
Preparation and mechanical properties of in situ Al2O3/Al composites by adding NH4AlO(OH)HCO3
JIANG Lan1, CUI Yan-zhou2, SHI Yu-juan3, DING You-dong1, YUAN Fang-lan1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. Bundy Fluid System (Shanghai) Co. Ltd, Shanghai 200131, China;
3. N. E. U Engineering & Research Institute Co. Ltd, Shenyang 110003, China
Received 30 October 2010; accepted 27 May 2011
Abstract: Aluminium matrix composite reinforced by Al2O3 particles was produced by adding NH4AlO(OH)HCO3 into molten aluminum. The mechanical properties and wear behavior of the as-fabricated composites were studied. The results show that during stirring γ-Al2O3 particles were formed via decomposition reaction of NH4AlO(OH)HCO3, and the distribution of Al2O3 particles is more uniform in the matrix aluminum than directly added Al2O3 into molten aluminum. The density and the hardness values of the as-fabricated composites increase with increasing the particle volume fraction, while the tensile strength of the composites decreases with increasing the volume fraction of the Al2O3 particles. The wear rate of the composites decreases with increasing the volume fraction of the particle and loading. The in situ formed Al2O3/Al composite by adding NH4AlO(OH)HCO3 shows more superior mechanical and wear behaviors than that prepared by directly adding Al2O3 particles.
Key words: aluminum matrix composite; NH4AlO(OH)HCO3; A12O3 particles; microstructure; hardness; tensile strength; wear behavior
1 Introduction
Particle reinforced aluminum matrix composites (Al-MMCs) have attracted much attention due to their excellent performance, such as lower density, high speci?c stiffness, high speci?c strength and good thermal stability compared with pure aluminum and aluminum alloys [1-3]. The Al-MMCs can be prepared by in situ or ex situ synthesis. In ex situ methods, the particles must be prepared prior to the fabrication of the composites. There are some defects and dif?culties in fabricating composites by ex situ methods. For example, the particle size is dif?cult to be controlled. Furthermore, the distribution of ?ne particles in matrix is generally inhomogeneous. These disadvantages bring about a detrimental effect on material properties [4]. To overcome these problems, in situ technique has been proposed to fabricate composites where the reinforcement phases are in situ formed within the matrix [5].
Recently, several in situ technologies of producing ceramic particles in metals have been proposed, e.g. direct melt reaction (DMR) process [1, 2, 6], direct decomposing reaction (DDR) process [7], reactive hot pressing (RHP) [8-9] and self-propagating high temperature synthesis (SHS) [10-11]. The DMR and DDR are considered one of the most promising in situ synthesizing techniques for commercial applications due to their simplicity, low cost and near net-shape forming capability [1].
In the present paper, Al2O3/Al composites were fabricated by a new in situ synthesizing technique where the reinforced phase Al2O3 particles were introduced by DDR process. In this process, the in situ Al2O3 particles were obtained from decomposition of NH4AlO(OH)HCO3 by adding it to aluminium melt. The microstructure was examined using scanning electron microscopy (SEM). Physical and mechanical properties of the composites were evaluated and the wear behavior was also investigated.
2 Experimental
2.1 Materials and processing
The raw materials used in this study were commercial purity Al (99.5% purity) and NH4AlO(OH)HCO3 (99.5% purity), which were employed as the matrix metal and in situ formed reinforcement phase by decomposing NH4AlO(OH)HCO3, respectively. In order to get a better comparison with Al2O3 reinforced pure aluminum composites, alumina(<1 μm, 99.7% purity) was used as comparative reinforcement. After melting at 667 °C, the molten metal was continuously reheated and stirred at a rotating speed of 200 r/min and 850 °C. Preheated powder of NH4AlO(OH)HCO3 was gradually added to the swirl of molten metal during stirring. As a result, in situ formed Al2O3 from NH4AlO(OH)HCO3 was produced within the metal matrix, and a molten composite formed. As the reaction was completed, the agitating was stopped for a while to let the gas out and the molten was then cast in the pressure die-machine with a maximum locking force of 1.5 MN. A comparative study was performed to prepare Al2O3/Al composite by directly adding superfine Al2O3 into molten Al under the same testing condition.
2.2 Material characterization
Specimens for SEM observations were taken from the as-cast composites. The specimens were polished in a conventional manner and the microstructure was observed on a SSX-550 scanning electron microscope (SEM). Philips PW 1840 X-ray diffractometer with a Cu Kα radiation was used to characterize the phase composition of the as-prepared composites.
The densities of the composites were measured using the water displacement technique. The theoretical density of composite can then be calculated according to the rule of mixtures. Brinell hardness (HBN) measurements were carried out using a ball of 5 mm in diameter under load of 498 N for 30 s. A Hounsfield H25KS tensile machine was used to evaluate the tensile behaviors in accordance with ASTM standard E-8-81. The strain rate was maintained to be 0.1 mm/min in tensile test till rupture. Wear tests were carried out using a pin-on-disk tester with the aluminum or composites as the pin and GCr15steel as the disk. The disk specimen was 70 mm in diameter and 5 mm in thickness. The pin test piece had a flat surface with a diameter of 6 mm. The diameter of the wear track was 60 mm. The surfaces of both the disk and the pin were polished with #1500 emery paper. The wear tests were performed under dry sliding conditions at loads of 5, 10 and 15 N, respectively.
3 Results and discussion
3.1 Microstructure and density
Figure 1 shows the microstructures of the composites prepared by adding NH4AlO(OH)HCO3 powder and the traditional composites by adding superfine Al2O3 particles at 8% (volume fraction), respectively.
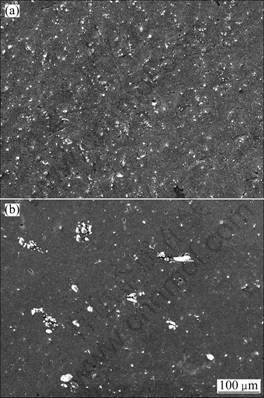
Fig. 1 SEM images of Al2O3/Al composites with 8% (volume fraction) Al2O3: (a) Composite by adding NH4AlO(OH)HCO3; (b) Composite by directly adding Al2O3
From Fig. 1(a), we can see that the Al2O3 particles in situ produced from NH4AlO(OH)HCO3 are uniformly distributed in the Al matrix. The particulate size of the Al2O3 was measured to be 0.5-2 μm in diameter, although some of them were much smaller. Figure 1(b) indicates that the Al2O3 particles agglutinate and distribute non-uniformly in the matrix.
The XRD pattern in Fig. 2 can be used to analyze reaction process, which reveals that γ-Al2O3 is the only resultant in the composite and residual NH4AlO(OH)HCO3 does not exist. According to XRD result, the in situ decomposition reaction in aluminum matrix can be expressed as the following reaction:
2NH4AlO(OH)HCO3(s) →
Al2O3(s) + 2NH3(g) + 2CO2(g) + 3H2O(g) (1)

Fig. 2 XRD pattern of composite fabricated by adding NH4AlO(OH)HCO3
NH4AlO(OH)HCO3 is known to be one of the raw materials for fabricating commercial superfine high-pure Al2O3 [12-13]. The mode of decomposition from NH4AlO(OH)HCO3 is γ-Al2O3 with a high specific surface area and sorption capacity [14]. At the same time, the Al2O3 particles are in spherical shape, which have better wettability with the molten aluminum, so they could bond firmly with the aluminum matrix. The morphology of this kind of composites is able to reduce stress concentration and easy to transfer load, so the properties of the composites could be improved.
The density was measured using the water displacement technique. The results of the density measurements of the matrix and the composites are presented in Fig. 3. Using the law of mixtures, the theoretical densities of the composites were calculated. Figure 3 shows the relationship between the densities and volume fraction of the Al2O3 particles. It can be observed from Fig. 3 that the densities of the composites increased by adding Al2O3 particles. According to Ref. [15], the matrix aluminium and Al2O3 particle have the densities of 2.7?103 and 3.9?103 kg/m3, respectively. Therefore, the density values of the composites increased by adding Al2O3 particles. Figure 3 also shows that while experimental and theoretical results are close to each other, there is a little deviation of the measured densities from theoretical calculations. This could be attributed to the change of volume fraction of matrix or Al2O3 particle in the sample. Figure 1 shows that there is no porosity in the composites, so it can be deduced that such a little divergence in the volume fraction of Al2O3 particle has no effect on the properties of the composites.
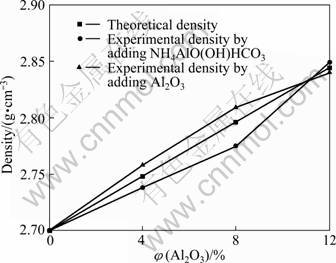
Fig. 3 Density as function of volume fraction of Al2O3 for composites obtained under different conditions
3.2 Hardness and tensile strength
After polishing, the hardness values of the composites and matrix were measured. The relationship between the particle volume fraction and the hardness of the composites is plotted in Fig. 4. Figure 4 shows that the hardness of the composites is increased with increasing particle volume fraction. The hardness of composites obtained by adding NH4AlO(OH)HCO3 is higher than that by directly adding Al2O3 particles. This is due to the fact that the small decomposed particles have more surface area in the matrix.

Fig. 4 Relationship between volume fraction of Al2O3 and hardness of composites
The ultimate tensile strength (UTS) of the composites is presented in Fig. 5, which shows that the Al2O3 in the matrix aluminium leads to a reduction in the tensile strength. The decrease in UTS of the MMCs with increasing Al2O3 particle content may be attributed to the fracture of the surfaces between Al2O3 and matrix Al during tensile loading. On the other hand, the lower UTS values were obtained in the composites by directly adding Al2O3 particles. This is due to the fact that large particles or agglomerated particles could have some ?aws with respect to small ones and easily crashed under loading. In such cases, this may be another reason why large particle reinforced composites show lower hardness than composites with small particles.
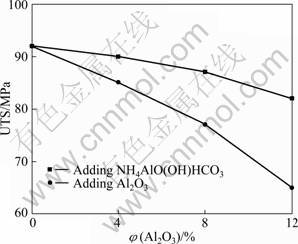
Fig. 5 Relationship between volume fraction of Al2O3 particles and ultimate tensile strength of composites
3.3 Wear behavior
Dry sliding wear tests were carried out at a sliding velocity of 0.5 m/s. Figure 6 shows the variation in mass wear rates of two kinds of MMC pin specimens as a function of the volume fraction of Al2O3 under different loads. It is observed that the wear rate of the matrix aluminium was more than that of the composite under all loads; the wear rates of the composites decrease with increasing particle volume fraction and the wear rates of the composites increase with the increase of the load. From Fig. 6, it can be observed that the wear rate of the composite prepared by adding NH4AlO(OH)HCO3 is smaller than that of the composite prepared by adding Al2O3. So the composite prepared by adding NH4AlO(OH)HCO3 is more wearable than the composite prepared by directly adding Al2O3. The main reason is that the Al2O3 particles decomposed from NH4AlO(OH)HCO3 are fine and uniform, and the bonding between the Al2O3 reinforcement and the Al matrix is improved.
4 Conclusions
1) An in situ Al2O3 reinforced Al matrix composite was successfully fabricated by adding NH4AlO(OH)HCO3, where g-Al2O3 particles distribute uniformly. Alumina particles in aluminium matrix lead to the increased density of the composites.
2) The hardness of the composites increases with increasing particle volume fraction, and is higher by adding NH4AlO(OH)HCO3 than by directly adding Al2O3 particles. The ultimate tensile strength is decreased with increasing the particle volume fraction.
3) Wear rate of the g-Al2O3/Al composites decreases with increasing the volume fraction of the reinforced particles and the load applied. The composite prepared by adding NH4AlO(OH)HCO3 is more wearable than the composite prepared by directly adding Al2O3.

Fig. 6 Wear rate of Al matrix and composites as function of volume fraction of Al2O3 at loads of 5 N (a), 10 N (b) and 15 N (c) at sliding velocity of 0.5 m/s
References
[1] WANG Hong-ming, LI Gui-rong, ZHAO Yu-tao, CHEN Gang. In situ fabrication and microstructure of Al2O3 particles reinforced aluminum matrix composites [J]. Materials Science and Engineering A, 2010, 527: 2881-2885.
[2] ZHAO Y T, ZHANG S L, CHEN G, CHENG X N, WANG C Q. In situ (Al2O3+Al3Zr)np/Al nanocomposites synthesized by magneto-chemical melt reaction[J]. Composite Science and Technology, 2008, 68: 1463-1470.
[3] ROY D, GHOSH S, BASUMALLICK A, BASU B. Preparation of Fe-aluminide reinforced in situ metal matrix composites by reactive hot pressing [J]. Materials Science and Engineering A, 2006, 415: 202-206.
[4] YU P, SNITOVSKY. Recrystallization behavior of aluminum layers in Al/Ti/Si and Al/Ti/SiOx/Si structures [J]. Materials Chemistry and Physics, 2005, 93: 109-111.
[5] KOCZAK M J, PREMKUMAR M K. Emerging technologies for the in-situ production of MMCs [J]. Journal of Metals, 1993, 45: 44-48.
[6] LI G R, ZHAO Y T, DAI Q X, CHENG X N, WANG H M, CHEN G. Fabrication and properties of in situ synthesized particles reinforced aluminum matrix composites of Al-Zr-O-B system [J]. Journal of Materials Science, 2007, 42: 5442-5447.
[7] WANG Hong, FU Gao-feng, JIANG Lan, SHI Yu-juan, LIU Chang-sheng. Study on in situ Al/ Al2O3 composites by adding NH4Al(SO4)2 into pure Al melt [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(5): 853-857.
[8] TANG F, HAGIWARA M, SCHOENUNG J M. Tensile deformation and fracture in a bulk nanostructured Al-5083/SiCp composite at elevated temperatures [J]. Materials Science and Engineering A, 2007, 30: 245–248.
[9] VIJIAN P, ARUNACHALAM V P. Optimization of squeeze cast parameters of LM6 aluminium alloy for surface roughness using taguchi method [J]. Journal of Materials Processing Technology, 2006, 180: 161–166.
[10] KOK M. Abrasive wear of Al2O3 particle reinforced 2024 aluminium alloy composites fabricated by vortex method [J]. Composites: Part A, 2006, 37: 457-464.
[11] YANA Z C, HUANGA Y, ZHANGA Y, HADJIPANAYIS G C, SOFFAB A W, WELLERC D. Magnetic and structural properties of MnAl/Ag granular thin films with L10 structure [J]. Scripta Materialia, 2005, 53: 463-468.
[12] LI Dong-hong, WEN Jiu-ba, LI Wang-xing. Study on preparation of nano-sized alumina by AACH thermal decomposition [J]. Journal of Henan University of Science and Technology, 2005, 26(4): 1-4. (in Chinese)
[13] FU Gao-feng, BI Shi-wen, YANG Yi-hong, LI Shang-ming, MI Zhen-zhen. Preparation of super-fine α-Al2O3 by NH4AlO(OH)HCO3 thermal decomposition [J]. The Chinese Journal of Nonferrous Metals, 1998, 8(S1): 64-66. (in Chinese)
[14] SHEN Xiao-qing, LI Zhong-jun, YAO Hong-chang, ZHANG Jun. Preparation of nanosized alumina powders by pyrolysis of ammonium aluminium carbonate hydroxide [J]. Chinese Journal of Inorganic Chemistry, 2003, 19(6): 651-654. (in Chinese)
[15] QIU Zhu-xian. Metallurgy [M]. Shenyang: Northeastern University Press, 2001. (in Chinese)
添加NH4AlO(OH)HCO3原位生成
Al2O3/Al复合材料的制备及其性能
姜 澜1, 崔焱洲2, 史玉娟3, 丁友东1, 袁芳兰1
1. 东北大学 材料与冶金学院,沈阳 110004;
2. 邦迪管路系统(上海)有限公司,上海 200131;
3. 东北大学设计研究院有限公司,沈阳 110013
摘 要:碳酸铝铵与熔融的铝液反应原位生成颗粒增强铝基复合材料,对复合材料的力学性能和摩擦磨损行为进行研究。结果表明:在搅拌的铝熔体中碳酸铝铵发生分解反应生成γ-Al2O3;该原位反应的增强颗粒比直接添加的Al2O3在铝熔体中分布得更均匀;复合材料的密度和硬度随着增强相加入量的增加而提高, 而强度则随着增强相加入量的增加而降低;磨损率随着增强相加入量的增加和载荷的增加而提高;原位反应生成的复合材料的力学性能和耐磨性明显优于直接添加Al2O3颗粒形成的复合材料的。
关键词:铝基复合材料;NH4AlO(OH)HCO3;氧化铝颗粒;微观组织;硬度;抗拉强度;摩擦磨损性能
(Edited by YANG Hua)
Foundation item: Project (2009BAE80B01) supported by the Ministry of Science and Technology, China
Corresponding author: JIANG Lan; Tel: +86-24-83681325; E-mail: jiangl@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)60992-8