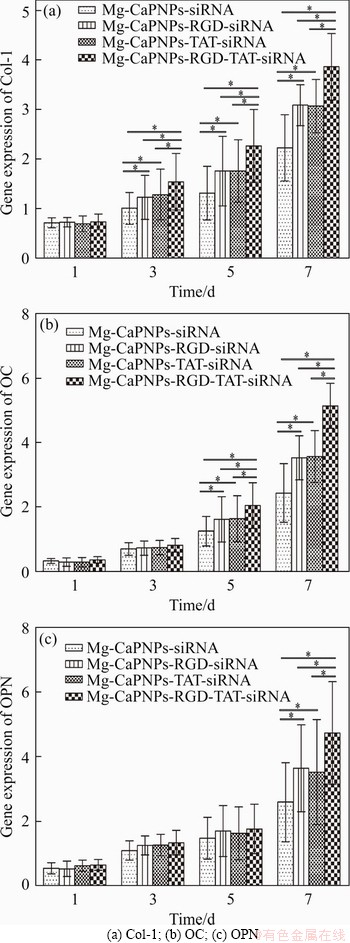
Figure 10 Effect of MCRTCR system on bone-related gene expression of MG63 after being cultured for 1, 3, 5 and 7 days (*: p<0.05):
After the 7th day, the expression of OPN mRNA was dramatically enhanced in the grafted groups, and the expression of the OPN gene in the MCRTCR group was higher than those in the other groups (Figure 10(c)). After the beginning of bone matrix mineralization, the level of the OPN gene in MG63 cells began to increase; furthermore, OPN was maintained at a high concentration in the process of bone remodeling, which played an important role in the osteogenesis and mineralization of the MG63 cells [42]. The results indicated that the Mg-CaPNPs-siRNA carrier system with grafted RGD and TAT promoted the mineralization function and the bone remodeling of osteoblasts.
In general, the results showed that both RGD and TAT improved the transfection efficiency of the Mg-CaPNPs-siRNA carrier system, which meant that more CKIP-1 siRNA was transfected into MG63 cells, thus, silencing intracellular CKIP-1 mRNA, and relieving the negative regulation of CKIP-1 from the bone. Consequently, it started the osteogenic process quickly and enhanced osteogenic differentiation. In addition, simultaneously grafting RGD and TAT had a beneficial synergistic effect.
4 Conclusions
Magnesium-doped CaPNPs with a uniform short rod-shaped morphology, 60 nm particle size and good dispersion are prepared. The effects of the Mg-CaPNPs-CKIP-1 siRNA carrier system with grafted RGD and TAT on cell endocytosis as well as the biological effects in vitro are evaluated. These findings indicate that the Mg-CaPNPs-siRNA carrier system simultaneously grafted with RGD and TAT is more likely to be phagocytized by cells and promoted osteoblast differentiation. Furthermore, the expressions of the osteogenesis-related genes COL-1, OC and OPN are stimulated, and thus the carrier system could be considered a promising scaffold coating material for further research.
Contributors
YI Man-fei contributed to the paper writing, designed the project, carried out data processing, and performed data analysis. CHEN Liang-jian, HE Hui-li, and SHI Lei contributed to the conception of the study. HE Hui-li, SHI Lei, SHAO Chun-sheng, and ZHANG Bo performed data analysis and offered some valuable suggestions for the contents of the manuscript. YI Man-fei, CHEN Liang-jian replied to reviewers’ comments and revised the final version.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
[1] ZHOU Z X, LIU X R, ZHU D C, WANG Y, ZHANG Z, ZHOU X F, QIU N S, CHEN X S, SHEN Y Q. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration [J]. Advanced Drug Delivery Reviews, 2017, 115: 115-154. DOI: 10.1016/j.addr.2017.07.021.
[2] AKHTAR S, BENTER I. Toxicogenomics of non-viral drug delivery systems for RNAi: Potential impact on siRNA-mediated gene silencing activity and specificity [J]. Advanced Drug Delivery Reviews, 2007, 59(2): 164-182. DOI: 10.1016/j.addr.2007.03.010.
[3] SZEBENI J, BARANYI L, SAVAY S, MILOSEVITS J, BUNGER R, LAVERMAN P, METSELAAR J M, STORM G, CHANAN-KHAN A, LIEBES L, MUGGIA F M, COHEN R, BARENHOLZ Y, ALVING C R. Role of complement activation in hypersensitivity reactions to Doxil and HYNIC PEG liposomes: Experimental and clinical studies [J]. Journal of Liposome Research, 2002, 12(1, 2): 165-172.
[4] van den HOVEN J M, NEMES R, METSELAAR J M, NUIJEN B, BEIJNEN J H, STORM G, SZEBENI J. Complement activation by PEGylated liposomes containing prednisolone [J]. European Journal of Pharmaceutical Sciences, 2013, 49(2): 265-271. DOI: 10.1016/j.ejps.2013.03. 007.
[5] BAKAN F, KARA G, COKOL CAKMAK M, DENKBAS E B. Synthesis and characterization of amino acid-functionalized calcium phosphate nanoparticles for siRNA delivery [J]. Colloids and Surfaces B: Biointerfaces, 2017, 158: 175-181. DOI: 10.1016/j.colsurfb.2017.06.028.
[6] ROJAS-SANCHEZ L, ZHANG E, SOKOLOVA V, ZHONG M, YAN H, LU M, LI Q, YAN H, EPPLE M. Genetic immunization against hepatitis B virus with calcium phosphate nanoparticles in vitro and in vivo [J]. Acta Biomaterialia, 2020, 110: 254-265. DOI: 10.1016/j.actbio. 2020.04.021.
[7] KHAN M A, WU V M, GHOSH S, USKOKOVIC V. Gene delivery using calcium phosphate nanoparticles: Optimization of the transfection process and the effects of citrate and poly(l-lysine) as additives [J]. Journal of Colloid and Interface Science, 2016, 471: 48-58. DOI: 10.1016/j.jcis.2016.03.007.
[8] SABOURIAN P, YAZDANI G, ASHRAF S S, FROUNCHI M, MASHAYEKHAN S, KIANI S, KAKKAR A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake [J]. International Journal of Molecular Sciences, 2020, 21(21): 8019. DOI: 10.3390/ijms21218019.
[9] LASKUS A, KOLMAS J. Ionic substitutions in non-apatitic calcium phosphates [J]. International Journal of Molecular Sciences, 2017, 18(12): 2542. DOI: 10.3390/ijms18122542.
[10] TITE T, POPA A C, BALESCU L M, BOGDAN I M, PASUK I, FERREIRA J M F, STAN G E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods [J]. Materials (Basel, Switzerland), 2018, 11(11): 2081. DOI: 10.3390/ ma11112081.
[11] HANIFI A, FATHI M H, SADEGHI H, VARSHOSAZ J M. Mg2+ substituted calcium phosphate nano particles synthesis for non viral gene delivery application [J]. Journal of Materials Science: Materials in Medicine, 2010, 21(8): 2393-2401. DOI: 10.1007/s10856-010-4088-3.
[12] KIM W, KIM W K, LEE K, SON M J, KWAK M, CHANG W S, MIN J K, SONG N W, LEE J, BAE K H. A reliable approach for assessing size-dependent effects of silica nanoparticles on cellular internalization behavior and cytotoxic mechanisms [J]. International Journal of Nanomedicine, 2019, 14: 7375-7387. DOI: 10.2147/IJN. S224183.
[13] NEUMANN S, KOVTUN A, DIETZEL I D, EPPLE M, HEUMANN R. The use of size-defined DNA-functionalized calcium phosphate nanoparticles to minimise intracellular calcium disturbance during transfection [J]. Biomaterials, 2009, 30(35): 6794-6802. DOI: 10.1016/j.biomaterials. 2009.08.043.
[14] CHEN Liang-jian, CHEN Tian, CAO Jun. Effect of Tb/Mg doping on composition and physical properties of hydroxyapatite nanoparticles for gene vector application [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(1): 125-136. DOI: 10.1016/S1003-6326(18)64645-X.
[15] ZOU L, PENG Q L, WANG P, ZHOU B T. Progress in research and application of HIV-1 TAT-derived cell-penetrating peptide [J]. The Journal of Membrane Biology, 2017, 250(2): 115-122. DOI: 10.1007/s00232-016-9940-z.
[16] ZHANG W J, MAO Z W, GAO C Y. Preparation of TAT peptide-modified poly(N-isopropylacrylamide) microgel particles and their cellular uptake, intracellular distribution, and influence on cytoviability in response to temperature change [J]. Journal of Colloid and Interface Science, 2014, 434: 122-129. DOI: 10.1016/j.jcis.2014.07.031.
[17] YAMANO S, DAI J, YUVIENCO C, KHAPLI S, MOURSI A M, MONTCLARE J K. Modified Tat peptide with cationic lipids enhances gene transfection efficiency via temperature-dependent and caveolae-mediated endocytosis [J]. Journal of Controlled Release, 2011, 152(2): 278-285. DOI: 10.1016/ j.jconrel.2011.02.004.
[18] LI Q, HAO X F, WANG H N, GUO J T, REN X K, XIA S H, ZHANG W C, FENG Y K. Multifunctional REDV-G-TAT-G-NLS-Cys peptide sequence conjugated gene carriers to enhance gene transfection efficiency in endothelial cells [J]. Colloids and Surfaces B: Biointerfaces, 2019, 184: 110510. DOI: 10.1016/j.colsurfb.2019.110510.
[19] HO A, SCHWARZE S R, MERMELSTEIN S J, WAKSMAN G, DOWDY S F. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo [J]. Cancer Research, 2001, 61(2): 474-477.
[20] MARCUCCI F, LEFOULON F. Active targeting with particulate drug carriers in tumor therapy: Fundamentals and recent progress [J]. Drug Discovery Today, 2004, 9(5): 219-228. DOI: 10.1016/S1359-6446(03)02988-X.
[21] LV H, ZHU Q, LIU K W, ZHU M M, ZHAO W F, MAO Y, LIU K H. Coupling of a bifunctional peptide R13 to OTMCS-PEI copolymer as a gene vector increases transfection efficiency and tumor targeting [J]. International Journal of Nanomedicine, 2014, 9: 1311-1322. DOI: 10.2147/IJN. S59726.
[22] CHEN Tian, CHEN Liang-jian, HE Hui-li. Influence of HAnps carrier system with doping Mg and grafting RGD on endocytosis of MG63 cells [J]. Journal of Central South University(Science and Technology), 2017, 48(3): 625-634. (in Chinese)
[23] WU D N, ZHANG Y N, XU X T, GUO T, XIE D M, ZHU R, CHEN S F, RAMAKRISHNA S, HE L M. RGD/TAT-functionalized chitosan-graft-PEI-PEG gene nanovector for sustained delivery of NT-3 for potential application in neural regeneration [J]. Acta Biomaterialia, 2018, 72: 266-277. DOI: 10.1016/j.actbio.2018.03.030.
[24] HUANG Z G, LV F M, WANG J, CAO S J, LIU Z P, LIU Y, LU W Y. RGD-modified PEGylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability [J]. International Journal of Pharmaceutics, 2019, 556: 217-225. DOI: 10.1016/ j.ijpharm.2018.12.023.
[25] LU K F, YIN X S, WENG T J, XI S L, LI L, XING G C, CHENG X, YANG X, ZHANG L Q, HE F C. Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1 [J]. Nature Cell Biology, 2008, 10(8): 994-1002. DOI: 10.1038/ncb1760.
[26] SAFI A, VANDROMME M, CAUSSANEL S, VALDACCI L, BAAS D, VIDAL M, BRUN G, SCHAEFFER L, GOILLOT E. Role for the pleckstrin homology domain-containing protein CKIP-1 in phosphatidylinositol 3-kinase-regulated muscle differentiation [J]. Molecular and Cellular Biology, 2004, 24(3): 1245-1255. DOI: 10.1128/MCB.24.3.1245-1255. 2004.
[27] GUO B S, ZHANG B T, ZHENG L Z, TANG T, LIU J, WU H, YANG Z J, PENG S L, HE X J, ZHANG H Q, YUE K K, HE F C, ZHANG L Q, QIN L, BIAN Z X, TAN W H, LIANG Z C, LU A P, ZHANG G. Therapeutic RNA interference targeting CKIP-1 with a cross-species sequence to stimulate bone formation [J]. Bone, 2014, 59: 76-88. DOI: 10.1016/j.bone.2013.11.007.
[28] SUZUKI T, ISHIGAKI K, MIYAKE M. Synthetic hydroxyapatites employed as inorganic cation-exchangers [J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1981, 5(5): 1059-1062.
[29] REN F, LENG Y, XIN R, GE X. Synthesis, characterization and ab initio simulation of magnesium-substituted hydroxyapatite [J]. Acta Biomaterialia, 2010, 6(7): 2787-2796. DOI: 10.1016/j.actbio.2009.12.044.
[30] LAURENCIN D, ALMORA-BARRIOS N, de LEEUW N H, GERVAIS C, BONHOMME C, MAURI F, CHRZANOWSKI W, KNOWLES J C, NEWPORT R J, WONG A, GAN Z, SMITH M E. Magnesium incorporation into hydroxyapatite [J]. Biomaterials, 2011, 32(7): 1826-1837. DOI: 10.1016/ j.biomaterials.2010.11.017.
[31] WANG Hui-yun, CUI Ya-nan, ZHANG Chun-yan. Influence factors on the zeta potential of colloid particle [J]. China Medical Herald, 2010, 7(20): 28-30. (in Chinese)
[32] RETAMAL M R R, BABICK F, HILLEMANN L. Zeta potential measurements for non-spherical colloidal particles – Practical issues of characterisation of interfacial properties of nanoparticles [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 532: 516-521. DOI: 10.1016/j.colsurfa.2017.04.010.
[33] ZHANG S, LI J, LYKOTRAFITIS G, BAO G, SURESH S. Size-dependent endocytosis of nanoparticles [J]. Advanced Materials, 2009, 21(4): 419-424. DOI: 10.1002/adma. 200801393.
[34] CHUA P H, NEOH K G, KANG E T, WANG W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion [J]. Biomaterials, 2008, 29(10): 1412-1421. DOI: 10.1016/j.biomaterials. 2007.12.019.
[35] TAUBENBERGER A V, WOODRUFF M A, BAI H, MULLER D J, HUTMACHER D W. The effect of unlocking RGD-motifs in collagen I on pre-osteoblast adhesion and differentiation [J]. Biomaterials, 2010, 31(10): 2827-2835. DOI: 10.1016/j.biomaterials.2009.12. 051.
[36] BELLIS S L. Advantages of RGD peptides for directing cell association with biomaterials [J]. Biomaterials, 2011, 32(18): 4205-4210. DOI: 10.1016/j.biomaterials.2011.02.029.
[37] WILKINSON A L, BARRETT J W, SLACK R J. Pharmacological characterisation of a tool αvβ1 integrin small molecule RGD-mimetic inhibitor [J]. European Journal of Pharmacology, 2019, 842: 239-247. DOI: 10.1016/j.ejphar. 2018.10.045.
[38] HUANG Ji-chun, CAI Hua-rong, JIANG Yao-quan. Construction of RGD-TAT modified liposomes and evaluation of its targeting on glioma [J]. Chinese Journal of Biochemical Pharmaceutics, 2014, 34(3): 1-3. (in Chinese)
[39] al SORAJ M, HE L, PEYNSHAERT K, COUSAERT J, VERCAUTEREN D, BRAECKMANS K, de SMEDT S C, JONES A T. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat [J]. Journal of Controlled Release, 2012, 161(1): 132-141. DOI: 10.1016/j.jconrel. 2012.03.015.
[40] FUSARO M, CREPALDI G, MAGGI S, D'ANGELO A, CALO L, MIOZZO D, FORNASIERI A, GALLIENI M. Bleeding, vertebral fractures and vascular calcifications in patients treated with warfarin: Hope for lower risks with alternative therapies.[J]. Current Vascular Pharmacology, 2011, 9(6): 763-769.
[41] MARELLI B, GHEZZI C E, BARRALET J E, BOCCACCINI A R, NAZHAT S N. Three-dimensional mineralization of dense nanofibrillar collagen-bioglass hybrid scaffolds [J]. Biomacromolecules, 2010, 11(6): 1470-1479. DOI: 10.1021/bm1001087.
[42] FU Ce-guang, LIU Jie, XIA Hai-bin. Biological characteristics of osteopontin and its role in bone remodeling [J]. Journal of Clinical Stomatology, 2012, 28(8): 506-508. (in Chinese)
(Edited by FANG Jing-hua)
中文导读
接枝TAT和RGD对Mg-CaPNPs-CKIP-1 siRNA载体系统的体外胞吞和生物学效应的影响
摘要:磷酸钙纳米粒(CaPNPs)作为基因载体具有良好的生物相容性;然而,CaPNPs通常表现出较低的转染效率。细胞穿透肽(TAT)可以增加纳米颗粒的摄取,但由于其非特异性而受到限制。纳米载体表面接枝细胞黏附肽(精氨酸-甘氨酸-天冬氨酸,RGD)可增强其靶向性。Plekho1基因编码酪蛋白激酶-2相互作用蛋白-1 (CKIP-1)可负调控成骨分化。在此基础上,本研究通过水热合成法、硅烷化法和吸附法制备了Mg-CaPNPs-RGD-TAT-CKIP-1 siRNA载体系统。通过体外细胞培养,评价了该载体系统对细胞胞吞及生物学效应的影响。结果表明,掺镁7%的CaPNPs(粒径60 nm,短棒形,分散性好)适合作为基因载体。载体系统促进了MG63细胞的内吞作用,有利于促进成骨细胞的分化,且双配体系统具有协同作用。结果表明,Mg-CaPNPs-RGD-TAT-CKIP-1 siRNA载体系统在高效转运进入细胞和诱导成骨方面具有巨大的潜力。
关键词:磷酸钙纳米颗粒;细胞穿透肽(TAT);黏附肽(RGD);胞吞作用;小分子干扰RNA
Foundation item: Project(81571021) supported by the National Natural Science Foundation of China; Project(2018zzts944) supported by the Graduate Student Independent Exploration Innovation Fund of the Central South University, China; Projects(2015WK3012, 2018SK2017) supported by the Hunan Provincial Science and Technology Department, China; Project(20160301) supported by New Talent Project of the Third Xiangya Hospital of Central South University, China
Received date: 2020-06-06; Accepted date: 2021-01-17
Corresponding author: CHEN Liang-jian, PhD, Professor; Tel: +86-13507405799; E-mail: jian007040@sina.com; ORCID: https://orcid. org/0000-0001-7038-9144

