碱式碳酸钴非等温热分解动力学
刘秉国1,2,彭金辉1,2,张利波1,2,张泽彪1,2,郭胜惠1,2,张世敏1,2
(1. 昆明理工大学 材料与冶金工程学院, 云南 昆明,650093;
2. 昆明理工大学 非常规冶金省部共建教育部重点实验室,云南 昆明,650093)
摘要:利用热重分析及X线衍射技术,考察不同升温速率下碱式碳酸钴的热分解特性,研究碱式碳酸钴热分解动力学。用模型函数法(Coats-Redfern和Achar法)和非模型法(Kissinger法和Flynn–Wall–Ozawa法)分析计算热分解过程第2阶段的动力学参数。研究结果表明:在空气气氛下,碱式碳酸钴的热分解过程分2个阶段进行(碱式碳酸钴在303 K左右开始失去结晶水;493 K时无水盐分解,至625 K以上基本分解完毕,产物为四氧化三钴),其中分解过程质量损失率为29.5%,与理论值相符;产物表观活化能E为106.139~144.537 kJ/mol,lg A(A为指前因子)为9.396~11.868,机理函数为成核与长大模型。
关键词:碱式碳酸钴;热分解;非等温动力学
中图分类号:O642;O643 文献标志码:A 文章编号:1672-7207(2011)02-0356-05
Non-isothermal kinetics of thermal decomposition of
basic cobalt carbonate
LIU Bing-guo1,2, PENG Jin-hui1,2, ZHANG Li-bo1,2, ZHANG Ze-biao1,2, GUO Sheng-hui1,2, ZHANG Shi-min1,2
(1. Faculty of Materials and Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. Key Laboratory of Unconventional Metallurgy, Ministry of Education,
University of Science and Technology, Kunming 650093, China)
Abstracts: Kinetics and thermal decomposition behavior of basic cobalt carbonate was investigated using TG and XRD at various heating rates. Kinetic parameters of decomposition of basic cobalt carbonate for the second-stage was calculated and analyzed using model-fitting (Coats-Redfern method and Achar method) and model-free (Kissinger method and Flynn-Wall-Ozawa method). The results show that the decomposition process of basic cobalt carbonate is divided into two stages: the crystallization water begins to dehydrate at 303 K, decomposition of basic cobalt carbonate follows at 493 K and changes into Co3O4 powder decomposition completely above 625 K in air atmosphere. The mass loss of decomposition of basic cobalt carbonate is 29.5%, which is in good agreement with the theoretical mass loss. Activation energy E and lg A are 106.139-144.537 kJ/mol and 9.396-11.868, respectively. The mechanism function is nucleation and growth model.
Key words: basic cobalt carbonate; thermal decomposition; non-isothermal kinetics
四氧化三钴作为重要的工艺材料,被广泛地应用于锂离子电池[1]、气体传感器[2]、催化剂[3]和磁性材 料[4]等领域。制备四氧化三钴的主要方法有热分解 法[5]、溶胶-凝胶法[6]和水热法[7]等。由于水热法制备四氧化三钴需要较高的温度和压力,同时对体系氧分压也有较高的要求,因此,限制了其工业化应用[8];溶胶-凝胶法尽管具有反应容易控制、产品纯度高、均匀性好等优点,但仍存在反应时间长、原料昂贵等不足[9-10]。目前,工业上多采用钴盐热分解制备四氧化三钴,该方法工艺简单,生产成本低,便于操作。碱式碳酸钴作为生产四氧化三钴的前驱体,尽管其热分解过程已有文献报道,且人们就温度对煅烧产物的影响也进行了详细研究[11],但在空气气氛下的热分解动力学参数研究尚不清楚,因此,开展碱式碳酸钴在空气气氛下的分解动力学研究对热分解法制备四氧化钴具有重要的意义。在此,本文作者用热分析技术对碱式碳酸钴在空气中的热行为进行研究,并利用非等温多重扫描速率法和模型函数法对热分解过程所遵循的动力学机理进行了探讨,旨在为碱式碳酸钴工业化应用提供理论依据。
1 试验部分
热重分析采用德国NETZSCH-STA 409 PC/PG型热分析仪,样品质量为20.0~20.5 mg,升温速率β分别为5,10,15和20 K/min,测量温度范围为300~1 073 K,空气气氛,流速为30 mL/min。采用日本理学公司的D/max-2200 Y型X线粉末衍射仪进行X线衍射分析,管电压为36 kV,管电流为30 mA,扫描速度为10 (°)/min。
碱式碳酸钴(2CoCO3?3Co(OH)2?xH2O)为上海国药集团化学试剂有限公司生产的化学纯,Co的质量分数为40%~50%,粒度小于150 μm,X线衍射分析结果见图1。

图1 碱式碳酸钴的X线衍射谱
Fig.1 XRD pattern of basic cobalt carbonate
2 结果与讨论
2.1 热行为和热分解机理
碱式碳酸钴在空气中不同升温速率下的TG和DTG曲线见图2和3。
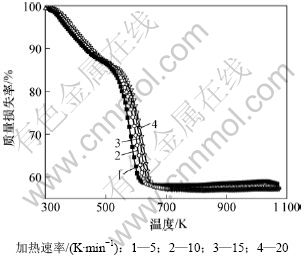
图2 碱式碳酸钴在不同加热速率下的TG曲线
Fig.2 TG curves of basic cobalt carbonate at various heating rates
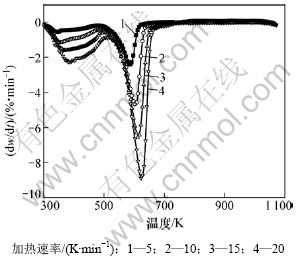
图3 碱式碳酸钴在不同加热速率下的DTG曲线
Fig.3 DTG curves of basic cobalt carbonate at various heating rates
由图2~3可知:当升温速率较小时,碱式碳酸钴在303 K左右开始分解,至607 K基本分解完全;随着升温速率的增大,碱式碳酸钴完全分解温度亦升高。但不同升温速率下的TG曲线均相吻合,说明其质量损失率基本一致,DTG曲线的2个峰与TG曲线上质量损失台阶一一对应。从碱式碳酸钴升温速率为10 K/min 的TG/DTG曲线计算可知,碱式碳酸钴在空气中的热分解过程为2步分解:第1个质量损失台阶出现在303~493 K,是一个缓慢的分解峰,为结晶水的脱除阶段,其质量损失率为13.16%,而碱式碳酸钴脱除结晶水的理论质量损失率为4%[11],造成实际质量损失率与理论质量损失率差异的原因可能是碱式碳酸钴吸附外在自由水所致;在493~625 K出现第2个质量损失台阶,质量损失率为29.5%(理论值为29.2%),归结于碱式碳酸钴无水盐的分解。碱式碳酸钴热分解过程可以表示如下[12]:
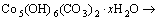
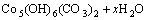 (1)
(1)
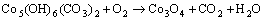 (2)
(2)
2.2 X线衍射分析
收集625 K下分解的残留物进行X线衍射分析,所得X线衍射谱如图4所示。由图4可知:625 K时分解产物的XRD谱与Co3O4标准谱相吻合,谱峰位置和强度与PDF卡(9-418)上的立方相Co3O4衍射数据(α=0.808 nm)一致,无任何杂质峰;所以,可以确定热分解产物为Co3O4,这与TG曲线所示结果一致。
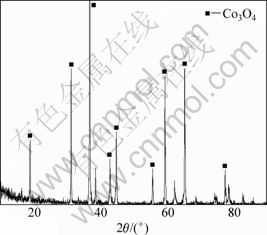
图4 热分解产物的X线衍射谱
Fig.4 XRD pattern of decomposition residues
2.3 非等温分解动力学
2.3.1 计算活化能E
固体分解反应动力学方程一般可表示为[13-14]:
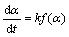 (3)
(3)
根据Arrhenius 公式 k=Aexp[E/(RT)],将其代入式(3)得:
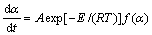 (4)
(4)
式中:α为转化率;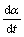 为分解速率;A为指前因子,s;E为活化能,kJ/mo1;T为热力学温度,K;f(α)为机理函数的微分形式。
为分解速率;A为指前因子,s;E为活化能,kJ/mo1;T为热力学温度,K;f(α)为机理函数的微分形式。
对于程序升温过程,升温速率 恒定,将
恒定,将 代入式(4)得Kissinger方程:
代入式(4)得Kissinger方程:
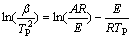 (5)
(5)
Flynn-Wall-Ozawa (F-W-O) 方程为:
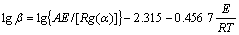 (6)
(6)
式中:R为气体摩尔常数;TP为峰顶热力学温度;g(a)为机理函数的积分形式。
通过非等温分解动力学研究可揭示碱式碳酸钴分解的反应机理,获得其最概然机理函数以及相应的动力学参数。在5,10,15和20 K/min不同升温速率下,碱式碳酸钴热分解第2阶段的DTG峰顶温度分别为588.358,603.462,613.316和623.469 K,用多重扫描速率法—Kissinger法和Flynn-Wall-Ozawa法处理这些数据,分别以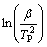 对
对 作图和以lg β对
作图和以lg β对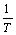 作图,如图5和图6所示。通过多项式拟合可获得第2阶段相应的活化能E和lg A,其中,由Kissinger法计算所得的活化能E为117.162 kJ/mol,lg A为9.464,相关系数r为0.998;由Flynn-Wall-Ozawa法计算所得的活化能见表1。表2列出了不同加热速率下碱式碳酸钴热分解过程的基本参数。
作图,如图5和图6所示。通过多项式拟合可获得第2阶段相应的活化能E和lg A,其中,由Kissinger法计算所得的活化能E为117.162 kJ/mol,lg A为9.464,相关系数r为0.998;由Flynn-Wall-Ozawa法计算所得的活化能见表1。表2列出了不同加热速率下碱式碳酸钴热分解过程的基本参数。
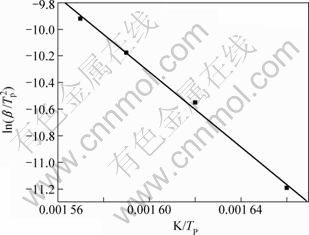
图5 碱式碳酸钴第2步热分解过程线性回归曲线
Fig.5 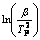 vs
vs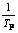 curve of thermal decomposition of basic cobalt carbonate of second steps
curve of thermal decomposition of basic cobalt carbonate of second steps
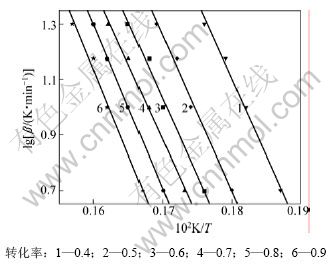
图6 不同转化率下的lg β-1/T曲线
Fig.6 lg β vs 1/T curves under various conversions
表1 用Flynn-Wall-Ozawa法得到的随转化率变化的表观活化能
Table 1 Apparent activation energy as function of conversion obtained by Flynn-Wall-Ozawa methods
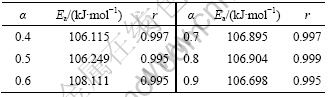
由表1可见:用Flynn-Wall-Ozawa法求得的反应活化能(0.4≤α≤0.9)比用Kissinger 法求得的略低。随着α的增大,分解反应的活化能变化不大,这说明在0.4≤α≤0.9范围内,碱式碳酸钴的分解反应可能是一步分解,这也与碱式碳酸钴的DTG曲线上的峰相吻合[15]。另外,碱式碳酸钴分解反应的相关系数r(0.4≤α≤0.9)几乎都在0.99~1.00之间,说明计算的活化能是可靠的。
2.3.2 机理函数的判定
对于单一的简单反应,最可几机理函数可以通过多元线性回归来判定。首先将实验数据进行微分和积分处理,然后应用Coats-Redfern法[16]和Achar法[17]结合40种机理函数[18],分别进行多元线性回归,计算出的活化能E与由Kissinger法得到的E最接近,且相关系数较好的反应机理即为该反应可能的动力学机理函数。
Coats-Redfern方程为:
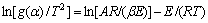 (7)
(7)
Achar方程为:
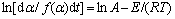 (8)
(8)
用积分法Coats-Redfern和微分法Achar结合40种机理函数,再根据热分解动力学三因子求算的比较法,得出碱式碳酸钴第2分解阶段的E,lg A和相关系数r见表3。
由表3可知:成核与长大模型机理函数对于Coats-Redfern方程和Achar方程的线性相关系数较好,且计算的活化能相近;同时与Kissinger法求得的活化能接近[19];因此,可以判定碱式碳酸钴第2步分解过程最可几机理函数的微分形式为:f(α)=5/2(1-α)? [-ln(1-α)]3/5;积分形式为:g(α)=[-ln(1-α)]2/5。
表2 不同升温速率下碱式碳酸钴热分解过程的基本数据
Table 2 Basic data of thermal decomposition of basic cobalt carbonate at various heating rates
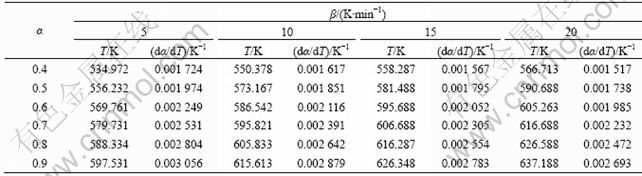
表3 积分法和微分法所得到的动力学参数
Table 3 Kinetics parameters of thermal decomposition obtained by integral and differential methods

3 结论
(1) 在空气气氛中,碱式碳酸钴的热分解过程分结晶水的脱除和无水盐的分解2个阶段,其最终分解产物为Co3O4。
(2) 在493~625 K时为碱式碳酸钴无水盐的分解过程,该过程质量损失率为29.5%,这与理论质量损失率相符。
(3) 用非等温多重扫描速率法和模型函数法分别计算碱式碳酸钴热分解过程第2分解阶段的动力学参数,其活化能E为106.139~144.537 kJ/mol,lg A为9.396~11.868,反应机理系成核与长大模型,机理函数为:f(α)=5/2(1-α)[-ln(1-α)]3/5;g(α)= [-ln(1-α)]2/5。
参考文献:
[1] Wang G X, Chen Y, Konstantinov K, et a1. Investigation of cobalt oxides as anode materials for Li-ion batteries[J]. Journal of Power Sources, 2002, 109(1): 142-147.
[2] LI Wei-yang, XU Li-na, CHEN Jun. Co3O4 nano-materials in lithium-ion batteries and gas sensors[J]. Advanced Functional Materials, 2005, 15(5): 85l-857.
[3] Wang C B, Tang C W, Gau S J, et a1. Effect of the surface area of cobaltic oxide on carbon monoxide oxidation[J]. Catalysis Letters, 2005, 101(1/2): 59-63.
[4] Ichiyanagi Y, Kimishima Y, Yamada S. Magnetic study on Co3O4 nanoparticles[J]. Journal of Magnetism and Magnetic Materials, 2004, 272/276(Suppl 1): E1245-E1246.
[5] WANG Wei-wei, ZHU Ying-jie. Microwave-assisted synthesis of cobalt oxalate nanorods and their thermal conversion to Co3O4 rods[J]. Materials Research Bulletin, 2005, 40(11): 1929-1935.
[6] CAO Jin-zhang, ZHAO Yan-chun, YANG Wu, et a1. Sol-gel preparation and characterization of Co3O4 nanocrystals[J]. Journal of University of Science and Technology Beijing, 2003, 10(1): 54-57.
[7] Meskin P E, Baranchikov A E, Ivanov V K, et a1. Synthesis of nanodisperse Co3O4 powders under hydrothermal conditions with concurrent ultrasonic treatment[J]. Doklady Chemistry, 2003, 389(1/3): 62-64.
[8] 石迪辉. 四氧化三钴的制备对钴酸锂性能的影响[D]. 长沙: 中南大学冶金科学与工程学院, 2006: 3-7.
SHI Di-hui. The effect of preparation of Co3O4 on LiCoO2 property[D]. Changsha: Center South University. School of Metallurgical Science and Engineering, 2006: 3-7.
[9] 田秀淑, 吕臣敬, 王黔平. 溶胶-凝胶法制备氧化铝系复合膜的研究进展[J]. 江苏陶瓷, 2006, 39(1): 7-10.
TIAN Xiu-shu, L? Cheng-jin, WANG Qian-ping. Advances of Al2O3 composite membrane prepared by sol-gel method[J]. Jiangsu Ceramic, 2006, 39(1): 7-10.
[10] 宋继芳. 溶胶-凝胶技术的研究进展[J]. 无机盐工业, 2005, 37(11): 14-17.
SONG Ji-fang. Progress in research on sol-gel technology[J]. Inorganic Chemicals Industry, 2005, 37(11): 14-17.
[11] 杨幼平, 刘人生, 黄可龙, 等. 碱式碳酸钴热分解制备四氧化三钴及其表征[J]. 中南大学学报: 自然科学版, 2008, 39(1): 108-111.
YANG You-ping, LIU Ren-sheng, HUANG Ke-long, et al. Preparation and characterization of Co3O4 by thermal decomposition from Co2(OH)2CO3[J]. Journal of Central South University: Science and Technology, 2008, 39(1): 108-111.
[12] 杨毅涌, 孙聚堂, 袁良杰, 等. 碱式碳酸钴热分解产物的微量粉末X射线衍射分析[J]. 武汉大学学报, 2001, 47(6): 660-662.
YANG Yi-yong, SUN Ju-tang, YUAN Liang-jie, et al. Micro-method powder X-ray diffraction analysis of thermal decomposition product of basic cobalt carbonate[J]. Journal of Wuhan University, 2001, 47(6): 660-662.
[13] Brown M E, Dollimore D, Galwey, et a1. Reactions in the solid state in comprehensive chemical kinetics[M]. Amsterdam: Elsevier, 1980: 41-113.
[14] Galwey A K, Brown M E. Thermal decomposition of Ionic solids[M]. Amsterdam: Elsevier, 1999: 75-77.
[15] ZHANG Yu-dong, LI Bin jie, XU Xiang min. Thermal decomposition kinetics of ZnSn(OH)6[J]. J Acta Phys Chim Sin, 2007, 23(7): 1095-1098.
[16] Achar B N, Brindley G W, Sharp J H. Kinetics and mechanism of dehydroxylation processes(III): Applications and limitations of dynamic methods[C]//Proceedings of International Clay Conference. Jerusalem, 1996: 67-73.
[17] Coats A W, Redfern J P. Kinetic parameters from thermogravimetric data[J]. Nature, 1964, 201: 68-69.
[18] 胡荣祖, 史启祯. 热分析动力学[M]. 北京: 科学出版社, 2001: 151-155.
HU Rong-zu, SHI Qi-zhen. Thermalanalysis kinetics[M]. Beijing: Science Press, 2001: 151-155.
[19] 罗世永, 王德君, 张家芸, 等. 草酸氧钛锶的热分解动力学[J].功能材料, 2000, 31(6): 664-666.
LUO Shi-yong, WANG De-jun, ZHANG Jia-yun, et al. Thermolysis kinetics of strontium titanyl oxalate[J]. Functional Materials, 2000, 31(6): 664-666.
(编辑 陈爱华)
收稿日期:2009-11-03;修回日期:2010-03-22
基金项目:国家自然科学基金重点资助项目(50734007);云南省科技计划项目(2007GA002);昆明理工大学分析测试基金资助项目(2008-16)
通信作者:彭金辉(1964-),男,云南昆明人,教授,从事微波冶金及资源综合利用等研究;电话:0871-5191046; E-mail:Jhpeng.ok@Yeah.net