J. Cent. South Univ. (2016) 23: 3085-3091
DOI: 10.1007/s11771-016-3373-9
Solvent extraction mechanism and precipitation stripping of bismuth (III) in hydrochloric acid medium by tributyl phosphate
WANG Zhi-jian(王志坚), DING Feng-hua(丁风华), ZHAN Jing(湛菁), ZHANG Chuan-fu(张传福)
School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: Tributyl phosphate (TBP) was employed for the Bi(III) extraction from hydrochloric acid medium. The effects of extraction time and material concentration were examined. The replacement mechanism between the anion (Cl-) and TBP was proposed for extraction. The results show the species extracted into the organic phase were found to be mainly BiCl3·xTBP (x=2 or 3). Thermodynamic parameters of the extraction reaction were obtained from the thermodynamics analysis, which illustrates that higher temperatures show a negative effect on the extraction. Extraction isotherm was obtained with 2.16 mol/L TBP for a typical solution containing 0.1 mol/L of bismuth and 1.0 mol/L of hydrochloric acid. About 98.5 % of bismuth has been extracted from the leaching solution under the optimum condition. Moreover, oxalate was explored as a precipitation stripping agent for BiCl3·xTBP (x=2 or 3) complexes, by which Bi(III) was stripped in the form of Bi2(C2O4)3·7H2O. A stripping efficiency of 99.3% was obtained in only one stage at the phase ratio of 1 and TBP also could be recycled. Therefore, the method is an efficient, effective and highly selective approach to extract Bi(III) and to recover metal bismuth.
Key words: solvent extraction; bismuth (Bi); tributyl phosphate (TBP); precipitation stripping; extraction mechanism
1 Introduction
Bismuth is considered “green and ecologically clean” metal and used in cosmetics and pharmaceuticals [1-2]. However, very little bismuth directly comes from the primary processing of bismuth minerals. Most bismuth is obtained as a by-product in other metallurgical process for copper, silver, tin, lead and gold where it is usually present in sludge or in acidic liquors [3-4].
During the processing of these bismuth minerals, the acidic-chloride based leaching of these bismuth minerals is the most promising method. Hydrochloric acid is usually involved as the lixiviant in conjunction with an oxidant, such as ferric chloride, chlorine, and sodium hypochlorite, and highly acidic solutions containing base metals and bismuth are obtained [5-6]. Bismuth can be recovered from these solutions by hydrolytic precipitation, slovent extraction, ion exchange and electrolysis [7]. Among these methods, solvent extraction, as an effective separation and extraction of metals method from solution, has received much attention in recent decades. Many authors have proposed the separation of bismuth from copper through solvating extractants using the Acorga SB-50 extractant in chloride media and organic phosphorous extractants [8-11]. YANG et al [12] have studied the extraction of Bi from the hydrochloric acid leaching liquor of bismuth glance floatation concentrate using N235 as extractant. Other authors have proposed the separation and extraction of Bi(III) from acid or highly acidic solutions of HCl using Cyanex 925 [13], tri-n-octyl phosphine oxide (TOPO) [14], 4-n-Octylaniline [15], 2-bromoalkanoic acid [16] and Cyanex 302 [17] as extractant. Except for these single systems, synergisitic extraction effects on bismuth with mixtures of extractants, 2-ethylhexylphosphonic acid mono-(2-ethylhexyl) ester and 2,2’-bipyridyl, were also investigated [18]. For the aforementioned extraction processes, bismuth ions in the ogainc phase, are always stripped into the aquous acids by mixing the solutions with mineral acids (typically, hydrochloric acid or nitric acid ) or EDTA for further treatment. Little information on the bismuth ions stripped from organic phase directly as solid oxalate salt has been reported.
Tributyl phosphate (TBP) is a widely-used extractant and has been used to separate and recover iron [19], nickle [20], copper [21], molybdenum [22] and rare earth [23]. AHN et al [24] have reported the removal of impurity Bi, As, Cu, Pb and Zn from plating sludges containing Sn in hydrochloric acid solution by solvent extraction using TBP as extractant under different HCl concentrations conditions. However, the uncertainties regarding the species compostion and extraction mechanism about TBP have not been reported in the above literature.
Inspired by the preceding consideration, TBP is exploited as the extractant of Bi(III) in acidic chloride media in the present work,. The composition of the various extracted bismuth species and the stoichiometry of the metal-organic complexes in the organic phase consisting of TBP and kerosene will be investigated. In addition, the distribution coefficients and extraction constants of the metal-organic comlexes should be determined. To get a complete understanding of the system, Bi(III)-Cl--H2O diagram would be drawn to analyze the complexes between Bi(III) and Cl-. What is more, oxalic acid is explored as the stripping agent, by which Bi(III) can be stripped from the organic phase directly as solid oxalate salts. To our knowledge, no studies of extraction mechanism of Bi(III) by TBP from hydrochloric acid medium and the production of bismuth via precipitation stripping with TBP have been published.
2 Experimental
2.1 Reagent and solutions
Tributyl phosphate (TBP) was used as received without futher purification. Kerosene was used as diluent. Before use, the kerosene (density=786 kg/m3, boiling range=200-250°C, supplied by Sublimation Technology Co.) was washed twice with 1/5 volume ratio of 98% H2SO4 to remove aromatics and then washed with distilled water until neutral. As a source of Bi(III), stock solutions of BiCl3 were prepared with AR chemicals. The pH of the aqueous phase was adjusted by the addition of HCl to prevent BiCl3 from hydrolysis. The other chemicals, such as NaOH, H2C2O4·2H2O and NaCl, were of analytical reagent grade and used without further purification.
2.2 Extraction of Bi(III)
For the metal extraction, a required amount of TBP was added into sulfonated kerosene to form the organic phase. 20 mL the organic phase and 20 mL aqueous solution containing Bi(III) were added to a separatory funnel and then equilibrated mechanically in an orbital shaker for 10 min at 293 K. The volume ratio of the aqueous phase to the organic phase (Rw:o) was 1.0. Both of organic phase and aqueous phase had been pre-saturated. Afterwards, the organic phase and aqueous phase were separated. The separated organic phase and aqueous phase were both clear and transparent. Unless stated specially, all experiments were carried out at 293 K (except for effect of temperature experiments). The extraction efficiency (E) and distribution coefficient (D) of bimuth ions are expressed as the following equations:
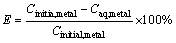 (1)
(1)
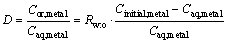 (2)
(2)
where Cinitial,metal and Caq,metal represent the initial concentration of bismuth ion and the instantaneous concentration in aqueouss, respectively.
2.3 Precipitation stripping
After the extraction, the Bi(III)-loaded organic phase was separated and scrubbed from the aqueous phase and then mixed with an equal volume of 20% oxalic acid solution. The precipitation was maintained at 293 K for 30 min and thus, white precipitation powders were formed. After the precipitation reaction, the mixtures solution allowed to settle until three distinct phases were noticeable (solid bismuth oxalate, aqueous precipitating solution, and stripped organic solution). The bismuth oxalate precipitation was filtrated from the other two phases and then stirred in ethyl alcohol for 10 min to remove any organic contaminates. The organic phase containing TBP was recovered and could be reused to extract Bi(III) from new feed solution again.
2.4 Analytical techniques
Before and after Bi(III) extraction, the Bi(III) concentration in aqueous solution was determined by titration with EDTA using xylenol orange as the indicator. Then the concentration of bismuth in organic phase was calculated based on mass balance. The percentage of precipitation and stripping efficience can be calculated after measuring the mass of bismuth in precipitates and filtrate by titration. The obtained sample by precipitation stripping was analyzed by an X-ray powder diffractometer (Rigaku-TTRIII) (Cu Kα, λ=0.154056 nm).
3 Results and discussion
3.1 Effect of extraction time
Extraction time was preliminary studied. With the use of the organic phase of 2.16 mol/L TBP and the aqueous phase of 0.1 mol/L Bi(III) in 1.0 mol/L HCl solution, Rw:O=1, the effect of vibration time on Bi(III) extraction by the TBP was studied (Fig. 1). The vibration time varied from 1 to 6 min. The Bi(III) extraction yield (E) reaches 80.4 % within 2 min, then the extraction is independent of the vibration time. Therefore, the vibration time of 10 min is sufficient in the following extraction experiments.
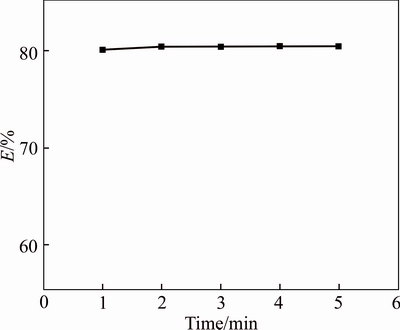
Fig. 1 Extraction yield (E) of Bi (III) as a function of vibration time (Aqueous phase, 0.1mol/L Bi (III) in 1 mol/L HCl at 293 K; Rw:O=1)
3.2 Effect of TBP concentration
The influence of the initial TBP concentration on Bi(Ⅲ) extraction yield(E) and distribution ratio (D) was studied from 1.44 mol/L to 3.60 mol/L at a temperature of 293 K, keeping Rw:o=1, BiCl3 concentration of 0.1 mol/L and initial hydrochloric acid concentration of 1.0 mol/L. The results are shown in Fig. 2. It is apparent that the Bi(III) extraction yield increases from 70% to 95% as the concentration of TBP from 1.44 mol/L to 3.60 mol/L. It was observed from Fig. 2 that the distribution coefficient increases with increasing extractant concentration in the organic phase.
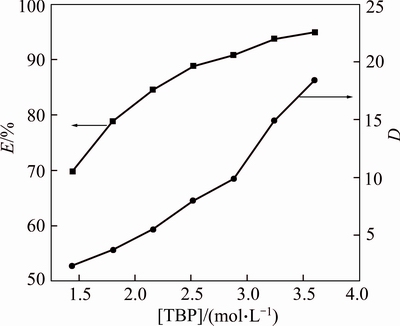
Fig. 2 Extraction yield (E) and distribution coefficient (D) of Bi (III) as a function of TBP concentration (Aqueous phase,0.1 mol/L Bi(III) in 1 mol/L HCl at 293 K; Rw:O=1)
3.3 Effect of initial HCl concentration
The extraction behaviors of Bi(III) by the TBP with various concentrations of hydrochloric acid in the aqueous phase were studied. The TBP concentration in the organic phase was 2.16 mol/L, while the HCl concentration varied from 1.0 mol/L to 5.0 mol/L with the concentration Bi(III) of 0.1 mol/L. The results are shown in Fig. 3. The increasing HCl concentration shows a negative effect on the Bi(III) extraction performance. The decrease in Bi(III) extraction is caused by the competition between TBP and Cl- to associate with BiCl3 by replacement reaction of the complexes.
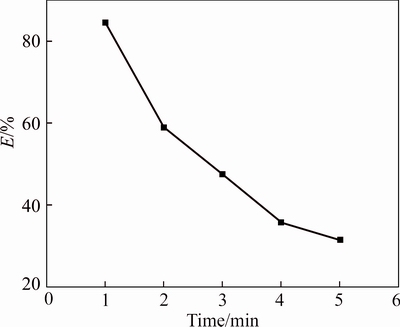
Fig. 3 Extraction yield (E) of Bi (III) as a function of HCl concentration (Aqueous phase, 0.1 mol/L Bi(III) at 293 K; Rw:O=1)
3.4 Determination of coordination number
In acidic chloride media, the extraction of bismuth with TBP can be expressed by basic species as following reaction:
 (3)
(3)
Equilibrium constant Ke for extraction reaction is
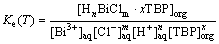 (4)
(4)
Substituting Eq. (2) into Eq. (4) yields
 (5)
(5)
Taking logarithm of Eq. (5), the relationship among distribution coefficient D and TBP concnetration can be expressed as follows:
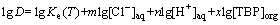 (6)
(6)
Therefore, the plot of the logarithm of TBP concentrations verse the logarithm D will yield x slope proportional to the coordination number. By plotting the lg[TBP] versus lgD, a line (R2=0.99) with a slope of 2.3 was obtained as shown in Fig. 4. Then, x=2 or x=3 was determined, which indicate the Bi(III)-TBP complexes with mole ratio of 1:2 or 1:3 are formed.
3.5 Extraction mechanism for extraction of Bi with TBP
To explain the extraction mechanism, it was necessary to discuss the aqueous chemistry of bismuth in acidic chloride media. In Bi-Cl--H2O, Bi-Cl complexes can co-exist in solution because Cl- is a good ligand for bismuth ions. Therefore, for acidic chloride media, the aqueous bismuth species are mainly Bi3+, BiO+, BiCli3-i (i=1-6). These species can be achieved under their equilibrium state according to the following chemical equations (Eqs. (7)-(8)).
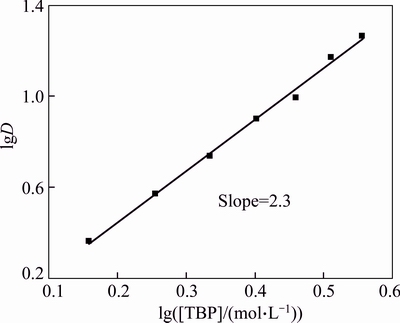
Fig. 4 Plot of lg D versus lg [TBP]

 (7)
(7)
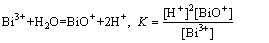 (8)
(8)
According to the thermodynamic calculation, the relative concentration (f) of each species at different [Cl-] was plotted as shown in Fig. 5. It is obvious that the species BiCl52- and BiCl63- predominate and maintain at a stable level in the solution when [Cl-]>1.0 mol/L. The results obtained above are in agreement with earlier reports [25].
Further experiments were carried out to address hydrogen ion concentration on the extraction of bismuth.
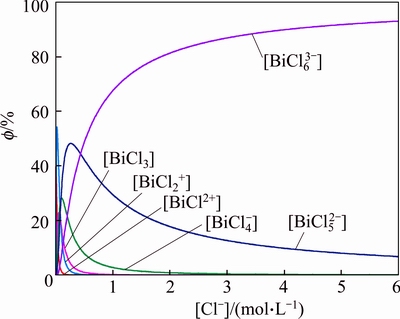
Fig. 5 Distribution of aqueous bismuth species as a function of [Cl-] ([Bi(III)]=0.1 mol/L, 298 K)
The H+ concentrations were ranged from 1.0 mol /L to 3.0 mol/L by addition NaOH solution with fixing Cl- concentration of 3.3 mol/L and TBP concentration of 2.16 mol/L. The results are shown in Fig. 6(a). The Bi(III) extraction yield, E increase from 34.64 % to 48.25% with the H+ concentration. The slight increase in Bi(III) extraction may be casued by the formation of H2BiCl3·2TBP and H2BiCl5·3TBP. According to the Fig. 6(b), the plots of lg D versus lg [H+] are nonlinearity, indicating bismuth extraction is slightly dependent on the H+ concentration.The n value in Eq. (3) can be deduced as 0 in Bi(III)-TBP extraction system with the HCl concentration of 1.0 mol/L.
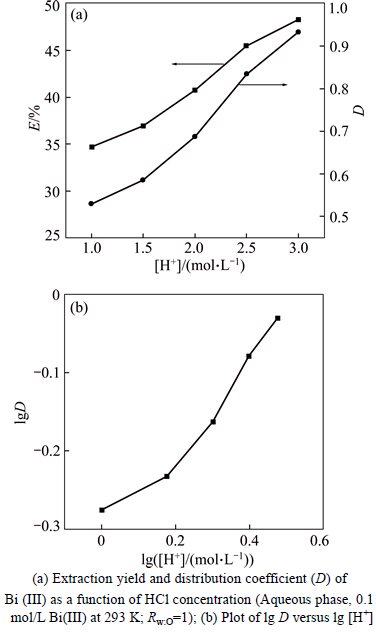
Fig. 6 Effect of hydrogen ion concentration on extraction of bismuth:
Therefore, according to Fig. 4, 1:2 or 1:3 associates are formed between Bi(III) and TBP in hydrochloric acid medium; meanwhile, a stronger interaction exists between BiCl3 and TBP. Furthermore, BiCl52- and BiCl63- are composed of BiCl3 neutral molecule and Cl- anion which is the ligand for Bi(III). So, replacement mechanism between the anion and extraction agent was supposed for Bi(III) extraction. Bi(III) extraction by TBP is deduced as the formation of the neutral complexes as BiCl3·2TBP or BiCl3·3TBP. The extraction reaction of Bi(III) by the TBP from the hydrochloric acid medium can be presented as the following equations:
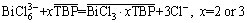 (9)
(9)
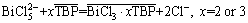 (10)
(10)
To further confirm the vality of the replacement mechanism between the anion and extraction agent, the effect of Cl- concentration on Bi(III) extraction in the aqueous phase was studied. The Cl- concentrations were adjusted by NaCl, and the organic phase was of the 2.16 mol/L TBP with Bi (III) concentration in the aquous phase of 0.1 mol/L, Rw:o=1 and H+ concentration of 1.0 mol/L.
As shown in Fig. 7, a sharp decrease in extraction yield of bismuth occurs from 84.5% to 15.6% (about six-fold) with the Cl- concentration increasing from 1.3 mol/L to 4.3 mol/L, indicating that extraction ratio of bismuth is firmly dependent on the Cl- concentration. The plots of lg D versus lg [Cl-] present a droping straight line with a slope of -2.8, which indicates that the coordination number for Bi(III)-TBP complexes is dependent on Cl- concentration, namely, coordination number of BiCl3-Cl- complex. These behaviors can be explained by the equilibrium shift of Eq. (9) and Eq. (10). The data further confirm that the extraction of Bi(III) with TBP as the extractant follows the replacement mechanism of the ligand, as represented by Eq. (9) and Eq. (10).
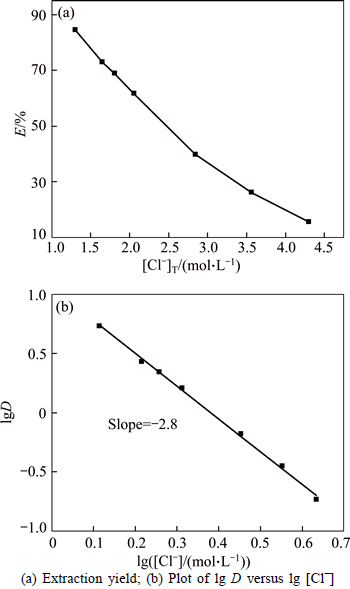
Fig. 7 Effect of chloride ion concentration on extraction of bismuth:(Aqueous phase, 0.1 mol/L Bi(III) at 293 K; Rw:o=1)
3.6 Thermodynamic study of extraction
The influence of temperature on the extraction of Bi3+ was investigated when the concentration of extractant was kept at 2.16 mol/L TBP with RO:W=1. The equilibrium constants and thermodynamic parameters were determined at different temperatures. According to Eq. (6), lgKe(T) values were calculated using the experiment data and listed in Table1. Furthermore, Gibbs energy is ΔGΘ=-RTlnKe(T) and ΔGΘ=ΔHΘ-TΔSΘ. The ΔG(T) values were calculated and also listed in Table 1. The plot of the natural logarithm of Ke(T) versus the inverse temperature will yield a slope proportional to the enthalpy.
 (11)
(11)
Table 1 Equilibrium constants and thermodynamic parameters of Bi(III) extraction
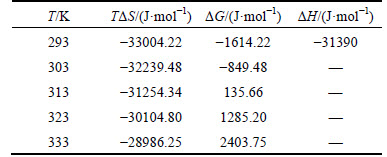
By plotting inverse temperature versus the natural logarithm of Ke(T), a line (R2=0.992) with a slope of 3.775 was obtained as shown in Fig. 8. Then ΔH=-31.39 kJ/mol was determined, which indicates that the reaction of Bi(III) extraction by TBP in hydrolytic acid system (Eq. (9) and Eq. (10)) should be exothermic. when T is 293 K and ΔGΘ and ΔSΘ could be determined as -1614.22 J/mol and -112.64 J/(mol·K), respectively,implying that the extraction is a spontaneous process. This theoretical result is consistent with the experiments.
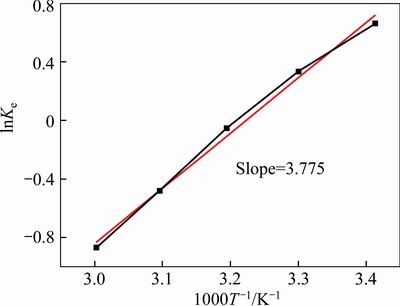
Fig. 8 Plot of ln Ke versus 1000/T
3.7 Extraction isotherm
To determine the number of stages, an organic solution consisting of 2.16 mol/L TBP was used to generate an extraction isotherm for bismuth from 0.1 mol/L BiCl3 in 1.0 mol/L HCl. The set of extraction experiments were carried out in separatory funnel of 125 mL. The McCabe-Thiele plot (Fig. 9) shows quantitative extraction of bismuth in three or four counter-current stages at 1:1 phase ratio.
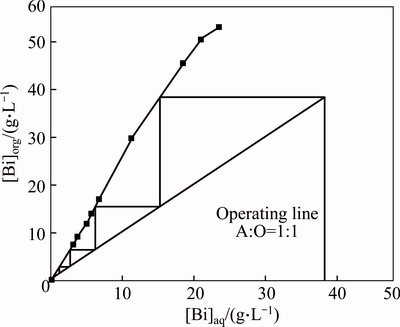
Fig. 9 McCabe-Thiele plot for bismuth extraction ([Bi]T=0.2 mol/L, [Cl-]T=2 mol/L; 2.16 mol/L TBP in sulfonated kerosene)
To confirm the prediction above, a four-stage count- current simulation study at Rw:o=1 was carried out. An extraction yield of 98.5% from the leaching solution (0.091 mol/L Bi3+) was obtained under the conditions of 60%TBP-40% sulfonated kerosene; ratio of O/A=1:1; temperature=293 K; time=5 min; [C1-1]T=46 g/L and pH=0.8. Thus, the loaded organic phase concentration of bismuth was 0.197 mol/L (41.173 g/L). A requisite quantity of bismuth loaded organic was obtained to carry out the stripping experiment.
3.8 Precipitation stripping
In the present study, the Bi(Ⅲ)-TBP complexes were stripped by the oxalic acid precipitation. The process was followed by Section 2.4. The precipitation stripping reaction between the BiCl3·xTBP (x=2 or 3) complexes and oxalic acid may be written as the following equation:


Under the conditions of 20% oxalic acid with a phase ratio (Vo/Va) of 1:1 and a contact time of 30 min, 99.20% bismuth in organic phase was stipped in the form of white precipitate. The surplus stripping agent can be recycled to use in the next time. Figure 10 shows the XRD paterns of the precipitates. It futher confirms the results, which indicates that Bi3+ in the Bi(III)-TBP complexes can be precipitated as Bi2(C2O4)3·7H2O at ambient temperature. This Bi2(C2O4)3·7H2O can be further decomposed to prepare the high-purity Bi2O3 powders.
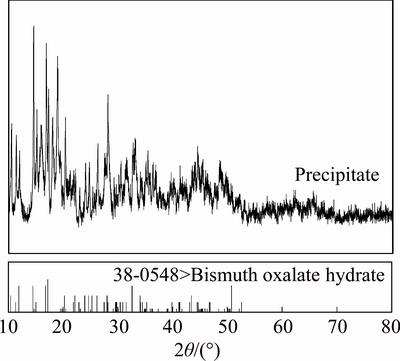
Fig. 10 XRD pattern of stripping precipitates
4 Conclusions
Bismuth can be extracted from acidic chlorid media using the commercially available tributyl phosphate (TBP) as extractant. The extracted complex was inferred to be BiCl3·xTBP (x=2 or 3) according to the mechanism analysis via thermodynamic calculation, the species analysis of extraction system, graphic and slope analysis techniques. Based on the study of thermodynamics, thermodynamic parameters of the Bi(III) extraction reaction were obtained. Higher temperatures show a negative effect on Bi(III) extraction. The extraction yield of bismuth from leaching solution (0.091 mol/L Bi3+) reached up to 98.5% after four stages count-current extraction at O:A ratio of 1:1, TBP concentration of 2.16 mol/L, time of 5 min, [Cl-1] of 0.1 mol/L and temperature of 293 K. Up to 99.2% of Bi(III) in the organic solution could be easily stripped by precipitation stripping in one stage under the condition of 20% oxalic acid with a phase ratio of 1 in the form of Bi2(C2O4)3·7H2O. After washing, the organic phase can be reused in the extraction process. This implies that TBP-sulfonated kerosene system has potential to be applied to separate and recover bismuth from chlorid media in the hydrometallurgical field.
References
[1] ROHR O. Bismuth—The new ecologically green metal for modern lubricating engineering [J]. Industrial Lubrication & Tribology, 2002, 54(4): 153-164.
[2] ZHAN Jing, WANG Zhi-jian, ZHANG Chuan-fu, HWANG Jian-yang, XIA Chu-ping. Separation and extraction of bismuth and manganese from roasted low-grade bismuthinite and pyrolusite: Thermodynamic analysis and sulfur fixing [J]. JOM, 2015, 67(5): 1114-1122.
[3] YANG Jian-guang, TANG Chao-bo, YANG Sheng-hai, HE Jing, TANG Mo-tang. The separation and electrowinning of bismuth from a bismuth glance concentrate using a membrane cell [J]. Hydrometallurgy, 2009, 100(1/2): 5-9.
[4] HA T K, KWON H B, PARK K S, MOHAPATRA D. Selective leaching and recovery of bismuth as Bi2O3 from copper smelter converter dust [J]. Separation & Purification Technology, 2015, 142: 116-122.
[5] KUNTER R S, BEDAL W E. Chloride process treatment of smelter flue dusts [J]. JOM, 1992, 44(12): 35-38.
[6] WANG Cheng-yan, QIU Ding-fan, JIANG Pei-hai. Bismuth hydrometallurgy technology in China [J]. Nonferrous Metals, 2001, 53(4): 15-18. (in Chinese)
[7] FLYNN C M, GARNAHAN T G, LINDSTROM R E. Recovery of bismuth from chloride process solutions: US, 4285912[P]. 1981-08-25.
[8] KIM D K, LEESE T A, SAITO M N, YOUNG B R, WEIDNER C J. Use of solvent extraction to remove bismuth and antimony from copper electrolyte at the San Manuel Refinery [C]// CROWSON A. Proceedings of Sessions and Symposia, the TMS Annual Meeting. San Antonio: EPD Congress, 1998: 301-315.
[9] DREINSIGER D B, LEONG B J Y, BAILINT B J, BEYAD M H. The solvent extraction of As, Sb and Bi from copper refining electrolytes using organophosphorus reagents [C]// Proceedings of International Solvent Extraction Conference. London: Elsevier Applied Science, 1993: 1271-1278.
[10] SZYMANOWSKI J. Removal of toxic elements from copper electrolyte by solvent extraction [J]. Miner Process Extr Metall Rev, 1998, 18(3/4): 389-418.
[11] WANG C, JIANG K, LIU D, WANG H. Purification of copper electrolyte with Cyanex 923 [C]// International Solvent Extraction Conference. Cape Town, 2002: 1039-1044.
[12] YANG Jian-guang, YANG Jian-ying, TANG Mo-tang, TANG Chao-bo, LIU Wei. The solvent extraction separation of bismuth and molybdenum from a low grade bismuth glance flotation concentrate [J]. Hydrometallurgy, 2009, 96(4): 342-348.
[13] TYER J N, DHADKE P M. Solvent extraction and separation studies of antimony (III) and bismuth (III) by using Cyanex-925 [J]. Ind J Chem Technol, 2003, 10(6): 665-669.
[14] ABDAR A K, VANJARA A K. Solvent extraction and separation of Bi(III) and Sb(III) from HCl and HBr media using tri-n-octyl phosphine oxide (TOPO) [J]. Ind J Chem Technol, 2001, 8(4): 239-243.
[15] KOKATE S J, SHELAR Y S, AHER H R, KUCHEKAR S R. Liquid-liquid extraction and recovery of bismuth(III) from hydrochloric acid media using n-octylaniline in chloroform [J]. Bulg Chem Commun, 2010, 42(2): 107-112.
[16] MORIYA Y, SUGAI M, NAKATA S, OGAWA N. Extraction of bismuth(III) with 2-bromoalkanoic acid in nondonating solvent from highly acidic aqueous solution [J]. Anal Sci, 2001, 17(2): 297-300.
[17] SARKAR S G, DHADKE P M. Solvent extraction separation of antimony (III) and bismuth (III) with bis (2, 4, 4-trimethylpentyl) monothiophosphinic acid (Cyanex 302) [J]. Separation & Purification Technology, 1999, 15(2): 131-138.
[18] SONG Nai-zhong, LI Wan-jun, JIA Qiong. Solvent extraction of bismuth with 2-ethylhexylphosphonic acid mono-(2-ethylhexyl) ester and 2,2'-bipyridyl [J]. Separation & Purification Technology, 2013, 104: 64-67
[19] PATNAIK P, BABA A A, NATHSARMA K C, SARANGI K, SUBBAIAH T. Separation of iron and zinc from manganese nodule leach liquor using TBP as extractant [J]. Mineral Processing and Extractive Metallurgy, 2013, 122(3): 179-185.
[20] KUMBASAR R A. Separation and concentration of cobalt from aqueous thiocyanate solutions containing cobalt-nickel by emulsion liquid membranes using TBP as extractant [J]. Journal of Membrane Science, 2009, 338(1): 182-188.
[21] SARANGI K, PARHI P K, PADHAN E, PALAI A K, NATHSARMA K C, PARK K H. Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using TBP, LIX 84I and Cyanex 923 [J]. Separation & Purification Technology, 2007, 55(1): 44-49.
[22] WU Jun, WEI Chang, LI Xing-bin, WANG Si-fu, WANG Ming-shuang, LI Cun-xiong. Selective extraction of Mo using Cyanex-272 and tributyl phosphate from low grade Ni-Mo ore leach liquor [J]. Separation & Purification Technology, 2012, 99(41): 120-126.
[23] GAGLIARDI F M, CASHION J D. Solvation of gold and rare earths by tributyl phosphate [J]. Hyperfine Interactions, 2012, 207(1/2/3): 13-17.
[24] AHN J W, LEE J C. Separation of Sn, Sb, Bi, As, Cu, Pb and Zn from hydrochloric acid solution by solvent extraction process using TBP (tri-n-butylphosphate) as an extractant [J]. Materials Transactions, 2011, 52(12): 2228-2232.
[25] CHEN Jia-yong. Handbook of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2005: 124-131, 1297-1327. (in Chinese)
(Edited by YANG Hua)
Foundation item: Project(2011AA061002) supported by the High-Tech Research and Development Program of China; Project(2010SK2010) supported by the Key Program of Science and Technology of Hunan Province, China; Project supported by the Hunan Nonferrous Metals Fund, China
Received date: 2016-06-14; Accepted date: 2016-10-06
Corresponding author: ZHAN Jing, Associate Professor; Tel: +86-13975147556; E-mail: zhanjing2001@hotmail.com