Bioleaching of chalcopyrite by UV-induced mutagenized Acidiphilium cryptum and Acidithiobacillus ferrooxidans
来源期刊:中国有色金属学报(英文版)2010年第2期
论文作者:徐爱玲 夏金兰 张 帅 杨 宇 聂珍媛 邱冠周
文章页码:315 - 321
Key words:Acidiphilium cryptum; Acidithiobacillus ferrooxidans; UV-induced mutagenesis; bioleaching; chalcopyrite
Abstract: The original strains Acidithiobacillus ferrooxidans GF and Acidiphilium cryptum DX1-1 were isolated from the drainage of some caves riched in chalcopyrite in Dexing Mine in Jiangxi Province of China. The optimum temperature and pH for growth were 30 ℃ and 3.5 for Ac. cryptum DX1-1, and 30 ℃ and 2.0 for At. ferrooxidans GF, respectively. For Ac. cryptum DX1-1, the optimum UV radiating time was 60 s and the positive mutation rate was 22.5%. The growth curves show that Ac. cryptum after mutagenesis reached stationary phase within 60 h, which was 20 h earlier than the original strain. For At. ferrooxidans GF, the optimum mutation time was 60 s and the positive mutation rate was 35%. The most active UV-mutated strain At. ferrooxidans GF oxidized all the ferrous after 48 h. The bioleaching experiments showed that bioleaching with the mixture of UV-mutated strains of At. ferrooxidans GF and Ac. cryptum DX1-1(1:1) could extract 3.01 g/L of copper after 30 d, while the extracted copper was 2.63 g/L with the mixture of the original strains before UV-mutation. At the end of the bioleaching experiments, the proportion of the cell density in the cultures of Ac. cryptum DX1-1 and At. ferrooxidans GF was approximately 1:5.
基金信息:the National Basic Research Program of China
the National Natural Science Foundation of China

XU Ai-ling(徐爱玲), XIA Jin-lan(夏金兰), ZHANG Shuai(张 帅),
YANG Yu(杨 宇), NIE Zhen-yuan(聂珍媛), QIU Guan-zhou(邱冠周)
Key Laboratory of Biometallurgy of Ministry of Education of China,
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 16 February 2009; accepted 8 April 2009
Abstract: The original strains Acidithiobacillus ferrooxidans GF and Acidiphilium cryptum DX1-1 were isolated from the drainage of some caves riched in chalcopyrite in Dexing Mine in Jiangxi Province of China. The optimum temperature and pH for growth were 30 ℃ and 3.5 for Ac. cryptum DX1-1, and 30 ℃ and 2.0 for At. ferrooxidans GF, respectively. For Ac. cryptum DX1-1, the optimum UV radiating time was 60 s and the positive mutation rate was 22.5%. The growth curves show that Ac. cryptum after mutagenesis reached stationary phase within 60 h, which was 20 h earlier than the original strain. For At. ferrooxidans GF, the optimum mutation time was 60 s and the positive mutation rate was 35%. The most active UV-mutated strain At. ferrooxidans GF oxidized all the ferrous after 48 h. The bioleaching experiments showed that bioleaching with the mixture of UV-mutated strains of At. ferrooxidans GF and Ac. cryptum DX1-1(1?1) could extract 3.01 g/L of copper after 30 d, while the extracted copper was 2.63 g/L with the mixture of the original strains before UV-mutation. At the end of the bioleaching experiments, the proportion of the cell density in the cultures of Ac. cryptum DX1-1 and At. ferrooxidans GF was approximately 1?5.
Key words: Acidiphilium cryptum; Acidithiobacillus ferrooxidans; UV-induced mutagenesis; bioleaching; chalcopyrite
1 Introduction
Recently, some microorganisms, which can grow in the extreme circumstance, have attracted considerable attention because of their unusual physiology and ecology. Yet, only a modest number of iron- and sulfur- oxidizing bacteria have been isolated from sulfide ores environments, characterised physiologically and phylogenetically, and deposited in data banks[1-3]. Among them, iron/sulfur-oxidizing At. ferrooxidans is considered to play the most important role in natural leaching of sul?de minerals[4-5] and is widely used in biohydrometallurgical processes[6-7]. In contrast, iron-reducing bacteria have been shown to accelerate the reductive dissolution of ferric iron-containing minerals, such as jarosites and goethite. These include heterotrophic mesophilic bacteria, such as Acidiphilium spp.[8-9]. Interestingly, all strains of Acidiphilium spp. appear to be able to catalyze the dissimilatory reduction of ferric iron to ferrous, though in some strains this is constitutive while in others it is inducible[10].
Leaching reactions that are catalyzed by these bacteria above, however, are relatively inef?cient because of their slow growth rate, low cell densities, and inhibition of iron oxidation by ferric iron[11]. Such drawbacks demand the improvement of leaching bacteria.
The UV-induced mutagenesis is a frequently-used and effective method for breeding. The pyrimidine bases have strong UV absorption. When UV ray is absorbed by them, the neighboring double thymines in the chain of DNA will form thymine dimer that mainly causes the mutations. The following is the mechanism. When the reproducing of DNA begins, if it is single thymine dimer, the site of the thymine dimer will possibly run over and the gap will be left, which makes the false bases inserted, causing the mutation of AT→GC. As the DNA of At. ferrooxidans contains 46%-47% of AT bases[11], while Ac. cryptum contains 37%-40% of AT bases in its DNA,there will be many neighbouring double thymines which possibly induce mutagenesis.
In this work, the original strains At. ferrooxidans GF and Ac. cryptum DX1-1 are collected from the drainages of some caves rich in chalcopyrite in Dexing Mine in Jiangxi Province, China[12]. The natural pH is 2.0, the temperature is 21 ℃, the bacteria quantity is 6×109 L-1, and the cupric concentration is 0.1 g/L. These show that these original strains are characterized by natural oxidative activity of copper-containing sulfides and high natural tolerance of cupric ions.
2 Materials and methods
2.1 Culture media and microorganisms
At. ferrooxidans GF(DQ062115) and Ac. cryptum DX1-1 (DQ529311) used in the experiments were isolated and conserved by our laboratory from acid mine drainage(AMD) taken from a reservoir in Dexing Mine, Jiangxi Province, China. Strain GF was grown in medium 9K (containing the following reagents: (NH4)2SO4 3 g/L, KCl 0.1 g/L, K2HPO4 0.5 g/L, MgSO4·7H2O 0.5 g/L, Ca(NO3)2 0.01 g/L) with initial pH value of 2.0 and with 4.5% FeSO4 as the energy source. Strain DX1-1 was maintained in 9K medium (pH 3.0) with 1.5% glucose as the energy source. Serial dilution was adopted to isolate the microorganism on the agar solid medium (1.5%) (w/v). The appropriately diluted samples were monitored by counting with Hematocyte Counter.
2.2 UV mutagenesis of Ac. cryptum DX1-1 and At. ferrooxidans GF
The experiment was carried out under the following conditions. The UV wavelength was 2 537 ?, the power was 15 W and the distance was 30 cm, and the radiation time was 0, 30, 60, 120, 180 and 240 s, respectively. The cultures were radiated by UV and stirred with bent rod. 1 mL of culture treated by UV was transferred to plate for kill rate assaying. 1 mL of culture treated by UV was transferred into 100 mL of liquid medium in a 250 mL of Erlenmeyer flask added with 0.3% lithium chloride to assist the mutagenesis. The effectiveness of the positive mutation for strain Ac. cryptum DX1-1 was determined by the generation time, and for strain GF by the oxidative rate of ferrous ion. The ability of strain At. ferrooxidans GF to oxidize Fe2+ was tested in 250 mL shake flasks with 100 mL medium at 30 ℃, pH 2.0. In the Fe2+ oxidation experiment, 30 g/L FeSO4·7H2O was added into the medium (with 6.08 g/L Fe2+).
2.3 Bioleaching
The mineral sample used in the experiments contains about 60.8% chalcopyrite and the component is listed in Table 1. The mineral was crushed, and subsequently washed with acetone and ethanol[13].
Table 1 Component of chalcopyrite (mass fraction, %)
![]()
Bioleaching tests were carried out in 250 mL flasks containing 100 mL medium. The 9K basal salts medium without iron was used in sulfide minerals bioleaching experiments. The mineral concentration was 5% (w/v). The experiments were carried out in triplicate.
Cells were harvested by centrifugation and washed twice in distilled water adjusted to pH 2.0 with sulfuric acid. The cells were then suspended in basal salts medium without energy sources. Bioleaching tests at 30 ℃ with initial pH 2.5 were performed using the following six inocula: 1) Ac. cryptum DX1-1 after UV-induced mutagenesis, 2) At. ferrooxidans GF before UV-induced mutagenesis, 3) At. ferrooxidans GF after UV-induced mutagenesis, 4) the mixture of the two strains (1?1) before UV-induced mutagenesis, 5) the mixture of the two strains (1?1) after UV-induced mutagenesis, and 6) control.
The cell density of each organism in culture medium after inoculation was about 1×107 cells/mL. The abiotic controls were also designed.
2.4 Analytical methods
Physicochemical analyses of the water samples were performed at the Testing Center of Central South University, China. Flame atomic absorption spectrometry was used for measuring metal ions. pH value was measured with a pH meter (pHs-25). Copper ion concentrations were determined by atomic absorption spectrometry within 30 d of incubation. The oxidative rate of ferrous ions was determined by titration with potassium dichromate (K2Cr2O7), the kill rate by plating, and the effectiveness of the positive mutation of Ac. cryptum DX1-1 and At. ferrooxidans GF by the generation time and the oxidative rate of ferrous ions, respectively.
2.5 Analysis of community structure in leachate at end of bioleaching
2.5.1 DNA extraction and purification[14]
The microorganisms of samples were isolated after filtration of leachate through 0.2 ?m nylon filters. DNA was extracted using EZ-10 Spin Column Genomic DNA Isolation Kit (Bio Basic Inc) and purified using Wizard DNA Clean-Up Kit (Promega).
2.5.2 16S rRNA gene amplification and cloning
Two primers were used to amplify 16S rRNA gene fragment: forward primer 27F (5′-AGAGTTTGATCC- TGGCTCAG-3′) and reverse primer 1492R (5′-GGT- TACCTTGTTACGACTT-3′)[15]. The PCR products were visualized by 1.0% low-melting-point agarose gel electrophoresis, and purified using Wizard DNA Clean-Up Kit (Promega). The purified 16S rRNA gene fragments were ligated into the vector PCR2.1 TOPO, then transformed into E. coli TOP10F’ competent cells according to the manufacturers’ instructions (Invitrogen). Recombinants were identified based on blue-white screening, and grown overnight in Luria-Bertani (LB) agar plates containing appropriate amounts of ampicillin, X-gal and IPTG at 37 ℃. Next, 60 white colonies were randomly selected, and the recombinant plasmids containing 16S rRNA gene fragments were reamplified by PCR using the vector primers M13F (5’-GTAAAA- CGACGGCCAGTG-3’) and M13R (5’-GGAAACAGC- TATGACCATG-3’).
2.5.3 Amplified ribosomal DNA restriction analysis (ARDRA)
The amplified rRNA PCR products were digested with HindI and MspI (Fermentas) overnight at 37 ℃.
The resulting ARDRA products were separated by gel electrophoresis in 3.0% agarose. The ARDRA patterns were visualized by UV excitation, and were compared with the ARDRA patterns from YANG et al[12].
3 Results and discussion
3.1 Geochemical analyses of AMD
There was 4.401 g/L sulfur in the AMD which was one of the substances that supplied energy for Ac. cryptum DX1-1 in the former experiments[13]. Fe3+ was one of those substances too, which was one reason for Ac. cryptum DX1-1 enhancing bioleaching. The pH value was 2.0 and temperature was 21℃ when AMD samples were collected.
3.2 Microorganism strains
The study on effects of temperature and pH on the growth of strains Ac. cryptum DX1-1 and At. ferrooxidans GF are shown in Figs.1 and 2, respectively. It shows that the optimum condition was 30 ℃, pH 3.5 for strain DX1-1, while that was 30 ℃, pH 2.0 for strain GF). Ac. cryptum DX1-1 and At. ferrooxidans GF had different optimum pH values. We chose pH 2.5 in the bioleaching experiment because At. ferrooxidans GF plays more important role in bioleaching. Phylogenetic tree based on 16S rDNA sequences shows the location of the two strains (Fig.3). GenBank accession numbers are provided in parentheses.
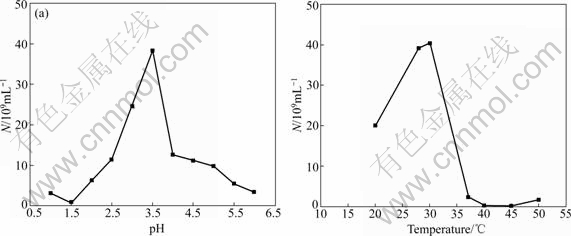
Fig.1 Effects of pH and temperature on growth of strain Ac. cryptum DX1-1
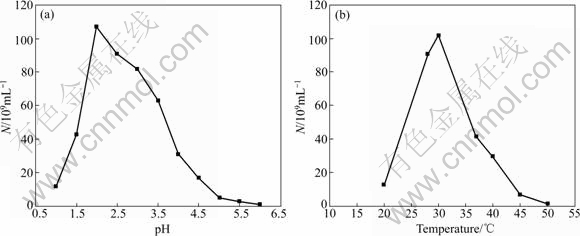
Fig.2 Effects of pH and temperature on growth of strain At. ferrooxidans GF
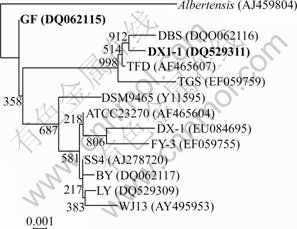
Fig.3 Phylogenetic tree based on 16S rDNA sequences, showing location of strain DX1-1 and strain GF (GenBank accession numbers are provided in parentheses)
3.3 UV-induced mutagenesis of Ac. cryptum DX1-1 and At. ferrooxidans GF
3.3.1 UV-induced mutagenesis of Ac. cryptum DX1-1
The inhibitive rates of colonies (UV kill rate) and the positive mutation rate for Ac. cryptum DX1-1 are listed in Table 2. It is shown that the longer the radiation time of UV, the higher the kill rate of the strain, i.e. the less the alive. For Ac. cryptum DX1-1 the optimization time was 60 s and the positive mutation rate was 22.5%. In this study, the positive mutated colonies had higher cell density at stationary phase than the original strain.
Table 2 Effect of UV-induced mutagenesis for Ac. cryptum DX1-1
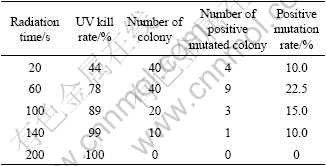
As we chose the big colonies to determine whether it was positive mutation or not, the process was also a rejuvenated process. So, the positive mutation rate we determined was higher than the actual rate.
From the positive mutation, we chose the strain that had the shortest generation time to leach the minerals. The growth curves of Ac. cryptum DX1-1 before and after UV-induced mutagenesis are shown in Fig.4. It is shown that the strain after mutagenesis reached stationary phase after 60 h, which was 20 h earlier than the original strain. It had higher cell density (1010/mL) than the original strain (109/mL).
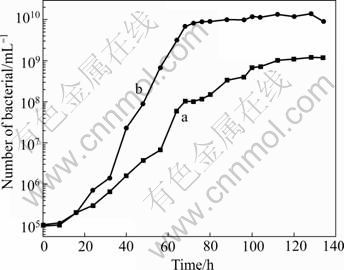
Fig.4 Growth curves of Ac. cryptum DX1-1 before (a) and after (b) UV-induced mutagenesis
3.3.2 UV-induced mutagenesis of At. ferrooxidans GF
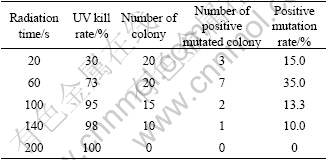
The inhibitive rates of colonies and the positive mutation rates for At. ferrooxidans GF are listed in Table 3. It was shown that, like Ac. cryptum DX1-1, the longer the radiation time of UV, the higher the kill rate of the strains. The optimization time was 60 s and the positive mutation rate was 35% for At. ferrooxidans GF. For At. ferrooxidans GF, the positive mutated colonies had higher oxidization rate than the original strain.
Table 3 Effect of UV-induced mutagenesis for At. ferrooxidans GF
From the positive mutation colonies, we chose the strain that had the highest oxidization rate to leach the minerals. The Fe2+ oxidization rate curves of the strain At. ferrooxidans GF before and after UV irradiation are shown in Fig.5. It is shown that the UV treated strain oxidized all the ferrous ions within 48 h, while the original strain needed 54 h.
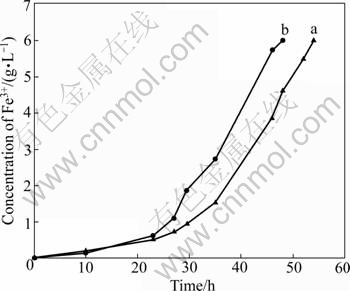
Fig.5 Fe2+ oxidation rate curves of strain At. ferrooxidans GF before (a) and after (b) UV-induced mutagenesis
The most favorable dose of UV irradiation is related to the character of strains, and to the property of genes as well. It is demonstrated by this study that the low dose of UV irradiation is the most suitable to the gene of At. ferrooxidans GF and Ac. cryptum DX1-1. The mechanism is probably that middle and high dose of UV irradiation damages the genes more seriously, so that it is hard to rescue the function of these genes, and more negative mutations are induced. At low temperature, not only the activity of the repairing enzymes can be inhibited, but also the time of reproducing, isolation and the forming of homozygous mutants can be satisfied.
3.4 Bioleaching experiment
As an acid-soluble metal sulfide, chalcopyrite is probably dissolved by the ferric iron and proton attack [16-19]. Ferric iron oxidizes chalcopyrite to copper, ferrous ions and elemental sulfur in solution. This reaction is illustrated as follows:
CuFeS2+4Fe3+→Cu2++2S+5Fe2+ (1)
At. ferrooxidans are capable of oxidizing the ferrous ions formed in the leaching reaction by the following reaction:
4Fe2++4H++O2→4Fe3++2H2O (2)
The sulfur formed in the above reaction can be oxidized to sulfate by At. ferrooxidans as the following reaction:
2S+3O2+2H2O→2SO42-+4H+ (3)
In addition to the bioleaching progress, chalcopyrite may be oxidized as Eq.(4) in sulphuric acid solution, where the sulfur formed by chemical leaching is also oxidized by Eq.(3).
CuFeS2+4H++O2→Cu2++Fe2++2S+2H2O (4)
From the above results it can be seen that At. ferrooxidans are propitious to incessancy of the reaction. At the beginning of bioleaching, sulfuric acid is added into reactor to keep pH value around 2.5 for acid consumption. After 6 days, since acid is produced during the oxidation of sulfur and the hydrolysis of ferric iron to ferric species such as Fe(OH)2+ and Fe(OH)2+[20], the pH decreases continuously.
As the bioleaching continues, the precipitation of jarosite and sulfur compounds (forming a layer on mineral surface) may hinder chalcopyrite dissolution[21]. Acidiphilium is able to reduce a wide range of Fe(Ⅲ)- (hydr)oxides including akagenite, jarosite, natrojarosite, amorphous ferric hydroxide, and the mixed ferrous/ferric mineral magnetite[10]. The existence of Acidiphilium dissolves the hard layer on mineral surface, which leads to further extraction of copper.
The bioleaching result of chalcopyrite with the strains Ac. cryptum DX1-1 and At. ferrooxidans GF is shown in Fig.6. In the whole process, the copper extraction continuously increases. In the first 15 d, the copper extraction rate increased quickly. From the 16th day to the 30th day, the copper extraction became slow. The first 15 d was the main stage of bioleaching of chalcopyrite, when the copper extraction increased remarkably. The jarosite and sulfur compounds hinder the chalcopyrite dissolution. Therefore, copper extraction in the last 15 d became slow. Bioleaching with strain Ac. cryptum DX1-1 after UV-induced mutagenesis could extract copper 0.49 g/L in 30 d. With the strain At. ferrooxidans GF before UV-induced mutagenesis, the extraction of copper reached 1.77 g/L in 30 d, while it was 1.98 g/L with the UV-induced mutant strain. Bioleaching with the mixture of strain At. ferrooxidans GF and Ac. cryptum DX1-1 (1:1) before UV-induced mutagenesis could extract copper 2.63 g/L after 30 d, while we got 3.01 g/L copper with the mixture of the two strains after UV-induced mutagenesis. UV-induced mutagenesis is helpful to improving the oxidization activity of At. ferrooxidans and makes it propitious to bioleaching. When the two strains were used in bioleaching, the effect was not the simple sum of the effect of bioleaching by the two strains independently, but they can stimulate each other. Ac. cryptum accelerated the reductive dissolution of jarosites and goethite, and they also consumed the organic compound which restrained the growth of At. ferrooxidans. So, they can assist At. ferrooxidans in bioleaching. They do not oxidize ferrous iron[22]. So Ac. cryptum had bad performance when they were used as the only bacterium in bioleaching.
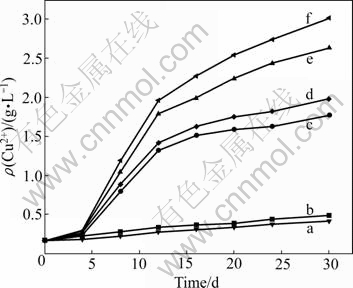
Fig.6 Copper extracted from chalcopyrite by Ac. cryptum DX1-1 after UV-induced mutagenesis (b), At. ferrooxidans GF before UV-induced mutagenesis (c) , At. ferrooxidans GF after UV-induced mutagenesis (d), mixture of two strains before UV-induced mutagenesis (e), mixture of two strains after UV-induced mutagenesis (f), and control (a)
3.5 Analysis of community structure in leachate at end of bioleaching
ARDRA patterns are shown in Fig.7. It shows that, at the end of the bioleaching experiments, the proportion of the cell density of Ac. cryptum DX1-1 and At. ferrooxidans GF became 4:19 in the leaching culture by the two strains before UV-induced mutagenesis and 3?17 in the leaching culture by the two strains after UV-induced mutagenesis. Contrary to that, it is worth to note that at the beginning of the bioleaching experiment, Ac. cryptum DX1-1 had the same cell density as At. ferrooxidans GF. This means that Ac. cryptum DX1-1 is not a prevailing bacterium but an assistant in bioleaching and has a small proportion. So when design bioleaching experiments next, we can prepare a mixture in which At. ferrooxidans is in predominated number, and at the same time to which put some Ac. cryptum in an approximate proportion of 1?5 to At. ferrooxidans.

Fig.7 ARDRA patterns of Ac. cryptum DX1-1 (a) and At. ferrooxidans GF (b) (M was marker)
4 Conclusions
1) Strains Ac. cryptum DX1-1 and At. ferrooxidans GF were isolated. They had the optimal growth temperature 30 ℃, and the optimal pH values 3.5 and 2.0, respectively.
2) The optimum mutagenesis time was 60 s for both Ac. cryptum DX1-1 and At. ferrooxidans GF. After the mutagenesis the positive mutation rate for these two strains was 22.5% and 35%, respectively. Strain Ac. cryptum DX1-1 after mutagenesis reached the stationary phase after 60 h, which is 20 h earlier than the original strain. It also had higher cell density than the original strain. Strain At. ferrooxidans GF after mutagenesis had higher ferrous ion oxidizing capability than the original strain.
3) Bioleaching with the mixture of strains At. ferrooxidans GF and Ac. cryptum DX1-1 (1?1) after UV-induced mutagenesis could extract copper 3.01 g/L after 30 d, which was higher than that extracted with At. ferrooxidans GF only. And also more copper can be leached out by using the mixture after UV-induced mutagenesis than by the two strains before UV-induced mutagenesis.
4) The ARDRA patterns showed that the proportion of the cell density of strains Ac. cryptum DX1-1 and At. ferrooxidans GF became about 1?5 in the culture at the end of the bioleaching experiments. We can follow this proportion to inoculate these two strains during bioleaching.
References
[1] RAWLING D E. Heavy metal mining using microbes [J]. Annu Rev Microbiol, 2002, 56: 65-91.
[2] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84(1/2): 81-108.
[3] JOHNSON D B, HALBERG K B. The microbiology of acidic mine waters [J]. Res Microbiol, 2003, 154: 66-473.
[4] KELLY D P, WOOD A P. Reclassi?cation of some species of Thiobacillus to the newly described genera Acidithiobacillus gen.nov., Hallothiobacillus gen. nov. and Thermithiobacillus gen. nov [J]. Int J Syst Evol Microbiol, 2000, 50: 11-516.
[5] RAWLING D E, SILVER S. Mining with microbes [J]. Biotechnology, 1995, 13: 73-778.
[6] RAWLING D E. The molecular genetics of Thiobacillus ferrooxidans and other mesophilic, acidophilic, chemolithotrophic, iron- or sulfur-oxidizing bacteria [J]. Hydrometallurgy, 2001, 59(2/3): 87-201.
[7] TOMA A E. The role of Thiobacillus ferrooxidans in hydrometallurgical processes [J]. Adv Biochem Eng Biotechnol, 1977: 1-37.
[8] BRIDGE T A M, JOHNSON D B. Reductive dissolution of ferric iron minerals by Acidiphilium SJH [J]. Geomicrobiol, 2000, 17: 193-206.
[9] BRIDGE T A M, JOHNSON D B. Reduction of soluble iron and reductive dissolution of ferric iron-containing minerals by moderately thermophilic iron-oxidizing bacteria [J]. Appl Environ Microbiol, 1998, 64: 2181-2190.
[10] JOHNSON D B, BRIDGE T A M. Reduction of ferric iron by acidophilic heterotrophic bacteria: Evidence for constitutive and inducible enzyme systems in Acidiphilium spp. [J]. Appl Microbiol, 2002, 92: 15-321.
[11] KULPA C F, ROSKEY M, MJOL I. Construction of genomic libraries and induction of iron oxidation in Thiobacillus ferrooxidans [J]. Biotechnol Appl Biochem, 1986, 8: 330-341.
[12] YANG Yu, XU Ai-ling, ZHANG Yan-fei. Isolation and characteriation of a facultative autotrophic bacterial strain and its cellular polymer granules from acid mine drainage [J]. Wuhan University Journal of Natural Science, 2007, 53(6): 753-758. (in Chinese)
[13] McGUIRE M M, EDWARDS K J, BANFIELD J F. Kinetics surface chemistry, and structural evolution of microbially mediated sulfide mineral dissolution [J]. Geochim Cosmochim Acta, 2001, 65(8): 243-1258.
[14] YANG Yu, WAN Min-xi, SHI Wu-yang. Bacterial diversity and community structure in acid mine drainage from Dabaoshan Mine, China [J]. Aquat Microb Ecol, 2007, 47: 141-151.
[15] LANE D J. 16S/23S rRNA sequencing [M]// STACKEBRANDT E, GOODFELLOW M. Nucleic acid techniqe in Bacterial system. New York: Jone Wiley & Sons, Inc, 1991: 115-175.
[16] ROHWERDER T, GEHRKE T, KINZLER K. Bioleaching review (part A): Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Appl Microbiol Biotechnol, 2003, 63: 239-248.
[17] SUZUKI I. Microbial leaching of metals from sulfide minerals [J]. Biotechnol Adv, 2001, 19(2): 19-132.
[18] SCHIPPERS A, SAND W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur [J]. Appl Environ Microbiol, 1999, 65: 319-321.
[19] BEVILAQUA D, LEITE A, GARCIA O Jr. Oxidation of chalcopyrite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in shake flasks [J]. Process Biochem, 2002, 38: 587-592.
[20] SANDSTORM A. PETERSSON S. Bioleaching of a complex sulphide ore with moderate thermophilic and extreme thermophilic microorganism [J]. Hydrometallurgy, 1997, 46(1/2): 181-190.
[21] STOTT M B, WATLING H R, FRANZMANN P D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching [J]. Minerals Engineering, 2000, 13: 1117-1127.
[22] KIRSTEN K. Microbial cycling of iron and sulfur in acidic coal mining lake sediments [J]. Water Air Soil Pollut, 2003, 3: 67-90.
Foundation item: Project(2010CB630902) supported by the National Basic Research Program of China; Projects(50674101, 50974140) supported by the National Natural Science Foundation of China
Corresponding author: XIA Jin-lan; Tel: +86-731-88836944; E-mail: jlxia@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(09)60140-0
(Edited by YUAN Sai-qian)

