玻璃制作工具用5% ZrO2纳米铂材的机械合金化制备
来源期刊:中国有色金属学报(英文版)2014年第z1期
论文作者:Taek-Kyun JUNG Dong-Woo JOH Seung-Yub LEE Myung-Sik CHOI Soong-Keun HYUN Hyo-Soo LEE
文章页码:99 - 105
关键词:铂;氧化锆;纳米粒子;电丝爆炸法;机械合金化
Key words:platinum; zirconia; nanoparticle; electrical wire explosion; mechanical alloying
摘 要:对机械合金化制备的玻璃工业中用作结构零件的Pt-5%ZrO2(体积分数)的进行表征。通过锆丝电爆炸法制备氧化锆(ZrO2)纳米颗粒(粒径<100 nm),并在室温下与铂粉(粒径< 44 mm)球磨2~72 h。普通球磨至48 h时,铂粒径遵循经典的递减趋势,至72 h时观测到颗粒团聚现象。晶粒尺寸演变规律与颗粒尺寸规律相似,48 h后减至50 nm左右。但是,铂晶粒的均方根应变规律相反,一直在增长,直至48 h时得到最大值,然后出现弛豫。粉末球磨48 h后,采用放电等离子体烧结制成块体。根据测量得到烧结体的质量损失表明:合金虽然相对密度较低,但有较好的热稳定性。
Abstract: Synthesis and characterization of mechanically alloyed Pt-5%ZrO2 (volume fraction) for structural components in the glass industry were described. Zirconia (ZrO2) nanoparticles (<100 nm) were produced by the electrical explosion of zirconium (Zr) wires, and blended with platinum (Pt) powders (<44 mm) for 2-72 h in ambient atmosphere. The Pt particle size followed the typical decreasing trend of the normal ball milling process up to 48 h, but particle agglomeration was observed at 72 h. The grain size evolution was similar to that of the particle size, dropping down to around 50 nm at 48 h. The root mean square strain of the Pt crystallites showed the opposite behavior, maximizing at 48 h with a subsequent relaxation process. For the 48 h ball milled powders, spark plasma sintering was carried out to form a bulk disk. The measured mass loss of the sintered bulk sample shows a decent thermal stability despite its relatively low density.
Trans. Nonferrous Met. Soc. China 24(2014) s99-s105
Taek-Kyun JUNG1, Dong-Woo JOH1, Seung-Yub LEE2, Myung-Sik CHOI1, 3, Soong-Keun HYUN3, Hyo-Soo LEE1
1. Korea Institute of Industrial Technology, 7-47 Songdo-dong, Yenosu-gu, Incheon 406-840, Korea;
2. Department of Applied Physics and Applied Mathematics, Columbia University, New York, NY 10027, USA;
3. School of Materials Science and Engineering, Inha University, 253 Yonghyun-Dong, Incheon 405-751, Korea
Received 18 June 2013; accepted 10 October 2013
Abstract: Synthesis and characterization of mechanically alloyed Pt-5%ZrO2 (volume fraction) for structural components in the glass industry were described. Zirconia (ZrO2) nanoparticles (<100 nm) were produced by the electrical explosion of zirconium (Zr) wires, and blended with platinum (Pt) powders (<44 mm) for 2-72 h in ambient atmosphere. The Pt particle size followed the typical decreasing trend of the normal ball milling process up to 48 h, but particle agglomeration was observed at 72 h. The grain size evolution was similar to that of the particle size, dropping down to around 50 nm at 48 h. The root mean square strain of the Pt crystallites showed the opposite behavior, maximizing at 48 h with a subsequent relaxation process. For the 48 h ball milled powders, spark plasma sintering was carried out to form a bulk disk. The measured mass loss of the sintered bulk sample shows a decent thermal stability despite its relatively low density.
Key words: platinum; zirconia; nanoparticle; electrical wire explosion; mechanical alloying
1 Introduction
Platinum (Pt) and platinum group metals are highly valuable because only a few hundred tons are produced annually. In spite of their high price, platinum metals have been widely used in the glass industry because of their physical properties and excellent corrosion resistance against aggressive glass melts in the high temperature range [1]. Typical applications of platinum and its alloys are feeder systems, bubble tubs, drain bushings, tank linings, melting crucibles and glass fiber bushings. Depending on the specific application, these components usually operate at temperatures of about 1223 K in atmospheres composed of a mixture of ammonia and air, 1523 K in the liquid glass environments, and up to 1873 K in atmospheres of air or industrial gases [2].
However, the use of pure platinum in the glass industry is not suitable for glass making tools owing to its low strength and coarsening at high temperature. A significant increase in strength can be achieved by alloying through a solid solution hardening mechanism. Rhodium (Rh) and iridium (Ir) have been considered promising elements in Pt-X solid solution hardened alloy because they are not detrimental to the chemical stability of the platinum. Nevertheless, the use of these elements is limited considering that the dissolution of the smallest traces of the alloying elements in the glass melts, resulting in an undesirable change in the optical properties of glass products [3]. Furthermore, at high temperature around 1873 K, Pt-Rh and Pt-Ir solid solution hardened alloys provide a limited increase in mechanical strength, and could not prevent extreme grain from growth [4].
For this reason, oxide dispersion strengthened (ODS) Pt has been developed for both pure Pt and Pt alloys [5]. The provided finely dispersed oxide particles of zirconia (ZrO2) or yittria (Y2O3) in the Pt matrix are very small (<1 μm) and their inter-particle spacing is also small (<10 μm), the particles will hinder the movement of dislocations in the matrix and thus give an increase in strength [6]. Furthermore, the dispersed particles hinder the movement of grain boundaries at high temperature and therefore restrict grain coarsening, leading to an increase of stress-rupture strength. ODS Pt alloys can be manufactured by two different approaches. One is the typical powder metallurgy route [5], and the other is the internal oxidation path, commercially named as DPH (dispersion hardened) platinum materials [6]. Both methods have their own advantages and disadvantages in terms of material properties or processing conditions. And also, a precipitation hardened Pt-based alloy, analogous to the Ni-based super alloy, was introduced as an another alternative solution [7]. As known from the existing literature, many properties, such as stress- rupture strength, creep resistance, ductility, formability, weldability and corrosion/oxidation resistance, have been satisfied for industrial applications. However, strengthening of Pt alloys has remained as a primary condition for the structural component at high temperature, with an increasing demand for better mechanical stability.
One possible way to improve mechanical stability is decreasing the size of the reinforcing particles. Recently, we have produced metallic nanoparticles less than 100 nm by the electrical explosion of metal wire [8]. Electrical explosion is a physical method which is effective in producing high purity nanoparticles, compared to those prepared by chemical methods [9]. In this method, a high current pulse is passed through a thin metal wire, which leads to explosion, evaporation and ionization. The vaporized and ionized metal expands outward, and it is cooled by interacting with the surrounding gas or liquid media. Finally, the condensed vapor forms into nanoparticles.
Another way of strengthening Pt alloys is minimizing the crystallite size without the addition of solute atoms, which are potential contamination sources to the glass melts. Mechanical alloying (MA), which was originally developed to produce ODS super alloys for the aerospace industry, would also be an effective way to produce ODS Pt alloys. MA is a solid-state powder processing technique involving repeated cold welding, fracturing, and re-welding of powder particles in a high-energy ball mill [10]. Several reports on mechanical alloying of platinum powder with micro-scale zirconia and/or yittria powders have been made [5, 11-13]. However, the mechanical alloying behavior of ODS Pt alloy with nano-scale oxides has not been well addressed yet.
In this work, we have produced nano-scale zirconia powders using an electrical wire explosion technique, investigated the microstructural properties of the ball milled Pt-5%ZrO2 (volume fraction) powders, and prototyped a bulk sample via spark-plasma sintering process.
2 Experimental
2.1 Raw materials
High purity (3N5) Pt powders with a particle size smaller than 44 μm were purchased from Colonial Metals, Inc (Elkton, Maryland, USA). ZrO2 nanopowders were synthesized by the electrical explosion of Zr wires (2N2) with a diameter of 0.25 mm. The wires were exploded in an argon/oxygen atmosphere (70%Ar+30%O2, volume fraction) with a 80 mm in feeding length, 24 kV in charging voltage, and 6 μF in capacitance. X-ray diffraction confirmed that the produced powders were Baddeleyite ZrO2 phase. Figure 1 shows scanning electron micrographs of the Pt powders and the ZrO2 nanoparticles. SEM images reveal that the size of produced ZrO2 powders is less than 100 nm, while Pt powders are less than 50 μm.

Fig. 1 SEM images
2.2 Mechanical alloying
Pt powders (95%, volume fraction) were blended together with ZrO2 (5% (volume fraction), or 1.37% (mass fraction)) nanoparticles, then ball milled at 100 r/min for 2, 8, 24, 48, and 72 h. ZrO2 ceramic balls (1 mm in diameter) and a ZrO2 container (500 mL) were used to suppress contamination during mechanical milling. The volume ratio of ball to powder was maintained at 100:1.
2.3 Synchrotron radiation experiment
Lab source X-ray can provide a general idea about the phase and lattice information. However, synchrotron radiation experiments with high flux and high resolution were proposed in order to investigate the precise microstructural behavior according to the milling time. Experiments were performed at the National Synchrotron Light Source (NSLS), beam line X10A in Brookhaven National Laboratory, USA. For high resolution, a single- bounce Ge monochromator (analyzer) was placed in front of the scintillator detector to produce 0.003° full width half maximum (FWHM) in 2θ, indicating that measurable grain size from the Scherrer equation goes up to 2 μm under a given beam conditions (beam size: 1 mm× 1 mm, energy: 11.31 keV; l=1.0964 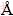 ).
).
2.4 Consolidation to bulk sample
The 48 h mechanically alloyed Pt-5%ZrO2 (volume fraction) powders were finally spark plasma sintered at 1673 K for 30 min under a 45 MPa load. The density of sintered sample was determined by Archimedes’ principle. Mass change in the sintered sample was measured from the initial and final masses after heat treatment at 1573 K for 96 h in ambient atmosphere.
3 Results and discussion
3.1 Microstructure from SEM
Figures 2(a)-(e) show the SEM micrographs of Pt-5%ZrO2 (volume fraction) composite powders ball milled for different time. In the early stages of milling (2 h), the powder shape became flattened due to cold welding effects. With increasing milling time (up to 48 h), powders became more rounded in shape and finer in particle size. This result indicates that predominant processes at this stage are cold welding and fracturing. Indeed, the powder size is estimated to be less than 5 μm after a milling for 48 h. However, after a milling time of 72 h, there is a rise in the particle size due to agglomeration.
Fig. 2 SEM micrographs of Pt-5%ZrO2 (volume fraction) composite powders mechanically milled for different time
Based on the SEM images, it is speculated that onset of particle agglomeration is driven by the accumulated strain and thermal energy during the ball milling process. It has been generally known that particle size becomes stable through a dynamic balance between welding and fracturing [10]. However, RAMAR et al [14] reported particle agglomeration at the later stage of ball milling. They observed constant hardness values and crystallite sizes by FWHM of diffraction peaks even at the point of particle growth. This means that particles are in touch with each other without microstructural change, but our SEM image, Fig. 2(e), seems to suggest internal change in microstructure.
3.2 Grain size and RMS strain from diffraction analysis
In order to quantify recrystallization and growth kinetics, time resolved ex-situ synchrotron diffraction measurements and peak shape analysis have been performed. The reference Pt and ZrO2 powders are displayed in Figs. 3(a) and (b), respectively. Pt was identified as a pure FCC (JCPDS 4-802) phase, but ZrO2 turned out to be a mixture of major monoclinic (JCPDS 37-1484) and minor tetragonal (JCPDS 79-1763) phases. Figures (c)-(e) illustrate the phase evolution of ZrO2 according to the milling time. Monitoring ZrO2 peaks is important because the oxides dispersion strengthening would require ZrO2 to be intact for the subsequent consolidation process. Otherwise, it is likely to cause solid solution hardening via decomposition of ZrO2 into zirconium and oxygen within Pt matrix.

Fig. 3 Full synchrotron diffraction patterns of Pt and ZrO2 powders as starting materials and evolution of low-angle regions with increasing milling time
The synchrotron data show that ZrO2 peaks lose their intensity with peak broadening up to 8 h, as shown in Fig. 3(e), but it was difficult to observe after 24 h milling. Peak broadening is due to the grain size minimization or local strain among crystallites, while the intensity drop corresponds to phase extinction or amorphization of the crystals. OKUDA and FUJIWARA [15] reported that carbide, nitride or intermetallic compounds can dissolve in metal during mechanical alloying by force, and oxides such as Y2O3, which do not have any solubility limit, can dissolve in metal during a mechanical alloying process [15]. ALINGER et al [16] also showed from small angle scattering data that Y2O3 dissolved into ferric alloys during MA process. However, RAMAR et al [14] commented that oxides can still be maintained as particles, and the disappearance of the ZrO2 peaks can be due to the very small ZrO2 particles size, which is reduced by the milling. Authors share the same idea as RAMAR because it is possible to lose coherent scattering power of the crystallites, considering the nano-scale small volume fraction of ZrO2. Secondary ion mass spectroscopy (SIMS) would reveal more details, but at this point, there is no direct evidence indicating that zirconium and oxygen are decomposed into Pt matrix (solid solution hardening), or maintained as amorphous or very fine nano-clusters outside Pt particles (oxide dispersion strengthening).

Fig. 4 Time-resolved Pt (111) diffraction profile (a) and FWHM and integrated intensity vs ball milling time (b)
Whether or not ZrO2 powders are dissolved in Pt matrix, Pt powders experience peak broadening with decreasing intensity at all five reflections. Figure 4(a) shows time-resolved Pt (111) diffraction profiles; FWHM and integrated intensity (fitted to Lorentzian profiles) for each milling step are plotted in Fig. 4(b). It is clearly seen that (111) peak loses its intensity up to 48 h, but increases again at 72 h. FWHM shows the opposite behavior, indicating strain relaxation via recrystallization and/or grain growth.
Both instrument and sample contribute to the FWHM of the diffracted intensity. Since the incident beam is very sharp (0.003° in 2θ), it was assumed that diffracted peak profiles are mostly from samples. However, it is still necessary to separate size and strain broadening in the sample; hence, Williamson-Hall (W-H) method [17,18] (Eq. (1)) was employed based on the fact that grain size is not dependent on hkl, but strain is. Equation (1) contains size (Scherrer equation) and strain components as follows.
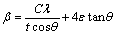 (1)
(1)
where t is the averaged dimension of crystallites; C is Scherrer constant (here C@1); λ is the wavelength of X-ray; β is the FWHM of the peak.
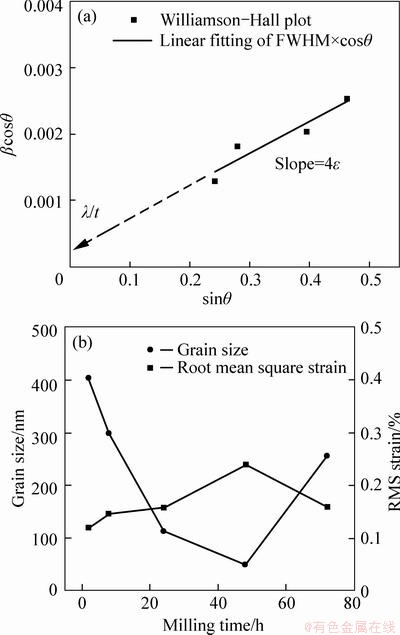
Fig. 5 Williamson-Hall plot (a) and calculated grain size and RMS strain information (b)
If one plots βcosθ against sinθ as shown in Fig. 5(a), the y-intercept will be l/t, and the strain broadening can be calculated from the slope. By applying W-H plots in all cases, grain size and root mean square (RMS) strain values are extracted and plotted in Fig. 5(b). The results show that grain size starts decreasing from 400 nm at 2 h ball milling conditions, down to 50 nm at 48 h. Then, it increases again back to 250 nm. RMS strain values also increase up to 0.25% with relaxation at 72 h. Therefore, it can be concluded that the ball milling process causes both particle and grain size to decrease while increasing RMS strain within particles. At 72 h, the relaxation phenomenon was more obvious in grain size and strain profile (obtained from synchrotron data) than particle size/shape information (from SEM micrographs). This suggests that agglomeration of the particles is the secondary process after recrystallization & grain growth unlike the observation from Ref. [14], or at least a concurrent event with microstructural relaxation. It should be noted that W-H method reliably predicts size and strain evolution within an order of magnitude, but the absolute value should not be taken seriously due to the several assumptions made in W-H analysis.
3.3 Mass loss measurement from bulk sample
For the 48 h ball milled Pt-5%ZrO2 (volume fraction) powders, a spark plasma sintering was carried out at 1673 K for 30 min under a load of 45 MPa. No visible defects such as crack or macro-pore are seen in the 50 mm diameter, 1 mm thick disk. The density of sintered sample was estimated to be 89.5 % of the nominal value of Pt-5%ZrO2 (volume fraction). Mass loss of platinum-group metals at high temperature is an important estimate for the life of the Pt products and it has been known to occur by the formation of volatile oxides [19]. Table 1 shows the mass loss after heat treatment at 1573 K for 96 h. For comparison, Pt and Pt-20%Rh (mass fraction) alloy were prepared by the casting process and compared with sintered Pt-5%ZrO2 (volume fraction). Note that the sintered Pt-5%ZrO2 (volume fraction) is not fully dense, while the cast Pt and Pt-20%Rh (mass fraction) alloy are fully compact.
Table 1 Comparative result of mass loss for sintered sample after heat treatment at 1573 K
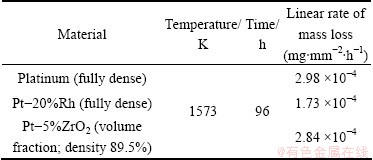
Our measurements show that mass loss of the Pt-20%Rh (mass fraction) alloy is smaller than any other samples, and the sintered Pt-5%ZrO2 (volume fraction) shows a bit less mass loss than pure Pt. It has been known that a linear rate of mass loss of rhodium is lower than that of platinum [20], which is consistent with our measurement. For the sintered Pt-5%ZrO2 (volume fraction) to be a promising alternative to pure Pt, its mass loss value should be comparable to that of Pt-20%Rh (mass fraction), but it is only slightly better than Pt. Considering the fact that the sintered powder is not fully dense compared to others, this result is not so disappointing. Since loose grain boundary or free volume can act as an efficient diffusion path for oxygen, it is expected to have more mass loss with less dense materials. As the density can be controlled to some extent by rolling conditions, mass loss can be improved, but it may affect other mechanical or chemical properties. Therefore, more comprehensive study in terms of processing conditions and materials selection (for example, amount of ZrO2 nanoparticles) must be performed, and a variety of measurements, such as tensile strength, rupture strength, creep rate, corrosion resistance, need to be understood for the actual applications.
4 Conclusions
In an attempt to develop a strengthened Pt-based alloy (or composites) system for the glass industry, 95% Pt (volume fraction; less than 44 mm) and 5% ZrO2 (volume fraction; less than 100 nm) powders are ball milled for 2, 8, 24, 48, and 72 h. ZrO2 powders are synthesized from the electrical explosion of zirconium wire to ensure high quality nanoparticles. SEM images illustrate that the average size of Pt particles decreases during the first 48 h of ball milling, but between 48 and 72 h the particle size grows again due to agglomeration. Williamson-Hall analysis of the synchrotron diffraction data shows the same trend in grain size evolution, and the opposite behavior for the root mean square strain of the Pt crystallites, suggesting that optimum ball milling time would be between 48 and 72 h to obtain powder of the minimum grain size and with a maximum internal strain.
Having determined that a 48 h milling time was optimal, spark plasma sintering was employed to form a 50 mm diameter disk from the mechanically alloyed Pt-5%ZrO2 (volume fraction) powders. Mass loss measurement seems to indicate decent thermal stability from the bulk sample although it is not completely dense. Since numerous materials properties and processing conditions have to be satisfied for the actual glass making tool in industry, more systematic mechanical alloying designs, such as materials selection and processing optimization, should be investigated. Such experiments are in progress.
Acknowledgements
Authors appreciate Dr. Steve Bennett for his help with synchrotron measurements. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.
References
[1] LUPTON D F, MERKER J, FISCHER B, VOLKL R. Platinum materials for the glass industry [C]//24th International Precious Metals Conference. Williamsburg, Virginia, USA, 2000.
[2] RDZAWSKI Z, CIURA L, NIKIEL B. Metallographic examination of catalyst gauzes and catchment gauzes from platinum and palladium alloys [J]. J Mater Process Technol, 1995, 53(1-2): 319-29.
[3] RAUB E. Metals and alloys of the platinum group [J]. Journal of the Less-Common Metals, 1959, 1(1): 3-18.
[4] FISCHER B, FREUND D, VOLKL R, LUPTON D F. Oxide dispersion hardened platinum materials for high temperature applications [C]//Thermec 2000. Las Vegas, Nevada, USA, 2000.
[5] JANSEN H A, THOMPSON F A. Use of oxide dispersion strengthened platinum for the production of high-quality glass [J]. Glastech Ber-Glass Sci Technol, 1992, 65(4): 99-102.
[6] FISCHER B. New platinum materials for high temperature applications [J]. Adv Eng Mater, 2001, 3(10): 811-820.
[7] CORNISH L A, FISCHER B, VOLKL R. Development of platinum- group-metal superalloys for high-temperature use [J]. MRS Bull, 2003, 28(9): 632-638.
[8] JUNG T K, JOH D W, LEE H S, LEE M H. Fabrication of ni nanopowder using wire explosion process and its characterization [J]. Rev Adv Mater Sci, 2011, 28(2): 171-174.
[9] CHO C, CHOI Y W, KANG C, LEE G W. Effects of the medium on synthesis of nanopowders by wire explosion process [J]. Appl Phys Lett, 2007, 91: 14501.
[10] SURYANARAYANA C. Mechanical alloying and milling [J]. Prog Mater Sci, 2001, 46(1-2): 1-184.
[11] WHALEN M V. Space station resistojets the compatibility of dispersion-strengthened platinum with candidate propellants [J]. Platin Met Rev, 1988, 32(1):2-10.
[12] STOKES J. Platinum in the glass industry zgs materials supplement conventional alloys [J]. Platin Met Rev, 1987, 31(2): 54-62.
[13] VOLKL R, FREUND D, FISCHER B, GOHLKE D. Comparison of the creep and fracture behavior of non-hardened and oxide dispersion hardened platinum base alloys at temperatures between 1200 degrees c and 1700 degrees c [C]//SAKUMA T, YAGI K. Creep and Fracture of Engineering Materials and Structures. Zurich-Uetikon: Transtec Publications Ltd, 2000: 77-83.
[14] RAMAR A, OKSIUTA Z, BALUC N, SCHAUBLIN R. Effect of mechanical alloying on the mechanical and microstructural properties of ods eurofer 97 [J]. Fusion Eng Des, 2007, 82(15-24): 2543-2549.
[15] OKUDA T, FUJIWARA M. Dispersion behavior of oxide particles in mechanically alloyed ods steel [J]. J Mater Sci Lett, 1995, 14(22): 1600-1603.
[16] ALINGER M J, ODETTE G R, HOELZER D T. The development and stability of Y-Ti-O nanoclusters in mechanically alloyed Fe-Cr based ferritic alloys [J]. J Nucl Mater, 2004, 329382-6.
[17] WILLIAMSON G K, HALL W H. X-ray line broadening from filed aluminium and wolfram [J]. Acta Metallurgica, 1953, 1(1): 22-31.
[18] BIJU V, SUGATHAN N, VRINDA V, SALINI S L. Estimation of lattice strain in nanocrystalline silver from X-ray diffraction line broadening [J]. J Mater Sci, 2008, 43(4): 1175-1179.
[19] COUPLAND D R, WILLIAMS P. New stirrer technology for the glass industry long-term benefits from the “diffusion choke” [J]. Platin Met Rev, 2005, 49(2): 62-69.
[20] KRIER C A, JAFFEE R I. Oxidation of the platinum-group metals [J]. Journal of the Less-Common Metals, 1963, 5(5): 411-431.
Taek-Kyun JUNG1, Dong-Woo JOH1, Seung-Yub LEE2, Myung-Sik CHOI1, 3, Soong-Keun HYUN3, Hyo-Soo LEE1
1. Korea Institute of Industrial Technology, 7-47 Songdo-dong, Yenosu-gu, Incheon 406-840, Korea;
2. Department of Applied Physics and Applied Mathematics, Columbia University, New York, NY 10027, USA;
3. School of Materials Science and Engineering, Inha University, 253 Yonghyun-Dong, Incheon 405-751, Korea
摘 要:对机械合金化制备的玻璃工业中用作结构零件的Pt-5%ZrO2(体积分数)的进行表征。通过锆丝电爆炸法制备氧化锆(ZrO2)纳米颗粒(粒径<100 nm),并在室温下与铂粉(粒径< 44 mm)球磨2~72 h。普通球磨至 48 h时,铂粒径遵循经典的递减趋势,至72 h时观测到颗粒团聚现象。晶粒尺寸演变规律与颗粒尺寸规律相似,48 h后减至50 nm左右。但是,铂晶粒的均方根应变规律相反,一直在增长,直至48 h时得到最大值,然后出现弛豫。粉末球磨48 h后,采用放电等离子体烧结制成块体。根据测量得到烧结体的质量损失表明:合金虽然相对密度较低,但有较好的热稳定性。
关键词:铂;氧化锆;纳米粒子;电丝爆炸法;机械合金化
(Edited by Lü-xiang DENG)
Foundation item: Project (10037339) supported by the Industrial Strategic Technology Development Program of the Ministry of Knowledge & Economy, Korea
Corresponding author: Hyo-Soo LEE; Tel: +82-32-850-0492; Fax: +82-32-850-410; E-mail: todd3367@kitech.re.kr
DOI: 10.1016/S1003-6326(14)63294-5

