Ni(Ⅱ)-NH3-Cl--H2O体系中Ni(Ⅱ)配合平衡热力学
王瑞祥1, 2,唐谟堂1,巨少华1,杨声海1,张文海1,唐朝波1,何 静1,杨建广1
(1. 中南大学 冶金科学与工程学院,湖南 长沙,410083;
2. 江西理工大学 材料与化学工程学院,江西 赣州,341000)
摘 要:根据质量平衡和电荷平衡的双平衡法,研究Ni(Ⅱ)-NH3-Cl--H2O体系中Ni(Ⅱ)配合平衡热力学;求出氨水浓度和氯离子浓度在0~5 mol/L范围内变化时,体系的pH值、体系中总镍、游离氯离子、游离氨以及各种镍-氨配合物等的平衡浓度,绘制热力学平衡图,并对热力学计算结果进行试验验证和差异分析。研究结果表明:镍离子浓度理论计算值与试验值之间的相对误差绝对平均值为6.91%,这说明该热力学模型是正确的,所选数据的准确性较好。
关键词:NH3-Cl--H2O体系;Ni(Ⅱ)配合平衡;双平衡法
中图分类号:TF815 文献标识码:A 文章编号:1672-7207(2008)05-0891-06
Thermodynamics of Ni(Ⅱ) complex equilibrium in
system of Ni(Ⅱ)-NH3-Cl--H2O
WANG Rui-xiang1, 2, TANG Mo-tang1, JU Shao-hua1, YANG Sheng-hai1, ZHAGN Wen-hai1,
TANG Chao-bo1, HE Jing1, YANG Jian-guang1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Materials and Chemical Engineering, Jiangxi University of Science and Technology,
Ganzhou 341000, China)
Abstract: Thermodynamics of Ni(Ⅱ) complex equilibrium in the system of Ni(Ⅱ)-NH3-Cl--H2O was studied by the double equilibrium method which was simultaneous equilibrium and aqueous electronic charge neutrality. Varying the concentration of ammonia and chloride ion, respectively, in range of 0-5 mol/L, pH value and the equilibrium concentration of total Ni, free Cl-, free NH3 and the complexes of 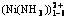 in the system were calculated, and thermodynamic diagrams were plotted. The relative errors between theoretic calculation and the experimental values of total concentration of nickel were analyzed. The results show that the relative average error is 6.91 %. The critical data and the thermodynamic model are reliable.
in the system were calculated, and thermodynamic diagrams were plotted. The relative errors between theoretic calculation and the experimental values of total concentration of nickel were analyzed. The results show that the relative average error is 6.91 %. The critical data and the thermodynamic model are reliable.
Key words: NH3-Cl--H2O system; Ni(Ⅱ) complex equilibrium; double equilibrium method
随着对镍需求的增长,以及硫化镍矿的日益匮乏,人们越来越重视对含镍氧化物料的开发利用[1-3]。自从Caron发明还原焙烧-氨浸工艺(Caron流程)后,氨法冶金在镍的提取冶金工业中得到了广泛应用[4-6],因此,深入开展氨浸法处理含镍氧化物料的理论研究,对推动镍冶炼技术的进步具有深远意义。
对于Ni(Ⅱ)水溶液体系的热力学,国内外科技工作者开展了一系列相关研究,ZHONG等[7]研究了Ni(Ⅱ)-NH3-H2O体系的热力学,但仅仅考虑了镍与氨生成的配合物离子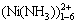 ,而没有考虑其他种类的镍配合物离子与物种;Plyasunova等[8]研究Ni(Ⅱ)-OH--H2O体系的热力学时,考虑了Ni(Ⅱ)与OH-的配合物,而没有考虑主体配离子的作用和电荷平衡;刘建华等[9]研究了NH3-Ni2+-pH之间的相互关系,主要是为了加氨沉淀Ni(OH)2,体系中不存在Cl-。曹华珍等[10]以NiCl2作为平衡固相,对Ni(Ⅱ)-NH3- NH4Cl-H2O体系中存在的物种进行了研究,但尚未涉及在工程上广为应用的镍氧化物物相在NH3-NH4Cl- H2O体系中的平衡问题。因此,在工程应用时,这些关于Ni(Ⅱ)水溶液体系的平衡热力学研究成果具有一定的局限性。本文作者综合考虑Ni(Ⅱ)与NH3,Cl-和OH- 3种配体的配合平衡[11],首先采用双平衡原理,对Ni(Ⅱ)-NH3-Cl--H2O体系的配合平衡进行热力学计算,然后用实验验证计算结果的正确性。
,而没有考虑其他种类的镍配合物离子与物种;Plyasunova等[8]研究Ni(Ⅱ)-OH--H2O体系的热力学时,考虑了Ni(Ⅱ)与OH-的配合物,而没有考虑主体配离子的作用和电荷平衡;刘建华等[9]研究了NH3-Ni2+-pH之间的相互关系,主要是为了加氨沉淀Ni(OH)2,体系中不存在Cl-。曹华珍等[10]以NiCl2作为平衡固相,对Ni(Ⅱ)-NH3- NH4Cl-H2O体系中存在的物种进行了研究,但尚未涉及在工程上广为应用的镍氧化物物相在NH3-NH4Cl- H2O体系中的平衡问题。因此,在工程应用时,这些关于Ni(Ⅱ)水溶液体系的平衡热力学研究成果具有一定的局限性。本文作者综合考虑Ni(Ⅱ)与NH3,Cl-和OH- 3种配体的配合平衡[11],首先采用双平衡原理,对Ni(Ⅱ)-NH3-Cl--H2O体系的配合平衡进行热力学计算,然后用实验验证计算结果的正确性。
1 热力学数据
Ni(Ⅱ)-NH3-Cl--H2O体系是一个十分复杂的体系,体系中存在的物种有Ni2+,Ni(NH3)2+,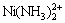 ,
,  ,
,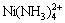 ,
,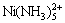 ,
,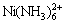 ,NiCl2(aq),Ni(OH)+,Ni(OH)2(aq),
,NiCl2(aq),Ni(OH)+,Ni(OH)2(aq),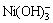 ,
,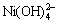 ,Ni2(OH)3+,
,Ni2(OH)3+,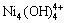 ,Cl-,NH3(aq),NH4+,H+和OH-共19种,镍配合物的稳定常数见表1[12],其他相关化合物的标准自由焓见表2[13]。
,Cl-,NH3(aq),NH4+,H+和OH-共19种,镍配合物的稳定常数见表1[12],其他相关化合物的标准自由焓见表2[13]。
表1 298 K时镍配合物稳定常数
Table 1 Critical stability constants of zinc complexes at 298 K

2 热力学分析和模型建立
2.1 平衡固相分析
在Ni(Ⅱ)-NH3-Cl--H2O体系中可能存在NiO(s)和Ni(OH)2(s) 2种固相,它们与Ni2+平衡的浓度方程分 别为:
c(Ni2+)1=exp(28.654 4-4.606 pH); (1)
c(Ni2+)2=exp(29.271 2-4.606 pH)。 (2)
表2 相关物种的标准自由焓(T =298 K)
Table 2 Gibbs free energy of related species at 298 K

比较式(1)和式(2)可知,在pH值相同时,与Ni(OH)2(s)平衡的Ni2+浓度总是大于与NiO(s)平衡的Ni2+浓度。这说明:在存在NiO(s)的NH3-NH4Cl-H2O体系中,不会有Ni(OH)2(s)固相出现。因此,在Ni(Ⅱ)-NH3-Cl--H2O体系中存在的惟一固相物质为NiO(s),根据同时平衡原理,每种镍配合离子或物种均与NiO(s)平衡:
NiO+ iNH3 + H2O=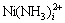 +2OH-; (3)
+2OH-; (3)
NiO+ (j-1)H2O=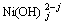 +(j-2)OH+; (4)
+(j-2)OH+; (4)
NiO+H2O+kCl-=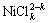 +2OH-; (5)
+2OH-; (5)
2NiO+H2O= Ni2(OH) 3+ +OH-; (6)
4NiO+4H2O=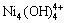 +4OH-。 (7)
+4OH-。 (7)
2.2 热力学模型的建立
根据双平衡电算指数法[14-15],Ni(Ⅱ)-NH3-Cl-- H2O体系中的离子或配合物的摩尔浓度可以用以下通式表示:
c(R)=exp[A+BpH+Cln c(NH3(aq))+Dln c(Cl-)]。 (8)
式中:R代表各种离子或物种;A为常数,可由反应式平衡方程求得;B为配合物得失质子数与ln10的乘积;C和D分别为氨和氯离子的配位数。根据以上表中数据和化学反应方程式,可以计算出各物种浓度表达式中的A,B,C和D,见表3。
另外,根据质量守衡定律建立镍量、氨量和氯量平衡方程:
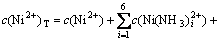
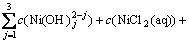
 ; (9)
; (9)
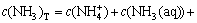
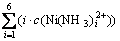 ; (10)
; (10)
 。 (11)
。 (11)
根据溶液电中性原理,建立电荷平衡方程:
2c(Ni2+)T+c(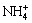 )+c(H+)=c(Cl-)T+c(OH-)。 (12)
)+c(H+)=c(Cl-)T+c(OH-)。 (12)
模型中,c(Ni2+)T和c(Ni2+)以及c(Cl-)T和c(Cl-)分别表示镍离子和氯根的总浓度以及游离镍离子和游离氯离子的浓度;c(NH3)T为溶液中氨和铵的总浓度;c(NH3(aq))表示游离氨的浓度;i和j分别表示氨和羟基等配体的配位数。
表3 各离子的电算指数常数
Table 3 Constants in exponential for calculating species concentration

在以上模型中,有式(9)~(12) 4个平衡方程,共有pH值,c(NH3(aq)),c(NH3)T,c(Ni2+)T,c(Cl-)T和c(Cl-) 6个未知数,因此,模型求解时,实际未知数的数量为6个,给定其中2个未知数,求解这个模型,便可以得到其他未知数的值。
在实际计算过程中,因为浸出剂的组成为NH4Cl和NH4OH(氨水),所以,在这个体系中,可以选择给定它们的初始浓度。其中c(NH4OH)=c(NH3)T- c(Cl-)T,c(NH4Cl)=c(Cl-)T。
这样,模型中就只有4个未知数、4个方程。将以上已知数据和方程组输入由MATLAB编写的程序,求出c(NH4Cl)和c(NH4OH)分别在0~5 mol/L的变化范围内其他未知的浓度和pH值。
3 结果与讨论
3.1 pH值随氨和氯化铵浓度的变化
根据计算结果,利用MATLAB得出体系平衡时pH值随c(NH4OH)和c(NH4Cl)的变化情况,如图1 所示。

图1 pH值与c(NH4OH)和c(NH4Cl)的曲面关系
Fig.1 Relationship among pH value and c(NH4OH) and c(NH4Cl)
由图1可以看出,体系pH值随c(NH4OH)的增加而呈上升趋势,最大达到13.17。随着c(NH4Cl)的增大,pH值呈下降趋势,最小达到7.16,这说明NH4Cl具有提供氢离子的能力。
3.2 游离氯离子浓度随氨和氯化铵浓度的变化
游离c(Cl-)随c(NH4OH)和c(NH4Cl)的变化情况如图2所示。由图2可以看出,游离氯离子浓度总的变化趋势是随着氯化铵浓度的增大而增大;当氨浓度较低时,游离氯离子浓度随着氯化铵浓度增加而增大的速度趋缓,这主要是因为氨离子浓度低,少量氯离子与镍离子配位,生成了镍与氯的配合物NiCl2(aq)。这一点可以由图3清楚地看出。在低氨浓度和高氯化铵浓度区域,有少量NiCl2(aq)生成。当氨浓度较高时,游离氯离子浓度随着氯化铵浓度增加而呈线性增大。主要是NH3与Ni的配位能力强,氨和镍优先配位,溶液中绝大多数Ni2+与NH3形成配合物,因而,氯离子浓度直线升高。
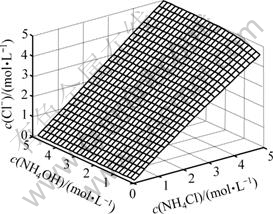
图2 c(Cl-)与c(NH4OH)和c(NH4Cl)的曲面关系
Fig.2 Relationship among c(Cl-) and c(NH4OH) and c(NH4Cl)
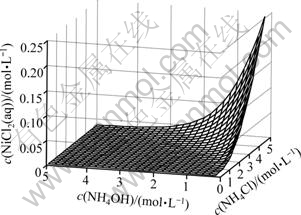
图3 c(NiCl2(aq))与c(NH4OH)和c(NH4Cl)的曲面关系
Fig.3 Relationship between c(NiCl2(aq)) and c(NH4OH) and c(NH4Cl)
3.3 游离氨浓度随氨和氯化铵浓度的变化
游离c(NH3(aq))浓度随c(NH4OH)和c(NH4Cl)的变化情况如图4所示。由图4可以看出,当c(NH4OH)/ c(NH4Cl)小于2?1时,游离氨浓度几乎为0;当c(NH4OH)/c(NH4Cl)大于2?1时,游离氨浓度快速增加。这是因为当c(NH4OH)/c(NH4Cl)小于2?1时,几乎所有的NH3都与Ni2+配位,形成了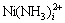 配合物。
配合物。

图4 c(NH3(aq))与c(NH4OH)和c(NH4Cl)的曲面关系
Fig.4 Relationship among c(NH3(aq)) and c(NH4OH) and c(NH4Cl)
3.4 总镍浓度随氨和氯化铵浓度的变化
c(Ni)T浓度随c(NH4OH)和c(NH4Cl)的变化情况如图5所示。由图5可以看出,在c(NH4Cl)小于2 mol/L时,若c(NH4Cl)一定,则c(Ni2+)T随着c(NH4OH)的增加而快速增大;当c(NH4OH)/c(NH4Cl)等于2?1时,趋于平缓,而后随着c(NH4OH)的继续增加,c(Ni2+)T变化不大;在c(NH4Cl)大于2 mol/L时,若c(NH4Cl)一定,则c(Ni2+)T随着c(NH4OH)的增加呈线性增大。当c(NH4OH)一定时,c(Ni2+)T随着c(NH4Cl)的增加总的变化趋势是快速增大。
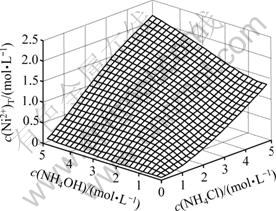
图5 c(Ni2+)T与c(NH4OH)和c(NH4Cl)的曲面关系
Fig.5 Relationship among c(Ni2+)T and c(NH4OH) and c(NH4Cl)
3.5 物种分布图
当c(NH4Cl)和c(NH4OH)均为0~5 mol/L时,在镍的各种配合物中,Ni(OH)+,Ni(OH)2(aq),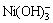 ,Ni2(OH)3+和
,Ni2(OH)3+和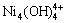 的浓度均小于1×10-5 mol/L。NiCl2(aq)的浓度如图3所示,其存在的优势区域为高氯化铵浓度与低氨水浓度的小范围区域,最高浓度小于0.25 mol/L;游离Ni2+存在的优势区域与NiCl2(aq)的类似,但其最高浓度低于0.014 mol/L;镍主要以
的浓度均小于1×10-5 mol/L。NiCl2(aq)的浓度如图3所示,其存在的优势区域为高氯化铵浓度与低氨水浓度的小范围区域,最高浓度小于0.25 mol/L;游离Ni2+存在的优势区域与NiCl2(aq)的类似,但其最高浓度低于0.014 mol/L;镍主要以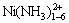 配合物离子形式存在。如图6所示,当氨水浓度较低时,镍与氨生成平衡浓度较低的
配合物离子形式存在。如图6所示,当氨水浓度较低时,镍与氨生成平衡浓度较低的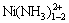 配合物,随着体系中氨水浓度的增加,镍与氨逐步生成
配合物,随着体系中氨水浓度的增加,镍与氨逐步生成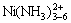 配合物,其平衡浓度也快速增大。
配合物,其平衡浓度也快速增大。
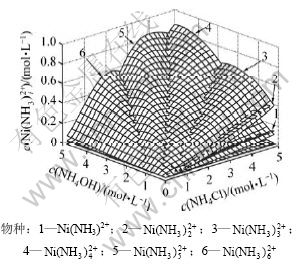
图6 c(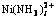 )与c(NH4OH)和c(NH4Cl)的曲面关系
)与c(NH4OH)和c(NH4Cl)的曲面关系
Fig.6 Relationship among c(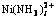 ) and c(NH4OH) and c(NH4Cl)
) and c(NH4OH) and c(NH4Cl)
4 实验验证
将配好的相应浓度的NH3+NH4Cl的水溶液和过量的分析纯NiO混合,在25 ℃搅拌72 h,液固分离,分析溶液中的镍浓度。实验值与理论计算值见表4。
从表4可以看出,在不同的氨和铵浓度下,镍平衡浓度相对误差的绝对平均值为6.91%,这说明该热力学模型是正确的,所选数据的准确性较好,产生相对误差大于5%的主要原因是用摩尔浓度代替活度,其次是试验和分析误差。所构建的热力学图可用来确定浸出剂的成分,优化浸出剂结构,在较高的氯化铵浓度下,大幅降低氨水浓度,同样可以获得较高的镍平衡浓度,可以有效减少氨水挥发。这对氧化镍物料的氨法冶炼具有重要的理论指导意义。
表4 不同c(NH4Cl)和c(NH4OH)下,溶液中平衡ρ(Ni2+)T的实验值和计算值比较
Table 4 Experimental and calculated values of equilibrium ρ(Ni2+)T at different c(NH4Cl) and c(NH4OH)

5 结 论
a. 根据电算指数法建立了较精确的Ni(Ⅱ)-NH3- Cl--H2O体系的热力学模型,通过对模型求解,绘制了各种重要的热力学关系图,得出体系的热力学规律。
b. 理论计算结果与实验数据符合较好,镍平衡浓度相对误差的绝对平均值为6.91%。
c. 在较高的氯化铵浓度下,大幅降低氨水浓度,同样可以获得较高的镍平衡浓度,可以有效减少氨法提镍过程中的氨挥发损失。
参考文献:
[1] 喻正军, 冯其明, 欧乐明, 等. 常压下硫酸体系中钴冰铜的浸出[J]. 中南大学学报: 自然科学版, 2006, 37(4): 675-679.
YU Zheng-jun, FENG Qi-ming, OU Le-ming, et al. Leaching of cobalt bearing metallic matte in sulphuric acid at normal pressure[J]. Journal of Central South University: Science and Technology, 2006, 37(4): 675-679.
[2] 王成彦, 江培海. 云南中低品位氧化锌矿及元江镍矿的合理开发利用[J]. 中国工程科学, 2005, 7(增刊): 147-150.
WANG Cheng-yan, JIANG Pei-hai. The rational exploitation of middle & low grade zinc oxide ore and Yuanjiang nickel oxide ore in Yunnan[J]. Engineering Science, 2005, 7(Suppl): 147-150.
[3] Lee H Y, Kim S G, Oh J K. Electrochemical leach of nickel from low-grade laterites[J]. Hydrometallurgy, 2005, 77(3/4): 263-268.
[4] Chander S, Sharma V N. Reduction roasting/ammonia leaching of nickeliferous laterites[J]. Hydrometallurgy, 1981, 7(4): 315-327.
[5] Panda S C, Sukla L B, Rao P K, et al. Extraction of nickel through reduction roasting and ammoniacal leaching of lateritic nickel ores[J]. Trans Indian Inst Met, 1980, 33(2): 161-165.
[6] Jana R K, Pandey B D, Premchand. Ammoniacal leaching of roast reduced deep-sea manganese nodules[J]. Hydrometallurgy, 1999, 53(1): 45-56.
[7] ZHONG Shao-lin, Malcolm T H. A calculation method for determining equilibria in metal-ammonia-water system[J]. Hydrometallurgy, 1995, 38(1): 15-37.
[8] Plyasunova N V, ZHANG Yu, Muhammed M. Critical evaluation of thermodynamics of complex formation of metal ions in aqueous solutions. Ⅳ. Hydrolysis and hydroxo-complexes of Ni2+ at 298.15 K[J]. Hydrometallurgy, 1998, 48(1): 43-46.
[9] 刘建华, 李新海, 郭炳昆, 等. 氢氧化亚镍制备中[NH3]-[Ni2+]-pH值的相互关系研究[J]. 湘潭矿业学院学报, 1997, 12(4): 61-64.
LIU Jian-hua, LI Xin-hai, GUO Bin-kun, et al. Study on the relation of [NH3]-[Ni2+]-pH in the preparation of nickel hydroxide[J]. Journal of Xiangtan Mineral Institute, 1997, 12(4): 61-64.
[10] 曹华珍, 郑国渠, 郑利峰, 等. Ni(Ⅱ)-NH3-NH4Cl-H2O体系中物种研究[J]. 浙江工业大学学报, 2003, 31(5):: 488-491.
CAO Hua-zhen, ZHENG Guo-qu, ZHENG Li-feng, et al. Study of species in Ni(Ⅱ)-NH3-NH4Cl-H2O system[J]. Journal of Zhejiang University of Technology, 2003, 31(5): 488-491.
[11] 巨少华. MACA体系中铜、镍和金的冶金热力学及其低品位矿的堆浸工艺研究[D]. 长沙: 中南大学冶金科学与工程学院, 2006.
JU Shao-hua. Study on hydrometallurgical thermodynamics of metal (Cu, Ni, Au) in the system of Me-NH4Cl-NH3-H2O and heap leaching process of their low-grade ores[D]. Changsha: School of Metallurgical Science and Engineering, Central South University, 2006.
[12] Smith R M, Matell A E. Critical stability constants inorganic complexes[M]. New York: Plenum Press, 1976.
[13] Dean A J. Lange’s handbook of chemistry[M]. 15th ed. New York: Plenum Press, 1985.
[14] TANG Mo-tang, ZHAO Tian-cong. A thermodynamic study on the basic and negative potential fields of the systems of Sb-S-H2O and Sb-Na-S-H2O[J]. J Cent South Inst Min Metall, 1988, 19(1): 35-43.
[15] TANG Mo-tang, ZHAO Tian-cong, LU Jun-le, et al. Principle and application of the new chlorination-hydrolization process[J]. J Cent South Inst Min Metall, 1992, 23(4): 405-411.
收稿日期:2007-12-25;修回日期:2008-02-20
基金项目:国家“973”计划资助项目(2007CB613604);江西省教育厅科技资助项目(GJJ08279)
通信作者:王瑞祥(1974-),男,河南登封人,博士研究生,讲师,从事低品位复杂矿综合利用研究;电话:0731-8830470;E-mail: wrx9022@163.com