文章编号:1004-0609(2015)10-2945-08
As(Ⅲ)在酸性水溶液中与金属铁的反应行为
刘志宏,潘庆琳,刘智勇,李玉虎,李启厚
(中南大学 冶金与环境学院,长沙 410083)
摘 要:从热力学分析和试验两方面研究As(Ⅲ)在酸性水溶液中与金属铁的反应行为。热力学计算结果表明:在酸性水溶液中,As(Ⅲ)与金属铁作用,分别生成As元素或AsH3气体的反应在热力学上均是可行的。试验结果表明:在温度为20~80 ℃、溶液初始pH值为-0.31~4、溶液初始浓度ρ[As(Ⅲ)]为1~20 g/L、铁粉大量过剩的条件范围内,由于动力学方面的原因,生成AsH3的反应实际并不会发生,铁粉仅能使As(Ⅲ)还原为As元素。铁粉“过量”系数、溶液pH值和温度对沉砷率有显著影响,提高铁粉“过量”系数和温度,可使沉砷率增大,在溶液pH值为3的条件下,沉砷效果最佳。在酸性溶液中,铁粉置换As(Ⅲ)生成As的反应难以进行到底的原因,可能是由于还原的As在铁粉表面沉积阻滞了反应的延续。而在初始pH值为3或较高温度条件下能达到较高的沉砷率,是由于在这些条件下,置换的砷呈疏散形态沉积,未能对铁粉表面形成紧密包裹,使得反应得以延续。
关键词:As(Ⅲ);金属铁;酸性溶液;反应行为
中图分类号:TF801.3 文献标志码:A
Reactive behaviors between As(Ⅲ) and metallic iron in acidic aqueous solution
LIU Zhi-hong, PAN Qing-lin, LIU Zhi-yong, LI Yu-hu, LI Qi-hou
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: The reactive behaviors between As(Ⅲ) and metallic iron in acidic aqueous solutions were investigated thermodynamically and experimentally. The thermodynamic calculation results show that the reactions between As(Ⅲ) and metallic iron in acidic aqueous solutions, which produce both elemental arsenic and AsH3 gas, respectively, are feasible thermodynamically. The experimental results show that, owing to the kinetic reason, the reaction producing AsH3 gas never occurs actually under a wide range of the conditions, such as, temperature from 20 to 80 ℃, initial solution pH value from -0.31 to 4, initial As(Ⅲ) concentration from 1 to 20 g/L, and a massive surplus in iron powders. Under these conditions, the iron powders can only reduce As(Ⅲ) ions from solution as elemental arsenic deposition. These factors, such as surplus coefficient of iron powders, solution pH value and temperature, all have obvious effects on the arsenic deposition rate. The arsenic deposition rate increases with the increase of the surplus coefficient of iron powders and temperature, while the best result of arsenic deposition rate is obtained when the pH value is 3. In acidic aqueous solutions, the reaction of iron powders replacing As(Ⅲ) is difficult to progress quickly to the end, the reason may be that arsenic reduced deposits on the surface of iron powders retard the reaction forward. Moreover, a higher arsenic deposition rate is obtained under the condition of pH=3 or higher temperature, the reason can be deduced that, under these conditions, arsenic deposits in loose form, and does not closely coat the surface of iron powders, thus making the reactions between As(Ⅲ) and iron powders sustainable.
Key words: As(Ⅲ); metallic iron; acidic aqueous solution; reactive behavior
砷属剧毒,为致癌元素,广泛伴生于铜、铅、镍、钴、锡和锑等有色金属矿中[1-3],研究砷在冶炼过程中的行为,对安全生产与环境保护,意义重大。
含砷复杂冶金原料,包括矿石或精矿[4-6]、各类烟尘[7]、阳极泥[8]、污酸硫化砷渣[9]和黑铜粉[10]等,广泛采用酸性体系湿法冶金方法处理,如湿法炼锌窑渣铁精矿的浸出[11]、复杂铋精矿湿法炼铋[12]等。在这些湿法冶金过程中,由于原料本身含有金属铁或作为还原剂加入,铁常常与酸性含砷溶液共存,使得体系的混合电势处于还原区,对砷的行为产生影响。
砷有-3、0、+3和+5等多种价态,在湿法冶金过程中行为十分复杂[13]。对其在还原电势下酸性体系湿法冶金中的行为,重点关注两点:1) 是否会生成AsH3气体;2) 在液相和渣相之间的分配行为。
AsH3属剧毒气体,GB 17055-1997(车间空气中砷及其无机化合物卫生标准)[14]规定,车间空气中AsH3浓度应低于0.3 mg/m3。在铜电解液净化电积脱铜[15]和锌粉置换[16]等湿法冶金过程中,由于满足生成AsH3的3个条件:溶液含砷、酸性体系和强还原电势,往往有AsH3气体产生,必须采取防护措施,否则可能造成人身伤亡。金属铁的还原性相对较弱,尚未见在其作用下导致AsH3从酸性含砷溶液中析出的报道。但是,从热力学和动力学两方面研究是否存在这种可能性,还是一个很有意义的课题。在酸性含砷溶液中有金属铁存在的条件下,As(Ⅲ)也可能被金属铁置换为元素As沉淀,由于As能够与Cu、Ni和Co等形成金属间化合物[17],在溶液中存在这些金属离子时,As(Ⅲ)的置换沉淀反应更易发生,其行为与温度、pH值、溶液组成及还原电势等密切相关。但是,目前这一方面仍缺乏系统研究。本文作者研究酸性溶液中As(Ⅲ)与金属铁的反应行为,以期揭示在不同技术条件下,砷在气相、液相和固相中的分配行为及其机理,研究结果对含砷物料的湿法冶金处理,具有一定的指导作用。
1 实验
1.1 实验原料
实验所用还原铁粉(纯度≥98%,质量分数)、三氧化二砷(As2O3)、氢氧化钠(NaOH)、98%(质量分数)硫酸(H2SO4)、30%(质量分数)双氧水(H2O2),均为分析纯,未经提纯直接使用。水为去离子水。图1所示为试验用还原铁粉的SEM像。由图1可见,还原铁粉呈多孔、类球形烧结体形貌,颗粒直径为20~30 μm。
As(Ⅲ)溶液配制过程:采用三氧化二砷、氢氧化钠和去离子水配制一定浓度的As(Ⅲ)溶液,用98%硫酸调节溶液pH至试验初始值。
AsH3吸收液配制过程:取30%双氧水40 mL,用去离子水稀释至200 mL,得到6%的双氧水溶液作为AsH3吸收液,用于检测试验中是否有AsH3气体产生及其产出量。

图1 试验用还原铁粉SEM像
Fig. 1 SEM image of reduced iron powders used in experiment
1.2 实验方法

图2 实验装置示意图
Fig. 2 Schematic diagram of experimental equipment
图2所示为实验装置示意图。试验在500 mL玻璃三口瓶中进行,3个开口分别装设机械搅拌器、pH/ORP测试电极和用作反应气体(H2或AsH3)出口,试验中三口瓶整体密闭,以使反应气体进入AsH3吸收液。三口瓶置于恒温水浴中,以控制试验温度在设定值±1 ℃波动。按实验预定浓度和pH值,将配制的300 mL含As(Ⅲ)溶液置于三口瓶中,加热至实验温度恒温,加入一定量还原铁粉,密闭三口瓶,开启搅拌,开始试验计时。实验结束后,分别取AsH3吸收液、反应溶液和渣样进行分析。
实验中,还原铁粉“过量”系数(n)为铁粉实际加入量/式(1)化学计量计算值。
 +3Fe+7H+=AsH3+3Fe2++2H2O (1)
+3Fe+7H+=AsH3+3Fe2++2H2O (1)
1.3 分析检测
采用萃取分离-碘滴定法测定试验溶液中砷含 量[18];采用美国热电公司生产的IRIS Intrepid II XSP型ICP-AES分析AsH3吸收液中砷的含量;取适量的试验渣样用浓硝酸溶解,采用 ICP-AES分析溶液砷浓度,计算确定渣中砷的含量。
采用Rigaku-TTRIII型X 射线衍射仪(Cu靶,Kα,λ=0.15406 nm)对试验渣样进行物相分析;采用扫描电镜(JSM-6360LV型,日本电子公司生产)观测渣样的显微形貌、表面状态、粒度大小;采用EDS-GENESIS 60S型X射线能谱仪测定试验渣样表面元素成分。
2 结果及讨论
2.1 Fe-As-H2O系的φ-pH图
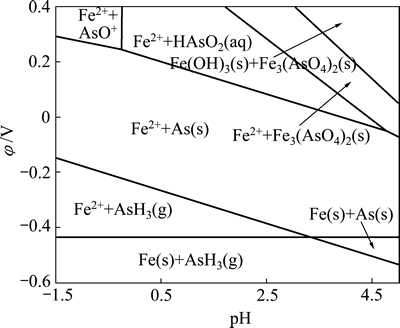
图3 Fe-As-H2O系φ-pH图(298.15 K, =1)
=1)
Fig. 3 φ-pH diagram of Fe-As-H2O system (298.15 K, =1)
=1)
采用FactSage 软件计算绘制了Fe-As-H2O系φ-pH图(298.15 K,
软件计算绘制了Fe-As-H2O系φ-pH图(298.15 K, =1),如图3所示。由图3可见,在Fe-As-H2O体系酸性区域,As(Ⅲ)在水溶液中依据pH值不同,以AsO+和HAsO2形态存在,热力学上它们都能被金属铁(Fe)还原,生成AsH3或As,如式(2)~(5)所示:
=1),如图3所示。由图3可见,在Fe-As-H2O体系酸性区域,As(Ⅲ)在水溶液中依据pH值不同,以AsO+和HAsO2形态存在,热力学上它们都能被金属铁(Fe)还原,生成AsH3或As,如式(2)~(5)所示:
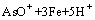 =
=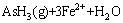 (2)
(2)
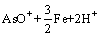 =
=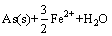 (3)
(3)
 =
= (4)
(4)
 =
=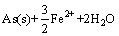 (5)
(5)
此外,在该体系中H+离子也可能被铁粉还原析出H2,如式(6)所示:
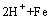 =
=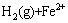 (6)
(6)
2.2 Fe粉“过量”系数对沉砷率的影响
在溶液初始pH值为0、c[As(Ⅲ)]为5 g/L、温度为40 ℃、反应时间为120 min的条件下,考察了Fe粉“过量”系数对沉砷率的影响,结果如表1、图4和图5所示。
由表1可知,在实验条件范围内,金属铁不能将溶液中的As(Ⅲ)离子还原为AsH3,这是由于虽然从热力学上讲,AsH3的析出是可能的,但众所周知,气体通过电化学反应析出,存在高达1000 mV左右的超电势[19],即动力学阻力很大,而金属铁的还原能力相对较弱。因此,AsH3气体实际上不会析出。此外,表1结果也表明,溶液中有5%~20%(质量分数)的As(Ⅲ)离子还原为固态进入渣中,其占总砷量的质量分数随铁粉“过量”系数增大而略有增加。
表1 不同Fe粉“过量”系数(n)下As的分配比及溶液终点pH值
Table 1 Distribution percent of arsenic and final solution pH value at different excess coefficients in iron powders (n)

在实验中,对溶液pH值和混合电势进行了测量。由表1可见,与其初始pH比较,溶液终点pH值仅略有增大,其值随铁粉“过量”系数增加略微升高,两方面变化幅度均较小。式(2)~(6)均为消耗H+、使pH值升高的反应,根据溶液初始和终点pH值变化较小的实验结果,加之实验中未观察到气体产生,可以断定式(6)并未大量发生,pH值升高主要是由于式(3)或式(5)消耗H+离子所致,随铁粉“过量”系数增大,沉淀的砷量略有增多,导致终点pH值稍有增加。图4所示为铁粉“过量”系数试验中,溶液混合电势随时间的变化。由图4可见,铁粉“过量”系数对溶液混合电势基本没有影响。在所有实验中,反应前60 min,溶液混合电势从-250 mV增高至200 mV,其后基本不再变化,说明As(Ⅲ)被铁粉置换沉淀的反应已基本停止,而此时依铁粉“过量”系数不同,仅有5%~20%的砷沉淀。这可能与砷的沉淀导致铁粉表面钝化相关。
在图5所示试验滤渣XRD谱中,仅有金属铁的衍射峰,而未见沉淀砷的衍射峰,可能由于其含量较低或结晶度较差。
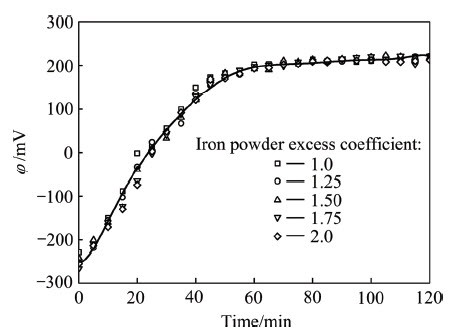
图4 不同铁粉“过量”系数下溶液混合电势随时间的变化曲线
Fig. 4 Changing curves of mixed electric potential in experimental solution with time at different iron powder excess coefficients
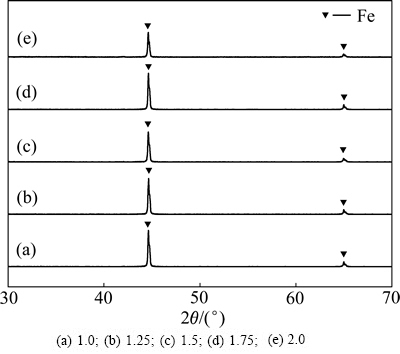
图5 Fe粉“过量”系数试验滤渣XRD谱
Fig. 5 XRD patterns of residues obtained at different iron powder excess coefficients
2.3 初始pH值对沉砷率的影响
在温度为40 ℃、铁粉“过量”系数为2、c[As(Ⅲ)]为5 g/L、反应时间为120 min的条件下,研究溶液初始pH值对沉砷率的影响,其结果如表2所列。
表2 不同溶液初始pH值下As的分配比及溶液终点pH值
Table 2 Distribution percent of arsenic and final solution pH value at different initial solution pH values

由表2可见,在实验条件下,溶液初始pH值从-0.31变化至4,均无ASH3生成,这进一步证实在酸性水溶液中,金属铁还原As(Ⅲ)生成AsH3的反应不会发生。从表2也可看出,溶液初始pH值对As在滤渣和溶液中的分配比有一定影响:溶液初始pH值从-0.31增大至3,沉淀进入滤渣的砷量占比从13.0%逐步增大至41.3%;而继续增大pH值到4,进入滤渣的砷量占比又逐步降低至18.1%。这说明在初始pH值为3的条件下,最有利于铁粉置换As(Ⅲ)使其沉淀,其原因可能与不同pH值下砷的沉淀形态不同,从而导致铁粉表面钝化情况不同有关。
图6所示为不同溶液初始pH值下实验滤渣的SEM像。由图6可见,不同溶液初始pH值下,所得滤渣形貌有显著差别。在pH值分别为-0.31、0、0.5、1和4的情况下,沉砷率均在20%以下,滤渣基本保持铁粉原有形貌(见图1);而在pH值为2、2.5、3和3.5的条件下,沉砷率较高,分别为27.6%、32.3%、41.3%和23.4%,滤渣形貌呈絮状,与铁粉原有形貌有显著差别。
图7所示为溶液初始pH值分别为0和3的滤渣的EDS分析结果。在图7(a)所示初始pH值为0的滤渣中只含有Fe、As,而在图7(b)所示的初始pH值为3.0的滤渣中,不仅含有Fe、As,还含有一定量O。在实验条件下,溶液中仅有Fe(Ⅱ)和As(Ⅲ)离子存在,文献[20]和实验研究均证实,它们之间不可能生成亚砷酸盐沉淀。该滤渣样品中含氧,可能是由于样品干燥中氧化所致。因此,可以推定砷的沉淀机理认为铁粉置换反应。
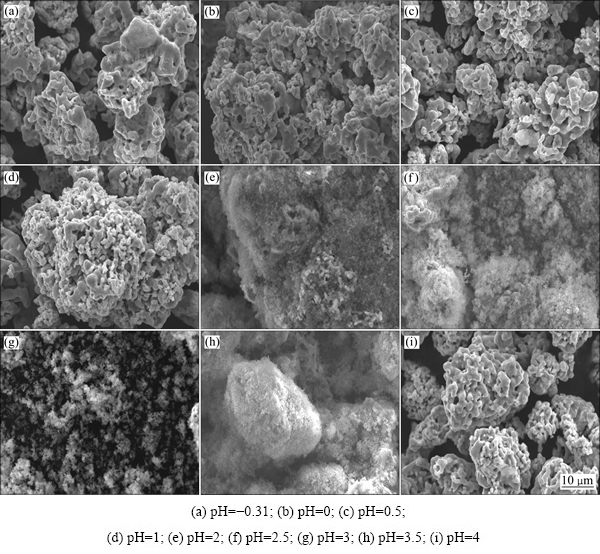
图6 不同溶液初始pH值下试验滤渣的SEM像
Fig. 6 SEM images of residues obtained in experiments at different initial solution pH values

图7 溶液初始pH值分别为0和3的滤渣的EDS分析结果
Fig. 7 EDS analysis results of residues obtained at different initial solution pH values
由图7及以上分析可知,置换沉淀的砷以单质砷(砷与铁之间无金属间化合物)存在,赋存状态有两种:一种为从铁粉表面脱落的元素砷,见图7(a)点A和图7(b)中点C;另一种为覆盖于铁粉表面的砷,见图7(a)中点B和图7(b)中点D。在pH为3的条件下,沉积的砷呈絮状松散附着在铁粉表面,既未对铁粉表面形成紧密包裹,使得反应可以持续进行,又较易从铁粉表面脱落,因此,可以达到较高的沉砷率;而在pH值为0的条件下,砷较为紧密地沉积在铁粉表面,阻滞了反应的延续,因而沉砷率较低。而图7(b)所示的初始pH值为3的试验滤渣中含有O,其原因可能在于以絮状存在的砷在滤渣干燥中氧化所致(见图7(b))。
图8所示为初始pH值分别为0和3的试验中,溶液混合电势随时间的变化。溶液初始pH值为0.0时,溶液混合电势随时间的变化,与图4所示规律相同,反应前60 min,溶液混合电势从-250 mV增高至200 mV,其后基本不再变化,提示铁粉置换As(Ⅲ)反应已终止;而当溶液初始pH为3时,在120 min试验中,溶液混合电势均维持在-300 mV左右,提示反应一直在持续进行。图8所示的结果有力地支持了砷的沉积形态不同,影响了沉砷反应发生和沉砷率的结论。
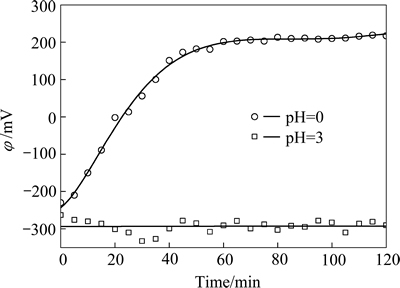
图8 初始pH值分别为0和3时溶液混合电势随时间的变化曲线
Fig. 8 Changing curves of mixed electro potentials in experimental solutions with time at different initial solution pH values
2.4 溶液初始As(Ⅲ)浓度对沉砷率的影响
在温度为40 ℃、溶液初始pH值为0、铁粉“过量”系数为2、反应时间为120 min的条件下,研究了溶液初始As(Ⅲ)浓度对沉砷率的影响,实验结果如表3所列,在实验条件范围内均无AsH3气体生成,溶液初始As(Ⅲ)浓度对沉砷率影响较小。
2.5 温度对沉砷率的影响
在溶液初始pH为0、初始As(Ⅲ)浓度为5 g/L、铁粉“过量”系数为2、反应时间为120 min的条件下,研究了温度对沉砷率的影响,结果如表4所列。由表4可见,在实验条件范围内,均无AsH3气体产生。温度对沉砷率有较大影响,升高温度有利于铁粉置换还原酸性溶液中的As(Ⅲ)。对不同温度下试验滤渣进行XRD分析,只有金属铁的衍射峰,说明砷以无定形元素砷沉淀。
表3 不同溶液初始As(Ⅲ)浓度下As的分配比
Table 3 Distribution percent of arsenic at different initial As(Ⅲ) concentrations

表4 不同温度下As的分配比
Table 4 Distribution percent of arsenic at different temperatures

图9所示为不同温度下实验滤渣的SEM像。由图9可见,在不同温度下,所得滤渣形貌有显著差别。在温度为20、40、60 ℃的情况下,沉砷率均在35%以下,滤渣基本保持铁粉原有形貌(见图1);而在80 ℃的条件下,沉砷率较高,为62.8%,而且该条件下的滤渣与铁粉原有形貌有显著差别,铁粉表面出现明显的丝絮状颗粒。
图10所示为80 ℃实验滤渣EDS分析结果。由图10可见,在80 ℃条件下,沉积的砷呈丝絮状松散附着在铁粉表面,既未对铁粉表面形成紧密包裹,使得反应可以持续进行,又较易从铁粉表面脱落,因此,在该条件下的沉砷率较高。而在20、40、60 ℃的条件下,砷较为紧密地沉积在铁粉表面,阻滞了反应的延续,因而,沉砷率较低。

图9 不同温度下滤渣的SEM像
Fig. 9 SEM images of residues obtained at different temperatures

图10 80 ℃下滤渣的EDS分析结果
Fig. 10 EDS analysis results of residues obtained at 80 ℃
3 结论
1) 在酸性水溶液中,As(Ⅲ)与金属铁反应生成元素As或AsH3气体,热力学分析结果均是可行的。
2) 在温度为20~80 ℃、溶液初始pH值为-0.31~4.0、溶液As(Ⅲ)浓度为1~20 g/L、铁粉大量过剩的条件下,实验中均未检测到Fe与As(Ⅲ)反应生成AsH3的反应发生。但铁粉可置换酸性溶液中的As(Ⅲ)离子,使其以元素砷的形态沉淀。
3) 铁粉“过剩”系数、溶液初始pH值和温度对Fe粉置换酸性溶液中As(Ⅲ)离子,生成元素砷的反应有显著影响。铁粉“过剩”系数大、温度高有利于沉砷,在初始pH值为3.0时,砷的沉淀率最高。
4) 实验初步证明,铁粉置换酸性溶液中As(Ⅲ)离子效率较低的原因在于随着反应的进行,元素砷覆盖于铁粉表面,阻滞了反应的延续。
REFERENCES
[1] MANDAL B K, SUZUKI K T. Arsenic round the world: A review[J]. Talanta, 2002, 58(1): 201-235.
[2] MATSCHULLAT J. Arsenic in the geosphere—A review[J]. Science of the Total Environment, 2000, 249(1): 297-312.
[3] HAN F X, SU Y, MONTS D L, PLODINEC M J, BANIN A, TRIPLETT G E. Assessment of global industrial-age anthropogenic arsenic contamination[J]. Naturwissenschaften, 2003, 90(9): 395-401.
[4] 王清宏, 翟秀静, 符 岩, 谢 锋. 镍, 锌, 铅及含砷金等矿物和渣的综合利用研究[J]. 有色矿冶, 2014, 30(1): 28-31.
WANG Qing-hong, ZHAI Xiu-jing, FU Yan, XIE feng. Comprehensive utilization research on nickle, zinc, lead, arsenic gold and some other minerals and slag[J]. Non-ferrous Mining and Metallurgy, 2014, 30(1): 28-31.
[5] BIAN X, YIN S H, LUO Y, WU W Y. Leaching kinetics of bastnaesite concentrate in HCl solution[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2306-2310.
[6] SOUZA A D, PINA P S, SANTOS F M F, SILVA C A, LEAO V A. Effect of iron in zinc silicate concentrate on leaching with sulfuric acid[J]. Hydrometallurgy, 2009, 95(3): 207-214.
[7] MORALES A, CRUELLS M, ROCA A, BERGO R. Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization[J]. Hydrometallurgy, 2010, 105(1): 148-154.
[8] AMER A M. Processing of copper anodic-slimes for extraction of valuable metals[J]. Waste Management, 2003, 23(8): 763-770.
[9] 李 倩, 成伟芳. 硫化砷渣的综合利用研究[J]. 广州化工, 2013, 41(13): 17-19.
LI Qian, CHENG Wei-fang. Study on comprehensive recycling of arsenic sulfide slag[J]. Guangzhou Chemical Industry, 2013, 41(13): 17-19.
[10] 王玉棉, 周 兴, 黄 雁, 赵忠兴. 黑铜泥酸性浸出及铜砷分离研究[J]. 兰州理工大学学报, 2012, 37(6): 19-22.
WANG Yu-mian, ZHOU Xing, HUANG Yan, ZHAO Zhong-xing. Research on acid leaching of black copper sludges and separation of copper and arsenic[J]. Journal of Lanzhou University of Technology, 2012, 37(6): 19-22.
[11] 何仕超, 刘志宏, 刘智勇, 李玉虎, 李启厚. 湿法炼锌窑渣铁精矿的浸出热力学分析[J]. 中国有色金属学报, 2013, 23(12): 3430-3439.
HE Shi-chao, LIU Zhi-hong, LIU Zhi-yong, LI Yu-hu, LI Qi-hou. Thermodynamic analysis of leaching of iron concentrate separated from kiln slag in zinc hydrometallurgy[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3430-3439.
[12] 王成彦, 邱定蕃, 江培海. 国内铋湿法冶金技术[J]. 有色金属, 2001, 53(4): 15-18.
WANG Cheng-yan, QIU Ding-fan, JIANG Pei-hai. Bismuth hydrometallurgy technology in China[J]. Nonferrous Metals, 2001, 53(4): 15-18.
[13] CULLEN W R, REIMER K J. Arsenic speciation in the environment[J]. Chemical Reviews, 1989, 89(4): 713-764.
[14] 1997GB. 车间空气中砷及其无机化合物卫生标准[S].
1997GB. Hygienic standard for arsenic and its inorganic compounds in the air of workplace[S].
[15] 仇勇海, 唐仁衡, 陈白珍. 砷化氢析出电势的探讨[J]. 中国有色金属学报, 2000, 10(1): 101-104.
QIU Yong-hai, TANG Ren-heng, CHEN Bai-zhen. Evolution potential of arsine[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(1): 101-104.
[16] 卢辉畴. 锌粉置换法从含高铜, 铅, 锌贵液中回收金的研究及生产实践[J]. 黄金, 2004, 25(4): 36-38.
LU Hui-chou. Research and productive practice on recovering gold from pregnant solution highly containing Cu, Pb, Zn by zinc dust precipitation[J]. Gold, 2004, 25(4): 36-38.
[17] 项斯芬, 严宣申, 庭 礼, 炳 南. 氮, 磷, 砷分族[M]. 北京: 科学出版社, 1995: 383-387.
XIANG Si-fen, YAN Xuan-shen, TING Li, BING Nan. Nitrogen, phosphorus and arsenic[M]. Beijing: Science Press, 1995: 383-387.
[18] 胡 杰. 萃取分离-碘滴定法测定粗铅中砷量[J]. 金属材料与冶金工程, 2010(3): 54-56.
HU Jie. Ascertaining of arsenium content in the rough lead by extracting separation-Iodine titration law[J]. Metal Materials and Metallurgy Engineering, 2010(3): 54-56.
[19] 李永常. 试论氢超电势[J]. 天津化工, 2003, 17(2): 61-62.
LI Yong-chang. Discussion about the hydrogen over potential[J]. Tianjin Chemical Industry, 2003, 17(2): 61-62.
[20] DRAHOTA P, FILIPPI M. Secondary arsenic minerals in the environment: A review[J]. Environment international, 2009, 35(8): 1243-1255.
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51404307)
收稿日期:2015-01-29;修订日期:2015-05-26
通信作者:刘智勇,讲师,博士;电话:0731-88830478;E-mail: csuliuzhiyong@163.com