Trans. Nonferrous Met. Soc. China 25(2015) 206-210
Effect of linear carboxylic ester on low temperature performance of LiMn2O4-graphite cells
Shu HONG, Jie LI, Guan-chao WANG, Zhi-an ZHANG, Yan-qing LAI
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 15 January 2014; accepted 28 April 2014
Abstract: To improve the low-temperature performances of Li-ion cells, three types of linear carboxylic ester-based electrolyte, such as EC/EMC/EA (1:1:2, mass ratio), EC/EMC/EP (1:1:2, mass ratio) and EC/EMC/EB (1:1:2, mass ratio), were prepared to substitute for industrial electrolyte (EC/EMC/DMC). Then, 18650-type LiMn2O4-graphite cells (nominal capacity of 1150 mA·h) were assembled and studied. Results show that the cells containing three types of electrolyte are able to undertake 5C discharging current with above 93% capacity retention at -20 °C. Electrochemical impedance spectra show that the discharge capacity fading of Li-ion cells at low temperature is mainly ascribed to the charge transfer resistance increasing with temperature decreasing. In comparison, the cells containing electrolyte of 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) have the highest capacity retention of 90% at -40 °C and 44.41% at -60 °C, due to its lowest charge-transfer resistance.
Key words: Li-ion cells; low temperature performance; electrolyte; linear carboxylic ester; ionic conductivity; charge-transfer resistance
1 Introduction
Li-ion cells have been widely applied in human’s daily life [1,2] since they were introduced into the market twenty years ago. Recently, NASA is considered introducing Li-ion cells to power Rovers and Landers in Mars exploration missions [3]. However, due to the poor performance of Li-ion cells at low temperatures, it is difficult to be well used in aerospace, military and other special areas. The freezing point of commercial electrolytes is about -30 °C, while the above-mentioned fields require Li-ion cells to be able to operate below -40 °C [4]. Therefore, it is of great significance to improve the low-temperature properties of Li-ion cells, which not only is related with electrodes, but also refers to electrolyte [5-9].
Adjusting the electrolyte formulation was known as one of the most effective and economic methods for improving the low-temperature performances of Li-ion cells [10-14]. With a certain proportion, EC/DMC/EMC ternary solvent mixture is commonly used as commercial electrolyte of Li-ion cells. A kind of electrolyte with 1.0 mol/L LiPF6 in EC/DMC/EMC (8.3:25:66.7, mass ratio) was optimized, and with this electrolyte composition, the cells retained 90.3% of the discharge capacity at 0.1C and -40 °C [15]. Another kind of electrolyte with 1.0 mol/L LiPF6 in EC/DEC/DMC/EMC (1:1:1:3, volume ratio) was proposed, with which cells delivered the improved low-temperature performance by comparison with a number of ternary and quaternary carbonate-based electrolytes [16]. Because the performance of the electrolyte based on the polybasic carbonate mixture at low temperatures is limited, linear carboxylic ester has been recommended as modifying agent, which has lower melting point and viscosity than the liner carbonate, guaranteeing a higher conductivity of electrolyte at low temperatures. The conductivity of binary and ternary mixtures containing EA or EB reaches 7 mS/cm at -20 °C and 5 mS/cm at -35 °C, respectively, while that of the base electrolyte was 2 mS/cm at -20 °C [17]. The performance at low temperatures of LiMn2O4-based Li- ion cells was studied. Results showed that cells with electrolyte compositions of 1.0 mol/L LiPF6 in EC/EMC/ MB (1:1:0.08, volume ratio) and EC/EMC/EB (1:1:0.08, volume ratio) discharged 80% capacity at -60 °C [18].
In this work, the electrolytes of 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio), 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio), 1.0 mol/L LiPF6 in EC/EMC/EP (1:1:2, mass ratio) and 1.0 mol/L LiPF6 in EC/EMC/EB (1:1:2, mass ratio) were prepared to study the effect of linear carboxylic esters on the low temperature discharge property and the kinetics behavior of the LiMn2O4-graphite cells. Focusing upon the improved rate capability at low temperatures (i.e., -20 °C to -40 °C), this approach was demonstrated well over a wide temperature range in LiMn2O4-graphite full cells. The most surprising finding from this work was that a decrease in charge-transfer resistance (Rct) rather than bulk resistance (Rb) was the main reason for improving the low temperature performance of the full cells.
2 Experimental
LiPF6, EC, EMC, EA, EP and EB were purchased from Guangzhou Tinci Materials Technology Co., Ltd., China. In a glove box (both oxygen and water contents <2×10-5), LiPF6 (1 mol/L) was dissolved in EC/EMC/ DMC (1:1:1, mass ratio), EC/EMC/EA (1:1:2, mass ratio), EC/EMC/EP (1:1:2, mass ratio), EC/EMC/EB (1:1:2, mass ratio) solvent mixture, respectively. The water content of the electrolytes was determined less than 10-5 by Karl-Fischer 798 MPT Titrino. An additional 2.0 % (mass fraction) of vinylene carbonate (VC) was added into each mixture to assist the formation of the solid electrolyte interphase (SEI). In the glove box, 18650 LiMn2O4-graphite cells were assembled.
In order to provide a constant temperature, a high-low temperature test-chamber (GDH-2005C) was used. The discharge performance at low temperatures was evaluated by Neware charge/discharge instrument. An electrochemical measurement system (PARSTAT 2273) was employed to measure the ionic conductivity of the electrolyte and the electrochemical impedance spectroscopy (EIS) of the cells. By applying a DC bias with its value equal to the open circuit voltage (OCV) of the cells and an AC oscillation of 5 mV over the frequencies from 100 kHz to 0.01 Hz, the AC impedance of the cells was potentiostatically recorded. The obtained EISs were analyzed by ZView software (Scribner and Associates).
3 Results and discussion
3.1 Ionic conductivity
Low ionic conductivity is one of the main factors deteriorating the low temperature performances of cells [19,20]. Considering the fact that the solubility of lithium salts will decrease with temperature decreasing, the concentration of 1 mol/L of LiPF6 was chosen. The ionic conductivities of electrolyte with the composition of 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio), 1.0 mol/L LiPF6 in EC/EMC/EP (1:1:2, mass ratio) and 1.0 mol/L LiPF6 in EC/EMC/EB (1:1:2, mass ratio) were measured and compared with those of commercial electrolyte of 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio), as shown in Fig. 1. The results show that ionic conductivity decreases with temperature decreasing, and the linear carboxylic esters-based electrolyte has a superiority of ionic conductivity at lower temperatures compared with commercial electrolyte of 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio). The electrolyte of 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) has the highest conductivity of 9.15 mS/cm at 25 °C, 4.01 mS/cm at -20 °C, 1.7 mS/cm at -40 °C and 0.66 mS/cm at -60 °C, respectively. However, the conductivity gap between the four electrolytes becomes narrower as temperature decreases. For example, the gap between electrolyte of 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) and commercial electrolyte of 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio) is 1.7 mS/cm at -20 °C, but it declines to 0.98 mS/cm as the temperature decreases to -40 °C, the conductivity gap is no more than 0.3 mS/cm at -60 °C .This phenomenon is in accordance with the results of XIAO et al [15].
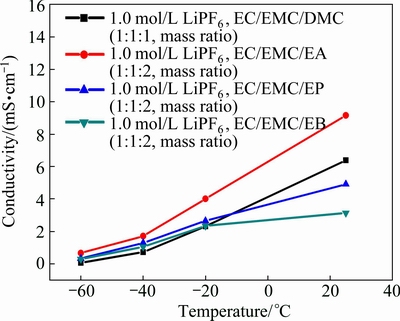
Fig. 1 Ionic conductivity-temperature curves of different electrolytes
3.2 Discharge capacity of LiMn2O4-graphite cells
The discharge curves of LiMn2O4-graphite cells with different electrolytes at -20, -40 and -60 °C are shown in Fig. 2. The cells are discharged to 2.5 V at 0.5C rate under -20 °C, discharged to 2.0 V at 0.2C rate under -40 °C and discharged to 2.0 V at 0.1C rate under -60 °C. It can be seen from Fig. 2(a) that the difference of the capacity retentions of LiMn2O4-graphite cells containing different electrolytes is no more than 5% at -20 °C. However, as shown in Fig. 2(b), the capacity retention (with respect to the capacity obtained at 25 °C) of the cells with electrolyte containing carboxylic ester is over 95% at -40 °C, while the capacity retention of the cell with 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio) base electrolyte is only 8.44% at -40 °C. As temperature further decreases to -60 °C, the capacity retention of the cells with 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) electrolyte can still reach 44.41%, whereas the cell with 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio) electrolyte cannot operate (see Fig. 2(c)). Furthermore, as to the discharge voltage plateaus at -20, -40 and -60 °C, that of the LiMn2O4-graphite cell using 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) electrolyte is the highest, which indicates that its polarization is the lowest among the cells with four types of electrolyte.
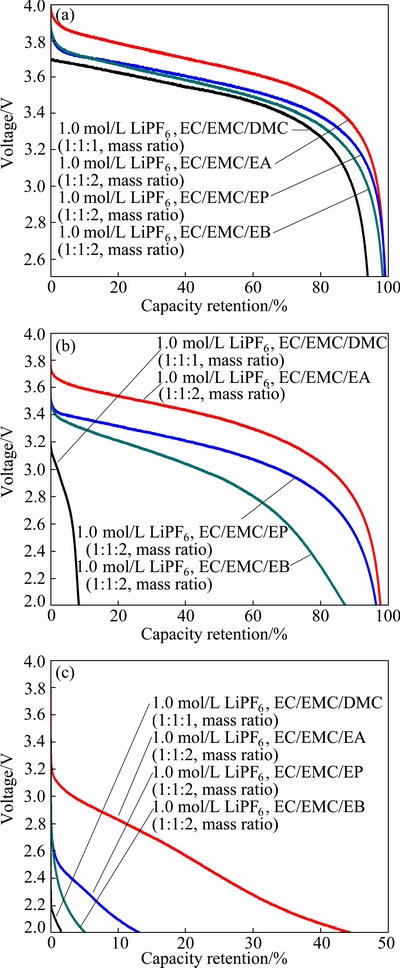
Fig. 2 Discharge curves at -20 °C (a), -40 °C (b) and -60 °C (c) of LiMn2O4-graphite cells containing different electrolytes
The rate discharge characteristic at low temperatures is determined as shown in Fig. 3. From Fig. 3(a), the capacity retentions with 5C (5.5 A) rate at -20 °C (with respect to the capacity obtained at 25 °C) of the cells with electrolytes of 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio), 1.0 mol/L LiPF6 in EC/EMC/EP (1:1:2, mass ratio) and 1.0 mol/L LiPF6 in EC/EMC/EB (1:1:2, mass ratio) are 95.5%, 96.1% and 93.3%, respectively. Whereas, the cell with electrolyte of 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio) cannot operate. The operational temperature range can be extended to -40 °C at the same rate, as illustrated in Fig. 3(b). Only the cell containing 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) electrolyte can support 5C (5.5 A) running with cut-off voltage above 2.0 V at -40 °C under 100% state of charge (SOC) status, the capacity retention can reach as high as 94.3%. Besides, it can be seen that the voltage drops at first and then rises to 3.2 V, finally drops rapidly for the high electrode polarization at low temperatures, which is consistent with the reported literature [13]. This also demonstrates that the carboxylic ester-based electrolytes is superior to the pure carbonate-based electrolyte at the temperatures below -40 °C.
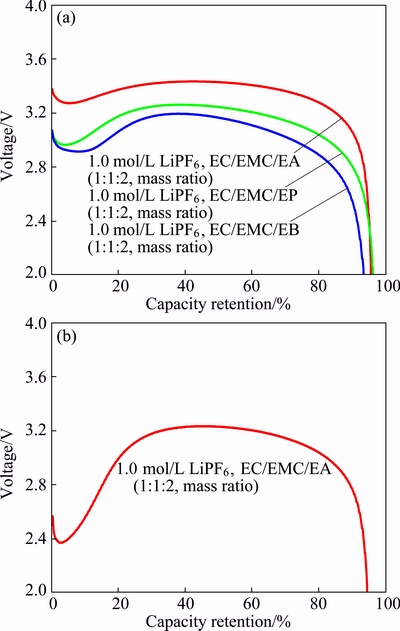
Fig. 3 High rate discharge curves of LiMn2O4-graphite cells at -20 °C (a) and -40 °C (b)
3.3 Impedance analysis of LiMn2O4-graphite cells
In order to understand the mechanism of the carboxylic esters used in Li-ion cells, the impedance spectra of the cells with four different electrolytes at various temperatures were analyzed, as shown in Fig. 4. A half-charged state was selected to analyze the effect of carboxylic ester on the low temperature performance of the cells. A typical EIS of LiMn2O4-graphite cell at 25 °C, which is composed of a partially semicircle and a straight line at the low frequency end, is well fitted by an equivalent circuit as shown in Fig. 4(a). However, the typical EIS spectra of LiMn2O4-graphite cells at -20 °C and -40 °C are different from that at 25 °C, as shown in Figs. 4(b) and (c), respectively, which are composed of a distinct semicircle and without a straight line at the low frequency end by reason of low temperature. As reported in other literatures [21,22], Rb denotes the resistance of the cell bulk including electrolyte, electrode and separator. RSEI and CSEI are the resistance and capacitance of the SEI, respectively. Rct is the charge-transfer resistance and Cdl is the double-layer capacitance. Wo is the Warburg impedance related to the diffusion of the lithium ions in the electrolytes. The resistance and capacitance of the SEI cannot be measured because the cell impedance is too low. It can be seen that the average value of Rb of the cells containing four different electrolytes at -20 °C and -40 °C is almost the same as that at 25 °C. However, the Rct of the cells significantly increases with the temperature decreasing. The average value of Rct of the cells containing four electrolytes at -20 °C is nearly 200 times that at 25 °C and two 2000 times that at -40 °C. Therefore, the Rct dominates the total impedance of the cells at temperatures below -20 °C. This indicates that an increase in Rct rather than Rb is the main reason for significant fading of capacity and energy at low temperatures for all the cells, which is consistent with other reports [23]. Furthermore, compared with cells containing EP- and EB-based electrolytes, the cells containing EA-based electrolyte show the lower value of Rct at low temperatures and the most excellent low temperature performance.
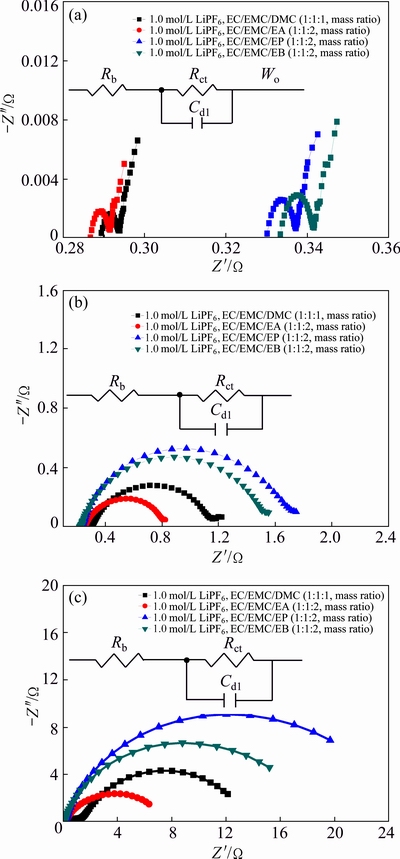
Fig. 4 Impedance spectra of LiMn2O4-graphite cell with different electrolytes at 25 °C (a), -20 °C (b) and -40 °C (c)
4 Conclusions
1) Compared with the commercial electrolyte of 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio), the linear carboxylic esters-based electrolytes show a superiority of ionic conductivity at low temperatures. In comparison, the electrolyte with 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) has the highest conductivity of 9.15 mS/cm at 25 °C, 4.01 mS/cm at -20 °C, 1.7 mS/cm at -40 °C and 0.66 mS/cm at -60 °C, respectively.
2) An improvement of low temperature performances of Li-ion cells was realized by linear carboxylic ester EA, EP and EB as a co-solvent in the electrolyte. The cells containing linear carboxylic ester-based electrolyte are able to undertake 5C discharge with above 93% capacity retention at -20 °C, whereas the cell with commercial electrolyte of 1.0 mol/L LiPF6 in EC/EMC/DMC (1:1:1, mass ratio) could not operate.
3) The electrochemical impedance spectra show that the increase in Rct rather than Rb is the main reason for significant fading of capacity and energy at low temperatures for the full cells. The cells containing electrolyte with 1.0 mol/L LiPF6 in EC/EMC/EA (1:1:2, mass ratio) have the highest capacity retention of 90% at -40 °C and 44.41% at -60 °C, due to the lowest charge-transfer resistance.
References
[1] TANG Xiao-hui, LI Guo-xi, GAO Gui-hong, HU Jin-feng, LIANG Guo-biao. Performance of LiCoO2-LiFePO4 cathode materials for lithium ion batteries [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 139-143. (in Chinese)
[2] LI J L, DANIEL C, WOOD D. Materials processing for lithium-ion batteries [J]. J Power Sources, 2011, 196(5): 2452-2460.
[3] NAGASUBRAMANIAN G. Electrical characteristics of 18650 Li-ion cells at low temperatures [J]. J Appl Electrochem, 2001, 31(1): 99-104.
[4] PLICHTA E J, BEHL W K. A low-temperature electrolyte for lithium and lithium-ion batteries [J]. J Power Sources, 2000, 88(2): 192-196.
[5] XING W B, BUETTNER-GARRETT J, KELLY J, KRYSIAK M, ZHANG J. High performance and safe electrolyte development [J]. The Society for Solid-state and Electrochemical Science and Technology, 2013, 45(29): 35-40.
[6] WU B R, REN Y H, MU D B, ZHANG C Z, LIU X J, WU F. Enhanced low temperature performance of LiFePO4 cathode with electrolyte modification [J]. Int J Electrochem Sci, 2013, 8: 8502-8512.
[7] HU Chuan-yue, LI Xin-hai, GUO Jun, WANG Xing-yan, YI Tao. Reactions between electrolyte and electrode of lithium ion batteries at elevated temperature [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(4): 630-635. (in Chinese)
[8] CHEN Zhao-yong, ZHU Hua-li, ZHU Wei, ZHANG Jian-li, LI Qi-feng. Electrochemical performance of carbon nanotube-modified LiFePO4 cathodes for Li-ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(4): 614-618.
[9] LIU Ping, LI Fan-qun, LI Jie, LU Hai, ZHANG Zhi-an, LAI Yan-qing. Compatibility between LiODFB/LiPF6 based electrolyte and activated carbon electrode [J]. J Cent South Univ, 2011, 42(4): 889-893. (in Chinese)
[10] HERREYRE S, HUCHET O. New Li-ion electrolytes for low temperature applications [J]. J Power Sources, 2001, 97(1): 576-580.
[11] ZHANG S S, XU K, JOW T R. The low temperature performance of Li-ion batteries [J]. J Power Sources, 2003, 115 (1): 137-140.
[12] LI Wen cheng, LU Shi gang. Thermal behavior of C/LiFePO4 power secondary battery [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(4): 1156-1162. (in Chinese)
[13] LIU Ping, LI Fan-qun, LI Jie, LU Hai, ZHANG Zhi-an, LAI Yan-qing. LiODFB-TEABF4 composite electrolyte for Li-ion battery and double-layer capacitor [J]. J Cent South Univ, 2010, 41(6): 2080-2085. (in Chinese)
[14] ZHU Wei xiong, LI Xin hai, WANG Zhi xing, GUO Hua jun. Synthesis and modification of Li rich cathode Li[Li0.2Ni0.2Mn0.6]O2 for Li ion batteries [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(4): 1047-1052. (in Chinese)
[15] XIAO L F, CAO Y L, AI X P. Optimization of EC-based multisolvent electrolytes for low temperature applications of lithium-ion batteries [J]. Electrochim Acta, 2004, 49(27): 4857-4863.
[16] SMART M C, RATNAKUMAR B V, WHITCANACKL D. Improved low temperature performance of lithium-ion cells with quaternary carbonated-based electrolytes [J]. J Power Sources, 2003, 119-121(1): 349-358.
[17] HERREYRE S, HUCHET O, BARUSSEAU S. New Li-ion electrolytes for low temperature applications [J]. J Power Sources, 2001, 97-98(1): 576-580.
[18] SMART M C, RATNAKUMAR B V, BEHAR A. Gel polymer electrolyte Lithium-ion cells with improved low temperature performance [J]. J Power Sources, 2007, 165(2): 535-543.
[19] PHICHTA E J, HENDRICKSON M, THOMPSON R. Development of low temperature Li-ion electrolytes for NASA and DoD application [J]. J Power Sources, 2001, 94(2): 160-166.
[20] SMART M C, RATNAKUMAR B V, SURAMPUDI S. Electrolytes for low temperature lithium batteries based on ternary mixtures of aliphatic carbonates [J]. J Electrochem Soc, 1999, 146(2): 486-492.
[21] LI J, YUAN C F, GUO Z H , ZHANG Z A, LAI Y Q, LIU J. Limiting factors for low-temperature performance of electrolytes in LiFePO4/Li and graphite/Li half cells [J]. Electrochim Acta, 2012, 59(1): 69-74.
[22] ZHANG S S, XU K, ALLEN J L, JOW T R. Effect of propylene carbonate on the low temperature performance of Li-ion cells [J]. J Power Sources, 2002, 110(1): 216-221.
[23] ZHANG S S, XU K, JOW T R. Electrochemical impedance study on the low temperature of Li-ion batteries [J]. Electrochim Acta, 2004, 49(7): 1057-1061.
线性羧酸酯对锰酸锂-石墨电池低温性能的影响
洪 树,李 劼,王冠超,张治安,赖延清
中南大学 冶金与环境学院,长沙 410083
摘 要:以线性羧酸酯EA、EP和EB分别替代工业用1.0 mol/L LiPF6 EC/EMC/DMC(1:1:1, 质量比)电解液中的DMC,配制了1.0 mol/L LiPF6 EC/EMC/EA (1:1:2, 质量比)、1.0 mol/L LiPF6 EC/EMC/EP (1:1:2, 质量比)和1.0 mol/L LiPF6 EC/EMC/EB (1:1:2, 质量比) 3种包含线性羧酸酯的电解液,采用18650全电池研究线性羧酸酯作为电解液溶剂组元对锰酸锂-石墨电池低温性能的影响。结果表明,采用3种包含线性羧酸酯的电解液,电池在-20 °C、5C倍率下放电容量保持率均大于93%,而采用工业用电解液时,电池无法在-20 °C、5C倍率下放电。电化学阻抗谱分析表明,在低温下电池放电容量和放电能量衰减的主要原因是电荷转移阻抗随温度的降低而增大。在3种含线性羧酸酯的电解液中,使用1.0 mol/L LiPF6 EC/EMC/EA (1:1:2, 质量比)电解液的电池因具有最低的电荷转移阻抗,表现出最好的电化学性能,在-40 °C下放电容量保持率大于90%,在-60 °C下放电容量保持率大于44.41%。
关键词:锂离子电池;低温性能;电解液;线性羧酸酯;离子电导率;电荷转移阻抗
(Edited by Xiang-qun LI)
Foundation item: Project (2007BAE12B01) supported by the National Key Technology Research and Development Program of China; Project (20803095) supported by the National Natural Science Foundation of China
Corresponding author: Zhi-an ZHANG; Tel: +86-731-88830649; E-mail: zza75@163.com
DOI: 10.1016/S1003-6326(15)63597-X