DOI:10.19476/j.ysxb.1004.0609.2019.03.20
铝酸钾溶液自发分解过程中的氢氧化铝晶型转变
江友法,刘程琳,罗孟杰,薛 金,李 平,于建国
(华东理工大学 国家盐湖资源综合利用工程技术研究中心,上海 200237)
摘 要:氢氧化铝作为生产金属铝的前驱体,其产品的多晶型控制对于下游操作以及产品性质存在至关重要的影响。考察了温度和苛性碱浓度对过饱和铝酸钾溶液自发分解产物及其晶型转化的影响。结果表明:温度对于溶液析出的初始晶相影响显著,在低温(30 ℃和40 ℃)下,主要晶型为拜耳石;高温(60 ℃和80 ℃)下,主要晶型为三水铝石和拜耳石的混合物;苛性碱浓度对初始晶相的类型影响并不显著。通过对三水铝石和拜耳石饱和浓度与溶液浓度的比较,结合低温下拜耳石作为主要晶相析出的现象,可知拜耳石的形成在动力学上要优于三水铝石。通过SEM像观测到了拜耳石的溶解,说明由拜耳石向三水铝石的晶型转变过程应为SMPT(以溶液为介质的晶型转化)过程,同时温度的提高与苛性碱浓度的增加均能加快晶型从拜耳石向三水铝石的转化,更验证这是一个溶解-再结晶过程。
关键词:铝酸钾溶液;结晶;拜耳石;晶型转变;三水铝石
文章编号:1004-0609(2019)-03-0606-11 中图分类号:TQ 133.1 文献标志码:A
铝作为第二重要的金属,被广泛应用于运输、建筑、机械以及国防材料等各个领域[1-2]。氢氧化铝作为生产氧化铝和金属铝的前驱体[3-4],存在4种多晶型:三水铝石、拜耳石、诺耳石和水铝石,其结晶产品的多晶型控制对于下游操作以及产品性质具有至关重要的影响[5]。三水铝石在热力学上最为稳定[6],大量稳定存在于自然中[7],并作为阻燃剂广泛应用在电力以及建筑产业[8],而拜耳石因其更少的碱挟带常被用于氧化铝催化剂的制备[9],目前,工业生产和实验研究主要以三水铝石和拜耳石两种晶型为主[10-13]。虽然有很多研究报道了三水铝石和拜耳石的析出机理及其两者之间的转化[14-18],但结论尚不统一,且研究内容多集中在铝酸钠溶液的晶种分解或碳酸化分解过程[19-25]。
现今关于氢氧化铝多晶型析出机理的讨论主要集中于温度和溶液浓度。LI等[14]总结了在铝酸钠溶液与碳酸氢钠溶液反应过程中的初始晶相取决于反应温度:在50 ℃时只有拜耳石成核析出,70 ℃时只有三水铝石成核析出,而与溶液过饱和度无关。KOCJAN等[15]证明了在稀的氮化铝粉末悬浮液水化过程中,随温度和时间的不同,存在不同的机理;当温度温度低于80 ℃、pH高于9.3时,一水薄铝石成核后,拜耳石作为主要晶相大量生成,同时发现三水铝石只在22~70 ℃下水解和老化的粉末中能检测到。LEE等[16]研究了铝酸盐溶液自发分解、碳酸化分解、盐酸化分解和晶种分解中氢氧化铝多晶型析出的影响因素,结果发现较高温度有利于三水铝石的形成,而在室温下分解产物主要晶相为拜耳石,同时发现阳离子类型对于三水铝石单晶的形貌有重要影响:在铝酸钠溶液中三水铝石表现为六角片状晶体,而在铝酸钾溶液中为六棱柱状晶体。文献[17]指出,溶液浓度对铝酸钠溶液中的氢氧化铝初始析出晶型有决定性作用:在稀铝酸钠溶液(c(NaOH)≤1.00 mol/L,c(Al3+)≤0.82 mol/L)中拜耳石作为主要晶相析出,在浓铝酸钠溶液 (c(NaOH)>2.50 mol/L,c(Al3+)>2.05 mol/L)中三水铝石作为主要晶相析出,而在中等浓度的铝酸钠溶液(c(NaOH)为1.00~2.50 mol/L, c(Al3+)为0.82~2.05 mol/L)中生成的是两者的混合物。WANG等[19]通过添加晶种改变介稳区宽度(MSZW),从而控制Al-H2O反应过程中氢氧化铝多晶型的生成,并且在不添加晶种的情况下,改变温度和碱浓度同样可以实现MSZW和产品中多晶型的控制。
有很多研究报道了由拜耳石向三水铝石的转化过程[14, 18, 20-21],而对于Al(OH)3多晶型之间的转化机理,目前并无研究对其加以探讨。van STRATEN等[26]在研究稀铝酸钾溶液(c(Al(OH)4)=4×10-3 mol/L)中氢氧化铝析出过程时,指出晶体析出顺序受过饱和度影响并遵循Ostwald规则:无定形→假一水薄铝石→拜耳石→三水铝石,LI等[14]在使用NaHCO3溶液酸化分解NaAl(OH)4溶液时也提出了这一论点。对于晶体多晶型间转化,其机理包括两种[27-29]:以溶液为介质的晶型转化(Solution-mediated polymorphic transformation, SMPT)和固态晶型转化 (Solid-state polymorphic transformation, SSPT)。SMPT机理认为晶型转化的过程要先经过亚稳态的溶解再形成热力学稳定的晶体。TONG等[30]在研究一种有机共晶体多晶型转化的过程中,通过综合使用ATR-FTIR和Raman发现此转化过程受稳态晶型的成核与生长所控制,并通过电镜观察到亚稳态晶体的溶解以及稳态晶型在亚稳态晶面上的生长。SSPT机理则认为固相中的离子或者分子在空间取向上的重新排列导致了多晶型之间的晶型转化。在以锂辉石精矿为原料的锂生产工艺中,需要将α-锂辉石在1150 ℃进行晶型转化焙烧,使其转化为高活性的β-锂辉石,从而利于后续的浸出工序[31-32]。
综上所述可知,铝酸盐溶液中氢氧化铝多晶型析出规律并不清晰,而且对于其转化过程的影响因素也并不明朗,且研究多集中与铝酸钠溶液。LI等[33]在考察铝酸钠/钾溶液的自发分解过程中,发现Al(OH)3晶核即Al—OH八面体结构的形成与发展是受金属阳离子影响的,析出晶体均表现为三水铝石和拜耳石的混合多晶,并进一步指出钠离子在Al(OH)3中的高吸附量降低了晶体的界面张力,从而导致了铝酸钠溶液自发分解初始晶相中的拜耳石占比更高,但其所考察温度(65 ℃、75 ℃)和浓度(4.00 mol/L [KOH]/[NaOH])有限,且并未探讨铝酸钾溶液中的Al(OH)3多晶型转化过程。本文将在更广的温度和浓度范围对饱和铝酸钾溶液自发分解过程进行研究和探讨,从温度和苛性碱浓度两方面考察,揭示其固相析出规律以及晶型转化过程。
1 实验
1.1 实验试剂与设备
实验用试剂为氢氧化钾(AR)和氢氧化铝(AR),均采购于上海泰坦科技股份有限公司。其中氢氧化钾主要杂质为碳酸盐,为保证所配置溶液浓度的精确性,使用自动电位滴定仪(Metrohm 888)确定氢氧化钾的准确含量。为充分考察不同浓度下的铝酸钾的分解过程[34],实验所选溶液浓度和温度如表1所示。
表1 过饱和铝酸钾溶液自发分解过程的实验条件
Table 1 Experimental conditions for spontaneous decomposition of supersaturated potassium aluminate solutions
实验中所用恒温振荡设备为SW23(Julabo),工作温度<100 ℃,控温精度±0.1 ℃,振荡频率选定为150 r/min。扫描电子显微镜(SEM)型号为Quanta250(美国FEI公司),X射线衍射仪(XRD)型号为D/MAX-B(日本Rigaku公司)。
1.2 实验方法
为避免引入杂质离子,选用聚四氟乙烯烧杯加热制备过饱和铝酸钾溶液,同时搅拌选用聚四氟乙烯转子。考虑到热历史将对结晶析出过程存在重要影响[35-36],因此,本实验中铝酸钾反应精液均严格按照如下过程制备:按照表1所设定的溶液浓度,称取相应质量的氢氧化钾(分析纯,上海泰坦科技股份有限公司)、三水铝石(分析纯,上海凌峰化学试剂有限公司)和去离子水于聚四氟乙烯烧杯中,加热溶解使其澄清,持续搅拌并于室温下冷却后将溶液转移至100 mL容量瓶定容。为抑制异相成核,使用0.22 μm亲水PTFE针式滤器(上海安谱实验科技股份有限公司)将溶液过滤后,注入聚四氟乙烯摇瓶中,并密闭置于恒温水浴振荡槽中。在恒温振荡的过程中,周期性的从摇瓶中取样,离心后将固体用去离子水清洗3次以移除碱残留,而后置于70 ℃下干燥。使用XRD鉴定析出固体晶相组成,使用SEM观测其晶体形貌。
2 结果与讨论
2.1 过饱和铝酸钾溶液自发分解初始晶相组成
在实验所考察条件下,析出产品主要含有三水铝石(Gibbsite,G)和拜耳石(Bayerite,B),同时伴随着少量的诺耳石(Norstrandite, N)。在本研究中,过饱和铝酸钾溶液自发分解的初始晶相组成分为两类:1) 拜耳石;2) 拜耳石、三水铝石或诺耳石的混合物。这两类产物组的典型XRD谱和SEM像分别如图1和2所示。
通过样品衍射峰与标准PDF卡片JCPDS 74—1775 三水铝石(Gibbsite)和JCPDS 74—1119拜耳石(Bayerite)的对比,可知拜耳石在2θ为18.8°、40.6°和50.3°处均有较强的衍射峰,分别对应于其(001)、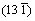 和(202)晶面,三水铝石则在2θ为18.3°、20.3°和20.5°处出现衍射峰,分别对应于其(002)、(110)和(200)晶面,其峰强在2θ为18.3°处尤为最大。诺耳石在2θ为18.3°处的衍射峰经常被前两者覆盖掉,不过通过放大可以清晰看到对应于诺耳石(001)晶面的衍射峰。由图1可明确地鉴别拜耳石和三水铝石作为主要晶相在不同实验条件下的出现:在实验条件1(30 ℃,
和(202)晶面,三水铝石则在2θ为18.3°、20.3°和20.5°处出现衍射峰,分别对应于其(002)、(110)和(200)晶面,其峰强在2θ为18.3°处尤为最大。诺耳石在2θ为18.3°处的衍射峰经常被前两者覆盖掉,不过通过放大可以清晰看到对应于诺耳石(001)晶面的衍射峰。由图1可明确地鉴别拜耳石和三水铝石作为主要晶相在不同实验条件下的出现:在实验条件1(30 ℃,  =1.3, ρ(K2Ok)=39.3 g/L)下,过饱和铝酸钾溶液自发分解初始产品主要为拜耳石;在实验条件11(80 ℃,
=1.3, ρ(K2Ok)=39.3 g/L)下,过饱和铝酸钾溶液自发分解初始产品主要为拜耳石;在实验条件11(80 ℃,  =1.3, ρ(K2Ok)=97.9 g/L)下,过饱和铝酸钾溶液自发分解初始产品主要为三水铝石。
=1.3, ρ(K2Ok)=97.9 g/L)下,过饱和铝酸钾溶液自发分解初始产品主要为三水铝石。
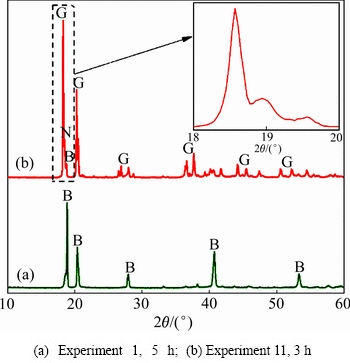
图1 不同实验条件下初始晶相组成的XRD谱
Fig. 1 XRD patterns of initial precipitated products under different experimental conditions
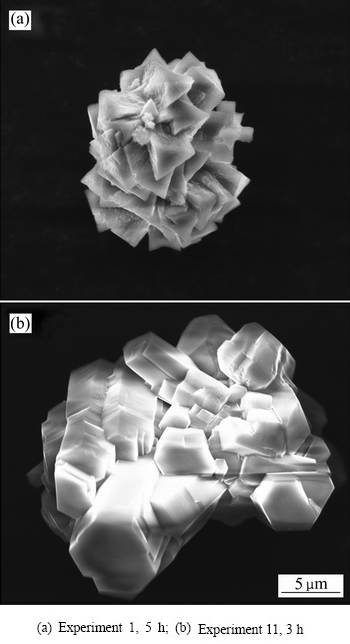
图2 不同实验条件下初始析出晶相的SEM像
Fig. 2 SEM images of initial precipitated products under different experimental conditions
通过析出晶体的SEM像同样可以观测到实验中出现的多晶型现象。如图2所示,实验1中晶体表现为锥形晶体的束状团聚物,这是典型的拜耳石晶体形貌,而实验11中则为假六棱柱形晶体的团聚物,这作为三水铝石的典型形貌已见于诸多研究中。
2.2 氢氧化铝晶型转变过程
通过XRD和SEM对不同实验条件和不同反应时间下过饱和铝酸钾溶液自发分解产物的鉴定,发现存在明显的拜耳石/诺耳石向热力学稳定相-三水铝石的晶型转变过程。图3和4所示分别为实验条件5(40 ℃,  =1.3, ρ(K2Ok)=97.9 g/L)下,溶液中析出产物的晶型转变及其形貌变化过程。在反应时间为5 h时,析出产物主要为拜耳石,同时伴随着少量的三水铝石,故其形貌表现为轮廓不清的晶体包裹着锥形晶体;在反应时间为2 d和4 d时,产物中的主要晶相均变为三水铝石,其形貌也表现为典型的棱柱状晶体团聚物。
=1.3, ρ(K2Ok)=97.9 g/L)下,溶液中析出产物的晶型转变及其形貌变化过程。在反应时间为5 h时,析出产物主要为拜耳石,同时伴随着少量的三水铝石,故其形貌表现为轮廓不清的晶体包裹着锥形晶体;在反应时间为2 d和4 d时,产物中的主要晶相均变为三水铝石,其形貌也表现为典型的棱柱状晶体团聚物。
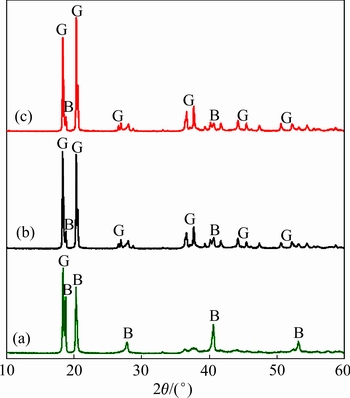
图3 实验5中不同反应时间下分解产物的XRD谱
Fig. 3 XRD patterns of precipitated products in experiment 5 obtained at 5 h(a), 2 d(b) and 4 d(c)
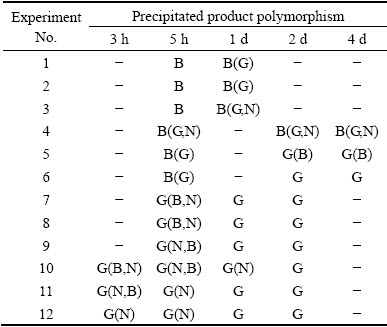
表2所列为各反应条件下观测到的晶型转化结果。由表2得出,在30℃、40℃的条件下,溶液浓度对于初始产品基本无影响,主要晶相均为拜耳石,而在40℃时,还伴随着少量的三水铝石或诺耳石;同时发现,溶液浓度对于由拜耳石向三水铝石的晶型转变过程存在明显的影响,且晶型转变速率随着浓度的升高而加快,如在实验4、5、6(θ=40 ℃)条件下,其初始产品的主要晶相均为拜耳石,在反应时间为2 d时,高浓度铝酸钾溶液中的固相为纯净的三水铝石,而在低浓度铝酸钾溶液中的主要晶相仍为拜耳石,中间浓度的铝酸钾溶液中主要晶相已转变三水铝石,但仍伴随着少量的拜耳石。在60 ℃、80 ℃的条件下,初始产品主要晶相为三水铝石伴随着少量的拜耳石或诺耳石,且晶型转变速率明显要高于低温下(30 ℃、40 ℃),其产品基本均在1 d之内全转变为三水铝石。
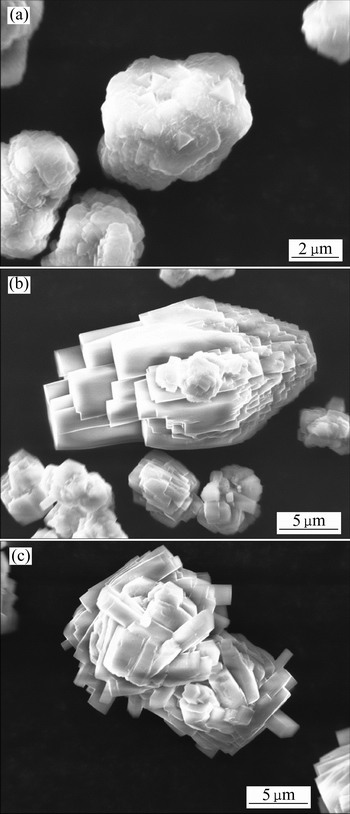
图4 实验5中不同反应时间下分解产物的SEM像
Fig. 4 SEM images of precipitated products in experiment 5 obtained at 5 h(a), 2 d(b) and 4 d(c)
表2 各实验条件下析出产品的晶型变化
Table 2 Polymorphism of precipitated products under different experimental conditions
本实验中所发现的多晶型现象与铝酸钠溶液体系中的一致[14, 17],在低温下初始析出晶相主要为拜耳石,而在高温下则主要为三水铝石,且都存在由拜耳石向三水铝石的晶型转化。同时可以观察到初始产品的主要晶相由低温下的拜耳石逐渐转化为高温下的三水铝石,这可以解释为一种协同作用的影响:一方面,拜耳石的析出证实了在特定的结晶条件下,动力学的影响甚至会大于热力学,使得拜耳石优先结晶析出,从而导致亚稳晶相出现在产品中,另一方面,三水铝石是热力学稳定晶相,由拜耳石向三水铝石的转变是不可逆的,所以理论上存在某一临界温度,使得初始产品中的主要晶相发生转变,但在本文所考察实验条件下,发现纯净的三水铝石并不能在初始晶相中得到,而是均夹杂着拜耳石或诺耳石,在稀铝酸钾溶液的自发分解中同样也有此现象的存在[26]。
2.3 三水铝石和拜耳石的饱和溶解度与相对过饱和度
对于铝酸钾溶液中氢氧化铝析出的多晶型现象,可通过比较两者饱和溶解度与溶液浓度的差别来进行解释。溶液的过饱和是结晶的必要条件,也就是说溶液中的溶质浓度必须要高于溶质的饱和浓度。氢氧化铝在碱液中的饱和浓度可以根据以下两步推导得出[19]:
1) 氢氧化铝在碱液中的溶解反应:
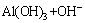 =
= (1)
(1)
则氢氧化铝的溶度积可表达为式(2):
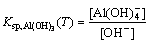 (2)
(2)
2) 三水铝石和拜耳石的饱和浓度可通过式(3)求得:
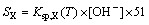 (3)
(3)
式中:X表示三水铝石或者拜耳石。
运用对数函数拟合三水铝石和拜耳石的溶度积文献数据[37],可得两者的溶度积表达式,如图5和式(4)、(5)所示。
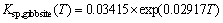 (4)
(4)
 (5)
(5)
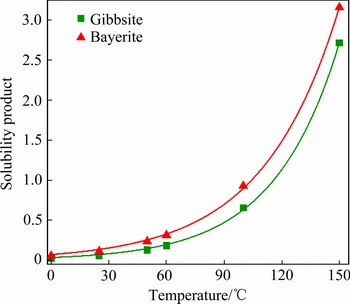
图5 三水铝石和拜耳石的溶度积曲线
Fig. 5 Solubility curves of gibbsite and bayerite
MULLIN等[38]指出,在给定温度下,热力学上最稳定的晶相在溶剂中的溶解度总是最小的,由此推出多晶型之间的转化存在两种情况(以晶型Ⅰ和晶型Ⅱ为例):互变型(晶型Ⅰ 晶型Ⅱ)和单向转变型(晶型Ⅰ→晶型Ⅱ)。互变多晶型存在临界转变温度(TC),也就是说,晶型Ⅰ和晶型Ⅱ的溶解度曲线存在交点;而单向转变多晶型的溶解度曲线并不相交。由图5可知,拜耳石向三水铝石的转变是不可逆的,并不存在临界转变温度(TC),这与前人结论一致[21]。同时,拜耳石的析出证实了在特定的结晶条件下,动力学的影响甚至会大于热力学,从而导致亚稳晶相出现在产品中。
晶型Ⅱ)和单向转变型(晶型Ⅰ→晶型Ⅱ)。互变多晶型存在临界转变温度(TC),也就是说,晶型Ⅰ和晶型Ⅱ的溶解度曲线存在交点;而单向转变多晶型的溶解度曲线并不相交。由图5可知,拜耳石向三水铝石的转变是不可逆的,并不存在临界转变温度(TC),这与前人结论一致[21]。同时,拜耳石的析出证实了在特定的结晶条件下,动力学的影响甚至会大于热力学,从而导致亚稳晶相出现在产品中。
同时,相对过饱和度的变化也会极大地影响结晶过程,比如多晶型现象[39]、晶体形貌[40]等。三水铝石或拜耳石在碱液中的相对过饱和度 可通过式(6)计算得出:
可通过式(6)计算得出:
 (6)
(6)
式中:X表示三水铝石或者拜耳石;A表示溶液中氧化铝浓度;SX表示三水铝石或拜耳石的饱和浓度。
2.4 溶液浓度与饱和浓度的比较
2.4.1 温度的影响
三水铝石和拜耳石在不同浓度下其饱和浓度随温度的变化如图6所示。由图6可知,在每一个实验条件下,溶液对于三水铝石和拜耳石都是过饱和的,表明三水铝石和拜耳石都存在结晶的可能性。图7所示为在 39.3 g/L下温度对相对过饱和度的影响。由图7可看出,在30 ℃时,拜耳石的相对过饱和度为3.99;而在80 ℃时,此项值却降为0.42。如2.2中所述,在30 ℃和40 ℃下,过饱和铝酸钾溶液分解产生的初始晶相主要为拜耳石,而不受溶液浓度的影响,而在60 ℃和80 ℃下,初始晶相却主要为三水铝石。结合图7可推知,这是由于拜耳石的相对过饱和度在低温下的明显升高造成的。需要指出的是,对于晶体的成核过程,温度的降低对于成核速率的影响是双重的,经典成核理论认为成核速率
39.3 g/L下温度对相对过饱和度的影响。由图7可看出,在30 ℃时,拜耳石的相对过饱和度为3.99;而在80 ℃时,此项值却降为0.42。如2.2中所述,在30 ℃和40 ℃下,过饱和铝酸钾溶液分解产生的初始晶相主要为拜耳石,而不受溶液浓度的影响,而在60 ℃和80 ℃下,初始晶相却主要为三水铝石。结合图7可推知,这是由于拜耳石的相对过饱和度在低温下的明显升高造成的。需要指出的是,对于晶体的成核过程,温度的降低对于成核速率的影响是双重的,经典成核理论认为成核速率 。一方面温度的降低增大了过饱和度,可参考图7所示,利于成核;另一方面,温度的降低减小了有效粒子碰撞的可能性,又会抑制成核[38]。因此,说明在低温下拜耳石相对过饱和度的升高对于晶体成核的促进作用要大于温度的降低对于成核的抑制作用。
。一方面温度的降低增大了过饱和度,可参考图7所示,利于成核;另一方面,温度的降低减小了有效粒子碰撞的可能性,又会抑制成核[38]。因此,说明在低温下拜耳石相对过饱和度的升高对于晶体成核的促进作用要大于温度的降低对于成核的抑制作用。
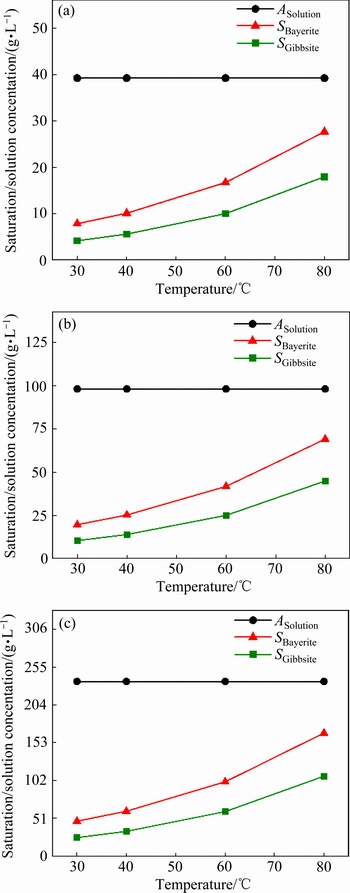
图6 三水铝石和拜耳石饱和浓度随温度的变化
Fig. 6 Saturation concentration change of gibbsite and bayerite with temperature at K2Ok concentration of 39.3 g/L(a), 97.9 g/L and 235.6 g/L(c)
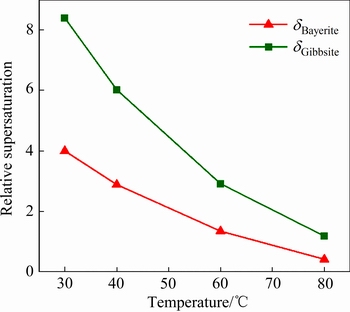
图7 三水铝石和拜耳石在K2Ok浓度为39.3 g/L下相对过饱和度随温度的变化
Fig. 7 Relative supersaturation of gibbsite and bayerite under different temperatures at K2Ok concentration of 39.3 g/L
如图6所示,在任一实验条件下,三水铝石的饱和溶解度均要低于拜耳石的饱和溶解度。由2.2中可知,三水铝石只有在较高的温度(60 ℃和80 ℃)下才成为初始产品的主要晶相,这说明拜耳石的形成在动力学上要优于三水铝石,从而导致了低温下初始产品中拜耳石作为主要晶相的现象,这与前人结论一致:WANG等[19]在研究利用晶种改变介稳区宽度来控制Al(OH)3多晶型的过程中,指出在溶液浓度超过拜耳石的饱和溶解度时,拜耳石由于具有更低的成核活化能,将优先析出;另外,SONTHALIA等[41]在详尽地综述三水铝石析出以及附聚现象中,总结得出三水铝石的晶体生长活化能为123.8 kJ/mol,而这相当于拜耳石晶体生长活化能(60~80 kJ/mol)[26]的1.5~2.0倍。当然,晶体的结晶过程并不完全有活化能所决定,还与温度、过饱和度、界面张力等因素有关,但具体结合实验结果,说明拜耳石的成核在动力学上要优于三水铝石。
2.4.2 苛性碱浓度的影响
苛性碱浓度对于铝酸钾溶液自发分解的初始晶相影响并不大。图8所示为三水铝石和拜耳石饱和浓度随浓度的变化。在同一温度下,三水铝石和拜耳石的饱和浓度均随着苛性碱浓度的增加而增加。然而,对于晶体成核有重要影响的相对过饱和度却保持不变,如图9所示:在30 ℃时,铝酸钾溶液中三水铝石的相对过饱和度为8.39,且并不随着苛性碱浓度的变化而变化。比对图7与9可知,显然温度对于过饱和度的影响要更加显著。同时,由表2可以看出,在同一温度下,浓度的变化并不影响铝酸钾溶液中初始析出产品的主要晶相,由此可推断,在本实验所考察的实验范围内,苛性碱浓度对于铝酸钾溶液自发分解初始产品的主要晶相并无明显影响。
2.5 Al(OH)3多晶型转化机理
根据热力学数据可知,三水铝石的吉布斯自由能比拜耳石的要小2 kJ/mol[42],这也与自然中大量稳定存在的三水铝石晶相相匹配。然而,由2.2可知,在过饱和铝酸钾溶液的自发分解中,其初始晶相均不是纯净的热力学稳定相——三水铝石,甚至在低温(30 ℃和40 ℃)下,其主要成分为拜耳石,但是都存在由拜耳石/诺耳石向三水铝石的晶型转变过程。
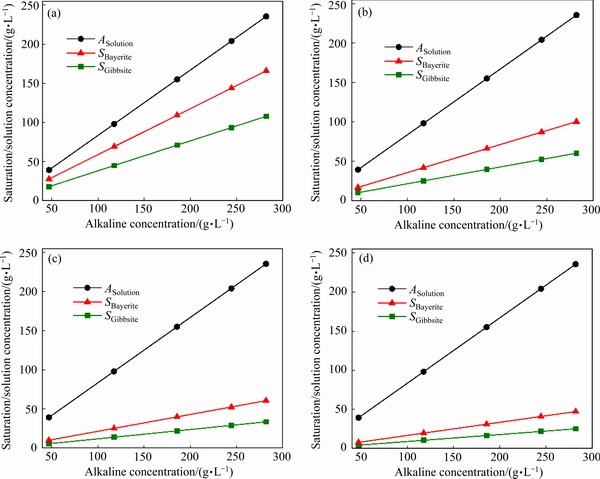
图8 三水铝石和拜耳石饱和浓度随浓度的变化
Fig. 8 Variation of saturation concentration of gibbsite and bayerite under different alkaline concentrations at 80 ℃(a), 60 ℃(b), 40 ℃(c) and 30 ℃(d)
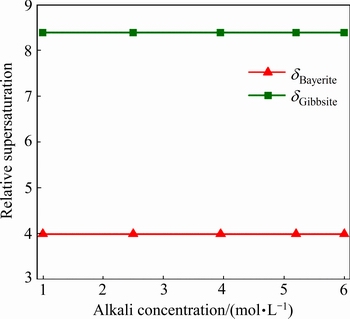
图9 三水铝石和拜耳石在30 ℃下相对过饱和度随苛性碱浓度的变化
Fig. 9 Relative supersaturation concentration of gibbsite and bayerite under different caustic concentrations at 30 ℃
图10所示为在30℃下含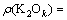 39.3 g/L铝酸钾溶液无晶种分解过程中的晶型转化过程。XRD分析显示,其初始析出的晶相中主要为拜耳石,而随着时间的推移,产品中主要晶相转变为拜耳石和三水铝石的混合晶相。另外,通过SEM像可观察到,在图10(a)中,三角锥形晶体的底边约为3.5~4.3 μm,而在图10(b)中,可明显发现锥形晶体颗粒尺寸的减小,其底边变约为1.4 μm。由此推断,在铝酸钾溶液自发分解的晶型转变过程中,存在拜耳石的溶解。
39.3 g/L铝酸钾溶液无晶种分解过程中的晶型转化过程。XRD分析显示,其初始析出的晶相中主要为拜耳石,而随着时间的推移,产品中主要晶相转变为拜耳石和三水铝石的混合晶相。另外,通过SEM像可观察到,在图10(a)中,三角锥形晶体的底边约为3.5~4.3 μm,而在图10(b)中,可明显发现锥形晶体颗粒尺寸的减小,其底边变约为1.4 μm。由此推断,在铝酸钾溶液自发分解的晶型转变过程中,存在拜耳石的溶解。
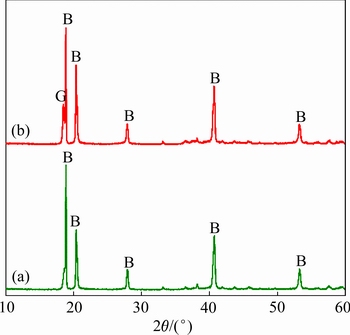
图10 实验1中不同反应时间下分解产物的XRD谱
Fig. 10 XRD patterns of precipitated products in experiment 1 obtained at 5 h(a) and 1 d(b)
图11 实验1中不同反应时间下分解产物的SEM像
Fig. 11 SEM images of precipitated products in experiment 1 obtained at 5 h(a) and 1 d(b)
如前所述,对于晶体多晶型间的转化,其机理解释包括以溶液为介质的晶型转化(Solution-mediated polymorphic transformation, SMPT)和固态晶型转化(Solid-state polymorphic transformation, SSPT)。在本实验中,并未发现固相状态拜耳石的晶型转化,这与前人报道一致[14-15, 19]。由此可推断,拜耳石向三水铝石的晶型转变并不是固态晶型转化过程。
结合图10中发现的拜耳石溶解可推导,铝酸钾溶液中的晶型转变应为以溶液为介质的晶型转化过程,同时由2.2节可知,温度的升高和苛性碱浓度的增加都促进此晶型转化过程,更佐证了这是一个溶 解-再结晶过程,且稳定相即三水铝石的成核与生长很可能是在亚稳定相即拜耳石的某一晶面上进行的。
3 结论
1) 对过饱和铝酸钾溶液自发分解过程的固相析出过程进行了考察,其初始晶相均不是纯净的热力学稳定相—三水铝石,甚至在低温(30 ℃和40 ℃)下,其主要成分为拜耳石,但是都存在由拜耳石/诺耳石向三水铝石的晶型转变过程。
2) 通过对三水铝石和拜耳石饱和浓度与溶液浓度的比较,可知温度对相对过饱和度的影响要比苛性碱浓度更加显著。三水铝石的饱和溶解度均要低于比拜耳石的饱和溶解度,而三水铝石只有在较高的温度(60 ℃和80 ℃)下才成为初始产品的主要晶相,这说明拜耳石的形成在动力学上要优于三水铝石,从而造成了低温下拜耳石作为主要晶相析出的现象。
3) 在铝酸钾溶液自发分解的过程中,均存在由拜耳石/诺耳石向三水铝石的晶型转变过程,且可观察到亚稳相的溶解,由此推断此晶型转变应为以溶液为介质的晶型转化过程,包括拜耳石的溶解、三水铝石的成核和三水铝石的生长。另外,温度的升高和苛性碱浓度的增加都促进此晶型转化过程,更佐证了这是一个溶解-再结晶过程。
REFERENCES
[1] CULLEN J M, ALLWOOD J M. Mapping the global flow of aluminum: From liquid aluminum to end-use goods[J]. Environmental Science & Technology, 2013, 47(7): 3057-3064.
[2] SVERDRUP H U, RAGNARSDOTTIR K V, KOCA D. Aluminium for the future: Modelling the global production, market supply, demand, price and long term development of the global reserves[J]. Resources, Conservation and Recycling, 2015, 103: 139-154.
[3] 聂玉娟, 王二东, 王许云. 制备高纯超细氧化铝粉体新方法[J]. 化工学报, 2017, 68(9): 3616-3622.
NIE Yu-juan, WANG Er-dong, WANG Xu-yun. New approach to prepare high purity and superfine alumina powder[J]. New Journal of Chemical Industry and Engineering, 2017: 68(9): 3616-3622.
[4] SASANI G M, ABDUL-HAMID M A, RADIMAN S, SASANI G A, RAHMANI S H. Carbonation effect on the synthesis of highly crystalline γ-alumina nanopowders[J]. Materials Letters, 2013, 106: 439-442.
[5] DEMICHELIS R, NOEL Y, UGLIENGO P, EICOVICH C, DOVESI R. Physico-chemical features of aluminum hydroxides as modeled with the hybrid B3LYP functional and localized basis functions[J]. The Journal of Physical Chemistry C, 2011, 115(27): 13107-13134.
[6] GALE J D, ROHL A L, MILMAN V, WARREN M C. An ab initio study of the structure and properties of aluminum hydroxide: gibbsite and bayerite[J]. The Journal of Physical Chemistry B, 2001, 105(42): 10236-10242.
[7] KLOPROGGE J T, DUONG L V, WOOD B J, FROST R. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite[J]. Journal of Colloid and Interface Science, 2006, 296(2): 572-576.
[8] 常志宏, 郭 奋, 陈建峰, 王国全, 李振刚, 赵超凡. 纳米氢氧化铝填充LDPE/EVA的力学和阻燃性能[J]. 化工学报, 2005, 56(9): 1771-1776.
CHANG Zhi-hong, GUO Fen, CHEN Jian-feng, WANG Guo-quan, LI Zhen-gang, ZHAO Chao-fan. Mechanical properties and flame retardancy of CG-ATH/LDPE/EVA[J]. Journal of Chemical Industry and Engineering (China), 2005, 56(9): 1771-1776.
[9] YOU S W, LI Y, ZHANG Y F, YANG C, ZHANG Y. Synthesis of uniformly spherical bayerite from a sodium aluminate solution reacted with sodium bicarbonate[J]. Industrial & Engineering Chemistry Research, 2013, 52(36): 12710-12716.
[10] 王熙慧, 于海燕, 吴玉胜, 毕诗文, 陈玉国. 添加剂对铝酸钠溶液种分产物质量的影响[J]. 化工学报, 2007, 58(8): 2120-2124.
WANG Xi-hui, YU Hai-yan, WU Yu-sheng, BI Shi-wen, CHEN Yu-guo. Effects of additive on product quality of seed precipitation in sodium aluminate solution[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(8): 2120-2124.
[11] 王子云, 邵 磊, 郭 奋, 陈建峰, 赵瑞红, 王东光. 超重力碳分法制备六角片状纳米级氢氧化铝[J]. 化工学报, 2006, 57(7): 1699-1703.
WANG Zi-yun, SHAO Lei, GUO Fen, CHEN Jian-feng, ZHAO Rui-hong, WANG Dong-guang. Preparation of hexagonal plate nano-aluminum trihydrate by hypergravity- carbonation[J]. Journal of Chemical Industry and Engineering (China), 2006, 57(7): 1699-1703.
[12] KOGA N, FUKAGAWA T, TANAKA H. Preparation and thermal decomposition of synthetic bayerite[J]. Journal of Thermal Analysis and Calorimetry, 2001, 64(3): 965-972.
[13] ADDAI M J. Surface and structural characteristics of gibbsite precipitated from pure, synthetic Bayer liquor[J]. Minerals Engineering, 1997, 10(1): 81-96.
[14] LI Y, ZHANG Y F, CHEN F F, YANG C, ZHANG Y. Polymorphic transformation of aluminum hydroxide precipitated from reactive NaAl(OH)4-NaHCO3 solution[J]. Crystal Growth & Design, 2011, 11(4): 1208-1214.
[15] KOCJAN A, DAKSKOBLER A, KOSMAC T. Evolution of aluminum hydroxides in diluted aqueous aluminum nitride powder suspensions[J]. Crystal Growth & Design, 2012, 12(3): 1299-1307.
[16] LEE M Y, PARKINSON G M, SMITH P G, LINCOLN F J, REYHANI M M. Characterization of aluminum trihydroxide crystals precipitated from caustic solutions[C]// ACS symposium Series. Washington, DC, America: American Chemical Society, 1997: 123-135.
[17] LI H X, ADDAI-MENSAH J, THOMAS J C, GERSON A R. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions[J]. Journal of Crystal Growth, 2005, 279(3/4): 508-520.
[18] LEE Y P, LIU Y H, YEH C S. Formation of bayerite, gibbsite and boehmite particles by laser ablation[J]. Physical Chemistry Chemical Physics, 1999, 1(19): 4681-4686.
[19] WANG H Q, WANG Z, LIU L, GONG X Z, WANG M Y. Alumina hydrate polymorphism control in Al-water reaction crystallization by seeding to change the metastable zone width[J]. Crystal Growth & Design, 2016, 16(2): 1056-1062.
[20] COUNTER J, GERSON A, RALSTON J. Caustic aluminate liquors: Preparation and characterisation using static light scattering and in situ X-ray diffraction[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1997, 126(2/3): 103-112.
[21] GERSON A R, COUNTER J A, COOKSON D J. Influence of solution constituents, solution conditioning and seeding on the crystalline phase of aluminium hydroxide using in situ X-ray diffraction[J]. Journal of Crystal Growth, 1996, 160(3/4): 346-354.
[22] SATO T. Preparation of gelatinous aluminium hydroxide[J]. Journal of Inorganic General Chemistry, 1972, 391(1): 69-78. (in German)
[23] 李小斌, 赵东峰, 王丹琴, 阎 丽. 铝酸钠溶液分解过程的理论及技术研究进展[J]. 中国有色金属学报, 2011, 21(10): 2577-2593.
LI Xiao-bin, ZHAO Dong-feng, YU Dan-qin, YAN Li. Research progress in theory and technology of gibbsite precipitation from sodium aluminate solution[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(10): 2577-2593.
[24] LI Y, ZHANG Y F, YANG C, ZHANG Y. Precipitating sandy aluminium hydroxide from sodium aluminate solution by the neutralization of sodium bicarbonate[J]. Hydrometallurgy, 2009, 98(1/2): 52-57.
[25] LEFEVRE G, PICHOT V, FEDOROFF M. Controlling particle morphology during growth of bayerite in aluminate solutions[J]. Chemistry of Materials, 2003, 15(13): 2584-2592.
[26] VAN STRATEN H A, DE B P L. Precipitation from supersaturated aluminate solutions.Ⅱ. Role of temperature[J]. Journal of Colloid and Interface Science, 1984, 102(1): 260-277.
[27] SIPOS P. The structure of Al(Ⅲ) in strongly alkaline aluminate solutions—A review[J]. Journal of Molecular Liquids, 2009, 146(1): 1-14.
[28] WEI D, YIN Q X, HAO H X, YING B, XIA Z, HUANG J T, XIANG L, XIE C, GONG J B. Solution-mediated polymorphic transformation of prasugrel hydrochloride from form Ⅱ to form Ⅰ[J]. Industrial & Engineering Chemistry Research, 2014, 53(14): 5652-5659.
[29] O’BRIEN L E, TIMMINS P, WILLIAMS A C, YORK P. Use of in situ FT-Raman spectroscopy to study the kinetics of the transformation of carbamazepine polymorphs[J]. Journal of Pharmaceutical and Biomedical Analysis, 2004, 36(2): 335-340.
[30] TONG Y, WANG ZZ, YANG E, PAN B C, DANG L P, WEI H Y. Insights into cocrystal polymorphic transformation mechanism of ethenzamide-saccharin: A combined experimental and simulative study[J]. Crystal Growth & Design, 2016, 16(9): 5118-5126.
[31] ROSALES G D, RUIZ M D C, RODRIGUEZ M H. Novel process for the extraction of lithium from β-spodumene by leaching with HF[J]. Hydrometallurgy, 2014, 147/148: 1-6.
[32] CHEN Y, TIAN Q Q, CHEN B Z, SHI X C, LIAO T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process[J]. Hydrometallurgy, 2011, 109(1/2): 43-46.
[33] LI J, PRESTIDGE C A, ADDAI-MENSAH J A. The influence of alkali metal ions on homogeneous nucleation of Al(OH)3 crystals from supersaturated caustic aluminate solutions[J]. Journal of Colloid and Interface Science, 2000, 224(2): 317-324.
[34] LI H X, ADDAI M J, THOMAS J C, GERSON A R. A study of colloidal Al(Ⅲ)-containing species in fresh/caustic aluminate solutions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2003, 223(1/3): 83-94.
[35] LI X B, ZHAO D F, YANG S S, WANG D Q, ZHOU Q S, LIU G H. Influence of thermal history on conversion of aluminate species in sodium aluminate solution[J]. Transactions of Nonferrous Metals Society of China, 2014, 10: 3348-3355.
[36] 戴国亮, 解 莹, 康 琦, 胡文瑞. 溶液热历史对蛋白质晶体生长影响的内在原因研究[J]. 化学学报, 2007, 65(13): 1202-1206.
DAI Guo-liang, XIE Ying, KANG Qi, HU Wen-rui. Study of intrinsic reason of effect of solution thermal history on protein crystal growth [J]. Acta Chemica Sinica, 2007, 65(13): 1202-1206.
[37] APPS J A, NEILL JM, JUN C H. Thermochemical properties of gibbsite, bayerite, boehmite, diaspore, and the aluminate ion between 0 and 350 ℃[J]. Lawrence Berkeley National Laboratory, 1988.
[38] MULLIN J W. Crystallization[M]. 4th ed. Oxford: Butterworth-Heinemann, 2001.
[39] JIANG Y F, LIU C L, XUE J, LI P, YU J G. Insights into the polymorphic transformation mechanism of aluminum hydroxide during carbonation of potassium aluminate solution[J]. Crystengcomm, 2018, 20: 1431-1442.
[40] SWEEGERS C, van ENCKEVORT W J P, MEEKES H, BENNEMA P, HIRALAL I D K, RIJKEBOER A. The impact of twinning on the morphology of γ-Al(OH)3 crystals[J]. Journal of Crystal Growth, 1999, 197: 244-253.
[41] SONTHALIA R, BEHARA P, KUMARESAN T, THAKRE S. Review on alumina trihydrate precipitation mechanisms and effect of Bayer impurities on hydrate particle growth rate[J]. International Journal of Mineral Processing, 2013, 125: 137-148.
[42] GALE J D, ROHL A L, MILMAN V, WARREN M C. An ab initio study of the structure and properties of aluminum hydroxide: Gibbsite and bayerite[J]. The Journal of Physical Chemistry B, 2001, 105(42): 10236-10242.
Polymorphic transformation of aluminum hydroxide during spontaneous decomposition of supersaturated potassium aluminate solution
JIANG You-fa, LIU Cheng-lin, LUO Meng-jie, XUE Jin, LI Ping, YU Jian-guo
(National Engineering Research Center for Integrated Utilization of Salt Lake Resource, East China University of Science and Technology, Shanghai 200237, China)
Abstract: As the precursor for production of aluminum, aluminum trihydroxide has four polymorphs and the control of polymorphism of crystal product is vital for downstream operation and product quality. The effects of temperature and caustic concentration on the initial crystalline product and polymorphism transformation were investigated during spontaneous decomposition of supersaturated potassium aluminate solution. The results show that the initial crystalline product under relatively lower temperature (30 ℃, 40 ℃) is bayerite and that under relatively higher temperature (60 ℃, 80 ℃) is a mixture of gibbsite and bayerite. The influence of alkaline concentration on the initial products is not significant. By comparison between the saturation concentration of the polymorphs and the solution concentration, it is concluded that the formation of bayerite is kinetically favored according to a predominance of the initial crystalline products under low temperature. The dissolution of bayerite was detected by SEM, which infers that the transformation of bayerite to gibbsite is attributed to the solvent-mediated polymorphic transformation (SMPT) process. The polymorphic transformation is promoted with the increase of temperature and caustic concentration, which further indicates a dissolution-recrystallization process.
Key words: potassium aluminate; crystallization; bayerite; polymorphic transition; gibbsite
Foundation item: Projects(22A201514058, 222201717008) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2018-02-18; Accepted date: 2018-07-18
Corresponding author: YU Jian-guo; Tel: +86-21-64252170; E-mail: jgyu@ecust.edu.cn
(编辑 李艳红)
基金项目:中央高校基本科研业务费专项资金资助项目(22A201514058,222201717008)
收稿日期:2018-02-18;修订日期:2018-07-18
通信作者:于建国,教授,博士;电话:021-64252170;E-mail:jgyu@ecust.edu.cn