J. Cent. South Univ. Technol. (2010) 17: 34-39
DOI: 10.1007/s11771-010-0007-5 
Adsorption isotherm mechanism of amino organic compounds as mild steel corrosion inhibitors by electrochemical measurement method
A. Y. MUSA1, A. A. H. KADHUM1, A. B. MOHAMAD1,
M. S. TAKRIFF1, A. R. DAUD2, S. K. KAMARUDIN1
1. School of Chemical and Process Engineering, Universiti Kebangsaan Malaysia,
Bangi 43600, Selangor, Malaysia;
2. School of Applied Physics, Universiti Kebangsaan Malaysia, Bangi 43600, Selangor, Malaysia
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: The inhibition ability of 4-amino-5-phenyl-4H-1, 2, 4-trizole-3-thiol (APTT), ethylenediaminetetra-acetic acid (EDTA) and thiourea (TU) for mild steel corrosion in 1.0 mol/L HCl solution at 30 ℃ was investigated. Tafel polarization and electrochemical impedance spectroscopy (EIS) were used to investigate the influence of these organic compounds as corrosion inhibitors of mild steel in 1.0 mol/L HCl solution at 30 ℃. The inhibition mechanism was discussed in terms of Langmuir isotherm model. Results obtained from Tafel polarization and impedance measurements are in a good agreement. The inhibition efficiency increases with the increase of the inhibitor concentration. The adsorption of the inhibitors on the mild steel surface follows Langmuir adsorption isotherm and the free energy of adsorption ΔGads indicates that the adsorption of APTT, EDTA, and TU molecules is a spontaneous process and a typical chemisorption.
Key words: corrosion inhibitor; Langmuir adsorption isotherm; mild steel
1 Introduction
The use of acid solution during pickling and industrial cleaning leads to corrosive attack on mild steel. Therefore, corrosion of mild steel and its inhibition in acidic solutions have attracted the attention of number of investigators [1-5]. The protection of mild steel against corrosion can be achieved by adding a small concentration of organic compounds to environment [6].
A survey of literature reveals that the applicability of organic compounds as corrosion inhibitors for mild steel in acidic media has been recognized for a long time. Compounds including triazole derivatives [7], bipyrazolic derivatives [8], surfactants [9], aromatic hydrazides [10], organic dyes [11], poly (4-vinylpyridine) [12] and thiosemicarbazide-type organic compounds [13] were reported as inhibitors. These compounds can be adsorbed on the mild steel surface and block the active sites to decrease the corrosion rate.
In this work, the inhibition ability of 4-amino-5- phenyl-4H-1, 2, 4-trizole-3-thiol (APTT), ethylene- diaminetetra-acetic acid (EDTA), and thiourea (TU) for mild steel corrosion in 1.0 mol/L HCl solution at 30 ℃ was evaluated. The inhibition mechanism of these organic compounds was discussed based on the analysis of the Langmuir adsorption isotherm.
2 Experimental
Tafel polarization and electrochemical impedance spectroscopy (EIS) were applied. The choice of the compounds was based on molecular structure, i.e., the chosen organic compounds should have the adsorption centre. The molecular structures of organic compounds are shown in Fig1.
The chemical composition of commercially mild steel metal with exposed area of 4.52 cm2 is as follows (in mass fraction): 0.08% C, 0.25% Si, 0.45% Mn, 0.03% P, 0.03% S, and balanced with Fe. The samples were mechanically polished using SiC paper in successive grades from 200 to 1 500 and rinsed in methanol to remove any organic contamination from metal surface.
A Gamry water-jacketed glass cell of capacity 175 mL was used, which contained three electrodes for working, graphite bar counter and reference electrodes. The reference electrode was a saturated calomel electrode (SCE). The measurements were carried out in aerated 1.0 mol/L HCl solution at 30 ℃. APTT, EDTA and TU were used as corrosion inhibitors through the experiments. The solution was freshly prepared from analytical grade chemical reagents using distilled water and used without further purification. For each run, a freshly prepared solution and a cleaned set of electrodes were used.
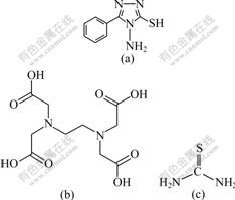
Fig.1 Molecular structures of APTT (a), EDTA (b) and TU (c)
Electrochemical measurements were performed using Gamry Instrument potentiostat/galvanostat/ZRA, which included Gamry framework system based on the Ref600. Gamry applications that include potentiodynamic scan and EIS are DC105 and EIS300 software. The potentiodynamic current-potential curves were swept from -0.2 to 0.2 V at a scan rate of 0.166 mV/s. Impedance measurements were carried out using AC signals of amplitude of ±10 mV (peak to peak) at open circuit potential in the frequency range from 100 kHz to 0.1 Hz.
3 Results and discussion
3.1 Tafel polarization
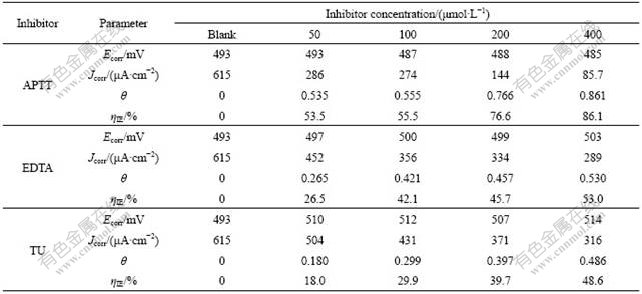
The effect of the inhibitor concentration is shown in Fig.2, which presents the anodic and cathodic Tafel curves of mild steel in 1.0 mol/L HCl solution at 30 ℃ with APTT, EDTA, and TU. The corrosion current density (Jcorr), coverage (θ), and corrosion potential (Ecorr) are listed in Table 1. Jcorr and Ecorr were calculated from the intersection of the anodic and cathodic Tafel lines of the polarization curve at Ecorr, while θ was obtained using the following expression [14]:
θ=(Jcorr(uninhibt)-Jcorr(inhibit))/(Jcorr(inhibit)-Jcorr(max)) (1)
where Jcorr(uninhibt) and Jcorr(inhibit) are the corrosion current densities without and with inhibitor, respectively, and Jcorr(max) is the corrosion current density for the maximum of inhibition. By considering Jcorr(uninhibt)>>Jcorr(max), for a low polarized electrode, Eq.(1) can be simplified to the following expression [14]:
θ=(Jcorr(uninhibt)-Jcorr(inhibit))/Jcorr(inhibit) (2)
It should be noted that the inhibitor efficiency (ηIE, %) is a function of θ by: ηIE=θ×100% [15]. Eq.(2) is valid based on the assumption that: (1) the adsorption sites on the mild steel surface are homogeneous, (2) a monolayer inhibitor adsorption is formed, and (3) dissolution is uniform and no localized attack takes place.
As observed from Table 1, Jcorr decreases with increasing inhibitor concentration, while ηIE rises with increasing the concentration of all inhibitors, which can be interpreted on the basis that the inhibitors exert the can by adsorbing themselves on the metal surface. In acidic solutions the anodic process of corrosion is the passage of metal ions from metal surface into the solution, and the principal cathodic process is the discharge of hydrogen ions to produce hydrogen gas or reduction of oxygen. The inhibitor may affect either of them or both anodic and cathodic processes [16].
Table 1 Polarization parameters for mild steel in 1.0 mol/L HCl solution at 30 ℃ with different concentrations of APTT, EDTA, and TU
In Fig.2, a decrease in both cathodic and anodic current densities is noted for all inhibitors. This result shows that the addition of these inhibitors reduces anodic dissolution and also retards the hydrogen evolution reaction. So, these inhibitors are considered to be mixed- type inhibitors [17].
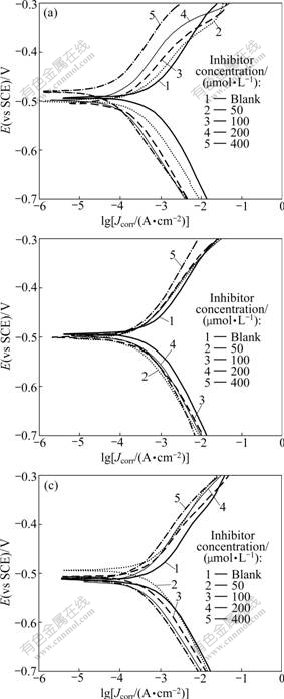
Fig.2 Polarization curves for mild steel in 1.0 mol/L HCl solution with different concentrations of inhibitors: (a) APTT; (b) EDTA; (c) TU
3.2 Electrochemical impedance spectroscopy (EIS)
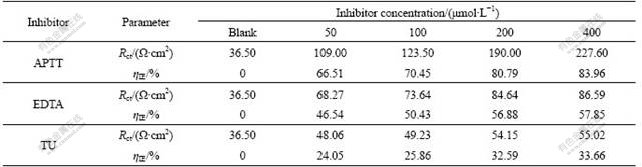
The EIS spectra were recorded on the working electrode at different inhibitor concentrations of APTT, EDTA and TU by Nyquist format. The results are presented in Fig.3. The impedance spectrum for blank solution (without inhibitor) shows that the Nyquist plot, in the capacitive quadrant, is similar to a semicircle with a small inductive behavior at low frequencies. This agrees with the result reported by MORETTI et al [18]. While adding APTT, EDTA and TU into solution, the spectrum becomes more complicated and the Nyquist plot shows two semicircles: one loop of which is much smaller than the other in high frequency range, and the other shows inductive behavior in low frequency range. The semicircle at lower frequency is due to the corrosion electrochemical process [18]. This is consistent with the results reported in Refs.[19-20]. The semicircle in high frequency range is formed due to the inhibitor film because a surface dielectric film normally has a small time constant and a phase angle shift takes place in the high frequency range [19]. The continuous increase in the semidiameter of Nyquist semicircles by the increase in inhibitor concentration suggests that the inhibition ability increases by the increase of the concentration of inhibitors. Charge transfer resistance (Rct) was obtained from the impedance, as described by MORETTI et al [18]. The ?tted values of Rct for these inhibitors are listed in Table 2.
Table 2 EIS parameters for mild steel in 1.0 mol/L HCl solution with different concentrations of APTT, EDTA and TU
ηIE can be calculated from the following relation:
ηIE=[(Rct(inhibt)-Rct(uninhibt))/Rct(inhibt)]×100% (3)
where Rct(inhibt) and Rct(uninhibt) are the charge transfer resistances with and without inhibitor, respectively.
It can be seen from Table 2 that the charge transfer resistance, Rct, and inhibition efficiency increase with increasing concentration of inhibitors. These results are in line with those of polarization measurements, indicating that the inhibitor does not alter the electrochemical reactions responsible for corrosion but inhibits corrosion primarily through its adsorption onto the metal surface.
4 Adsorption mechanisms
It is known that the adsorption isotherms are very important for the understanding of the mechanism of corrosion inhibition. The most frequently used isotherms are Langmuir, Freundlich, Temkin and Frumkin equations. Tafel polarization data were used to evaluate the surface coverage (θ), which was given by Eq.(2). We assumed that the adsorption of these inhibitors follows the Langmuir adsorption isotherm model, and can be described by the following equation [15]:
θ/(1-θ)=Kadsc (4)
Rearranging Eq.(4) gives:
c/θ=(1/Kads)+c (5)
where Kads is the equilibrium constant of the inhibitor adsorption process, and c is the inhibitor concentration. The surface coverage values (θ) were tested graphically to allow fitting for suitable adsorption isotherm. The best fitting straight line is obtained for the plot of c/θ vs c with slopes close to 1 for all inhibitors (Fig.4). This suggests that the adsorption of inhibitors on the metal surface for APTT, EDTA, and TU obeys Langmuir’s adsorption isotherm [9]. From the intercepts of the straight lines on the c/θ-axis, Kads values can be calculated, which are related to the standard free energy of adsorption, ΔGads, as given by the following equation [21]:
Kads=0.018exp[-?Gads/(RT)] (6)
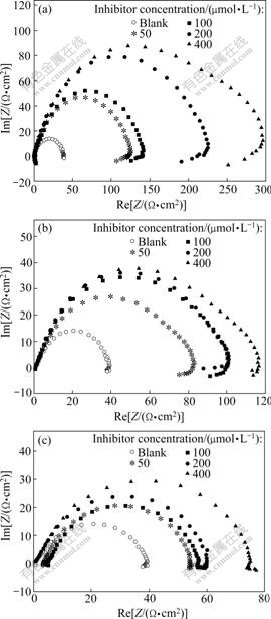
Fig.3 Nyquist plots for mild steel in 1.0 mol/L HCl solution of with different inhibitors: (a) APTT; (b) EDTA; (c) TU
The equilibrium adsorption constant and free energy (ΔGads) were obtained from these isotherms and listed in Table 3. Generally, values of ΔGads up to -20 kJ/mol are consistent with physisorption, while those around-40 kJ/mol or higher are associated with chemisorption sharing or transfer of electrons from organic due to molecules to the metal surface to form a coordinate type of bond (chemisorption). Thus, the adsorption mechanism of APTT, EDTA and TU on mild steel surface in 1.0 mol/L HCl solution was chemisorption [22]. This is in a good agreement with values of coverage (θ) or inhibition efficiency obtained from the electrochemical measurements. Therefore, the coverage or inhibition efficiency increases with increasing equilibrium constant. Moreover, the largest negative values of ΔGads indicate that these inhibitors are strongly adsorbed onto the mild steel surface.
Table 3 Langmuir isotherm adsorption parameters for APTT, EDTA and TU on mild steel surface in 1.0 mol/L HCl solution at 30 ℃
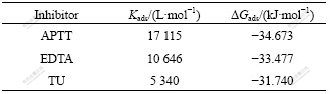
According to the increase in the absolute value for ΔGads and Kads the inhibitive action of the studied inhibitors can be written in the following order: TU<EDTA<APTT.
It is noted that a plausible mechanism of corrosion inhibition of mild steel in 1.0 mol/L HCl solution for the compounds under study may be deduced on the basis of adsorption. Further insight into the adsorption mechanism is offered by careful observation of the curves given in Fig.2. It can be seen that the increase in the concentration of APTT leads to the apparition of the irregularity of anodic polarization current density (overlapping). Fig.2 also reveals that the cathodic polarization curves follow normal trend when the concentration of APTT increases. The irregularity observed in the anodic region is probably due to variation effect in the adsorbed APTT on the surface. The most probable variation effect of the APTT inhibitor, which is responsible for the overlapping in anodic current, is the protonation process. The protonation of APTT results in the attachment of a proton to the highly negative S-atom [23]. Hence, it is expected that both unprotonated and protonated species of APTT, EDTA and TU could exist in acidic solution. Due to electrostatic attraction, the cationic species may be adsorbed on the cathodic sites of mild steel and reduce the evolution of hydrogen, thereby protecting the cathodic sites of steel. The adsorption of APTT, EDTA and TU at anodic sites can be attributed to the presence of unprotonated molecular species. The increase of the inhibitor concentration may increase the protonated species on the surface [24], thus decreasing the cathodic currents and limiting the decrease in the anodic currents. This could explain the strange anodic behavior of APTT, EDTA and TU observed at different concentrations.
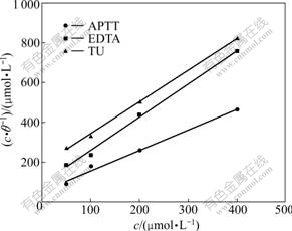
Fig.4 Langmuir isotherm adsorption model on mild steel surface in 1.0 mol/L HCl solution at 30 ℃
5 Conclusions
(1) The inhibition ability of APTT, EDTA and TU is evaluated for mild steel corrosion in 1.0 mol/L HCl solution at 30 ℃ using electrochemical measurements of Tafel polarization and electrochemical impedance spectroscopy.
(2) Tafel polarization and electrochemical impedance spectroscopy measurements are in a good agreement. Electrochemical investigation shows that the inhibition efficiency increases in the sequence of TU<EDTA<APTT. The inhibition efficiency increases with the increase of the inhibitor concentration. Polarization studies reveal that APTT, EDTA and TU are mixed-type inhibitors. The surface coverage increases with the increase of the adsorption equilibrium constant. The adsorption model obeys the Langmuir adsorption isotherm, and the values of ΔGads indicate that the adsorption of APTT, EDTA and TU molecules is a spontaneous process and a typical chemisorption.
References
[1] AL-SARAWYA A A, FOUDA A S, SHEHAB EL-DEIN W A. Some thiazole derivatives as corrosion inhibitors for carbon steel in acidic medium [J]. Desalination, 2008, 229(3): 279-293.
[2] FOUDAA A S, AL-SARAWY A A, EL-KATORI E E. Pyrazolone derivatives as corrosion inhibitors for C-steel in hydrochloric acid solution [J]. Desalination, 2006, 201(1):1-13.
[3] SAYED S, REHIM A, OMAR A, MOHAMMED A, KHALED K F. Inhibition of copper corrosion in acidic chloride pickling solutions by 5-(3-aminophenyl)-tetrazole as a corrosion inhibitor [J]. Corro Sci, 2008, 50(8): 2258-2271.
[4] ABDENNABI M S, ABDULHADI A I, ABU-ORABI S T. The inhibition action of 1(benzyl) 1-H-4,5-dibenzoyl-1,2,3-triazole on mild steel in hydrochloric acid media [J]. Corro Sci, 1996, 38(10): 1791-1800.
[5] ABBOUD Y, ABOURRICHE A, SAFFAJ T, BERRADA M, CHARROUF M. The inhibition of mild steel corrosion in acidic medium by 2,2′-bis(benzimidazole) [J]. Appl Surf Sci, 2006, 252(23): 8178-8184.
[6] MUSA A Y, KADHUM A A H, MOHAMAD A B, TAKRIFF M S, DAUD A R, KAMARUDIN S K. On the inhibition of mild steel corrosion by 4-amino-5-phenyl-4H-1, 2, 4-trizole-3-thiol [J]. Corro Sci, 2009, 52(2): 526-533.
[7] BENTISS F, TRAISNEL M, GENGEMBRE L, LAGRENE M. A new triazole derivative as inhibitor of the acid corrosion of mild steel: Electrochemical studies, weight loss determination, SEM and XPS [J]. Appl Surf Sci, 1999, 152(3): 237-249.
[8] TOUHAMI T, AOUNTI A, ABED Y, HAMMOUTI B, KERTIT S, RAMDANI A, ELKACEMI K. Corrosion inhibition of armco iron in 1 mol/L HCl media by new bipyrazolic derivatives [J]. Corro Sci, 2000, 42(6): 929-940.
[9] ALGABER A S, EL-NEMNA E M, SALEH M M. Effect of octylphenol polyethylene oxide on the corrosion inhibition of steel in 0.5 mol/L H2SO4 [J]. Mater Chem Phys, 2004, 86(1): 26-32.
[10] QURAISHI M A, SARDAR R, JAMEL D. Corrosion inhibition of mild steel in hydrochloric acid by some aromatic hydrazides [J]. Mater Chem Phys, 2001, 71(3): 309-313.
[11] OGUZIE E E, UNAEGBU C, OKOLUE C B, ONUCHUKWU A I. Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives [J]. Mater Chem Phys, 2004, 84(2): 363-368.
[12] LARABI L, HAREK Y, TRAISNEL M, MANSRI A. Synergistic influence of poly(4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1mol/L HCl [J]. J Appl Electroch, 2004, 34(8): 833-839.
[13] ITA B F, OFIONG O E. The study of the inhibitory properties of benzoin, benzil, benzoin-(4-phenylthiosemicarbazone) and benzil- (4-phenylthiosemicarbazone) on the corrosion of mild steel in hydrochloric acid [J]. Mater Chem Phys, 2001, 70(3): 330-335.
[14] SCENDO M. Inhibition of copper corrosion in sodium nitrate solutions with nontoxic inhibitors [J]. Corro Sci, 2008, 50(6): 1584-1592.
[15] OLIVARES O, LIKHANOVA N V, GO?MEZ B, NAVARRETE J. Electrochemical and XPS studies of decylamides of α-amino acids adsorption on carbon steel in acidic environment [J]. Appl Surf Sci, 2006, 252(8): 2894-2909.
[16] MUSA A Y, KADHUM A A H, MOHAMAD A B, DAUD A R, TAKRIFF M S, KAMARUDIN S K. A comparative study of the corrosion inhibition of mild steel in sulphuric acid by 4, 4-dimethyloxazolidine-2-thione [J]. Corro Sci, 2009, 51(10): 2393–2399.
[17] RAJA P, SETHURAMAN M. Atropine sulphate as corrosion inhibitor for mild steel in sulphuric acid medium [J]. Mater Lett, 2008, 62(10): 1602-1604.
[18] MORETTI G, GUIDI F, GRION G. Tryptamine as a green iron corrosion inhibitor in 0.5 mol/L deaerated sulphuric acid [J]. Corro Sci, 2004, 46(2): 387-403.
[19] TAN J, BAILEY S, KINSELLA B. An investigation of the formation and destruction of corrosion inhibitor films using electrochemical impedance spectroscopy (EIS) [J]. Corro Sci, 1996, 38(9): 1545-1561.
[20] MORETTI G, QUARTARONE G, TASSAN A, ZINGALES A. Some derivatives of indole as mild steel corrosion inhibitors in 0.5 mol/L sulphuric acid [J]. Brit Corro J, 1996, 31(1): 49-54.
[21] SOROR T Y. New naturally occurring product extract as corrosion inhibitor for 316 stainless steel in 5% HCl [J]. J Mater Sci Technol, 2004, 20(4): 463-466.
[22] BOUKLAH M, HAMMOUTI B, LAGRENE M, BENTISS F. Thermodynamic properties of 2, 5-bis(4-methoxyphenyl)-1, 3, 4- oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium [J]. Corro Sci, 2006, 48(9): 2831-2841.
[23] PILLAI K C, NARAYAN R. Anodic dissolution of mild steel in HCl solutions containing thio-ureas [J]. Corro Sci, 1983, 23(2): 151-166.
[24] AWAD M K. Semiempirical investigation of the inhibition efficiency of thiourea derivatives as corrosion inhibitors [J]. Electroanal Chem, 2004, 567(2): 219-225.
Foundation item: Project(UKM-GUP-BTT-07-25-170) supported by Universiti Kebangsaan Malaysia
Received date: 2009-03-24; Accepted date: 2009-08-06
Corresponding author: A. Y. MUSA, PhD; Tel: +60-3-89216148; E-mail: ahmed.musa@ymail.com
(Edited by CHEN Wei-ping)