A new chlorinated non-fullerene acceptor based organic photovoltaic cells over 12% efficiency
来源期刊:中南大学学报(英文版)2020年第12期
论文作者:邹应萍 曹睿 陈瑜 蔡方方 陈泓钢 刘玮 管慧兰 魏擎亚 李静 常秦 李哲
文章页码:3581 - 3593
Key words:non-fullerene acceptor; chlorination; electron-deficient core; device performance
Abstract: The method to fluorinate the terminal group has achieved remarkable success and been widely used to fine-tune the intrinsic properties of organic acceptor materials. Referring to chlorination, however, it gets less attention and remains ambiguous effect on organic photovoltaic (OPV) cells. Herein, a new non-fullerene acceptor named Y19 was reported with benzotriazole as the electron-deficient core and 2Cl-ICs as the strong electron-withdrawing end groups. Y19 exhibits a wide film absorption band from 600 nm to 948 nm and low LUMO (the lowest unoccupied molecular orbital) energy level of -3.95 eV. Photovoltaic devices based on PM6:Y19 show high-power conversion efficiency (PCE) of 12.76 % with high open-circuit voltage (Voc) of 0.84 V, short-circuit current density (Jsc) of 22.38 mA/cm2 and fill factor (FF) of 68.18 %. Broad external quantum efficiency (EQE) response of over 60 % in the range of 480-860 nm can be obtained. This study demonstrates that chlorination, as a low-cost molecular design strategy, has its own superiorities to improve device performance and promote the potential application in OPV.
Cite this article as: CAO Rui, CHEN Yu, CAI Fang-fang, CHEN Hong-gang, LIU Wei, GUAN Hui-lan, WEI Qing-ya, LI Jing, CHANG Qin, LI Zhe, ZOU Ying-ping. A new chlorinated non-fullerene acceptor based organic photovoltaic cells over 12% efficiency [J]. Journal of Central South University, 2020, 27(12): 3581-3593. DOI: https://doi.org/ 10.1007/s11771-020-4501-0.

J. Cent. South Univ. (2020) 27: 3581-3593
DOI: https://doi.org/10.1007/s11771-020-4501-0

CAO Rui(曹睿), CHEN Yu(陈瑜), CAI Fang-fang(蔡方方), CHEN Hong-gang(陈泓钢),
LIU Wei(刘玮), GUAN Hui-lan(管慧兰), WEI Qing-ya(魏擎亚), LI Jing(李静),
CHANG Qin(常秦), LI Zhe(李哲), ZOU Ying-ping(邹应萍)
College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: The method to fluorinate the terminal group has achieved remarkable success and been widely used to fine-tune the intrinsic properties of organic acceptor materials. Referring to chlorination, however, it gets less attention and remains ambiguous effect on organic photovoltaic (OPV) cells. Herein, a new non-fullerene acceptor named Y19 was reported with benzotriazole as the electron-deficient core and 2Cl-ICs as the strong electron-withdrawing end groups. Y19 exhibits a wide film absorption band from 600 nm to 948 nm and low LUMO (the lowest unoccupied molecular orbital) energy level of -3.95 eV. Photovoltaic devices based on PM6:Y19 show high-power conversion efficiency (PCE) of 12.76 % with high open-circuit voltage (Voc) of 0.84 V, short-circuit current density (Jsc) of 22.38 mA/cm2 and fill factor (FF) of 68.18 %. Broad external quantum efficiency (EQE) response of over 60 % in the range of 480-860 nm can be obtained. This study demonstrates that chlorination, as a low-cost molecular design strategy, has its own superiorities to improve device performance and promote the potential application in OPV.
Key words: non-fullerene acceptor; chlorination; electron-deficient core; device performance
Cite this article as: CAO Rui, CHEN Yu, CAI Fang-fang, CHEN Hong-gang, LIU Wei, GUAN Hui-lan, WEI Qing-ya, LI Jing, CHANG Qin, LI Zhe, ZOU Ying-ping. A new chlorinated non-fullerene acceptor based organic photovoltaic cells over 12% efficiency [J]. Journal of Central South University, 2020, 27(12): 3581-3593. DOI: https://doi.org/ 10.1007/s11771-020-4501-0.
1 Introduction
Organic photovoltaic (OPV) cells, as a clean and sustainable energy technology, have attracted widespread attention due to its unique advantages, such as light-weight, translucency, flexibility and large-area preparation through roll-to-roll printing [1-3]. Over the past three decades, the power conversion efficiency (PCE) has increased to more than 16 %, owing to the great scientific advances in materials science and device technology [4-6]. At present, the photoactive layer comprising wide band-gap polymer donors and narrow band-gap non-fullerene acceptors (NFAs) is promising as the dominant composition of high-performance organic photovoltaic (OPV) devices. This type of bulk heterojunction (BHJ) tends to obtain high photo-current with a wide range of harvesting photo from the visible (Vis) to near-infrared region (NIR) and the continuous interpenetrating network as well as the strong intermolecular interaction which mainly benefits from the development and application of NFAs [7-11]. Studies about NFAs suggest that compared with fullerenes and polymer acceptors, their superiorities originate from brilliant CAO Rui, CHEN Yu contributed equally to this work.
properties including (i) the good miscibility, (ii) the adjustability of solubility, energy levels and absorption range, (iii) the appropriate crystallinity in favor of high electron mobilities, (iv) exciton dissociated easily even under weak driving force, and (v) good reproducibility and low cost in synthesis [12-16].
Recent work on NFAs has contributed to some breakthroughs in OPV [17, 18]. Especially after the report of Y6 by ZOU et al [17], OPV has reached a higher level. When employing Y6 to blend the polymer PM6, the PCE of device surpassed 15.7%, and to blend the polymer D18, PCE increased to the amazing 18.2% [17, 19]. Compared with ITIC, the greater success of Y6 in the OPV research landscape can be attributed to advanced molecular design, such as the introduction of electron-deficient groups, like benzothiazole, into the central fused ring, forming a large ladder-type core with the donor-acceptor-donor (DA'D) structure to increase the electronic mobility [20]. Therefore, photovoltaic devices based on Y6 and its derivatives, achieving a universal high photocurrent and efficiency, are gradually becoming the focus in OPV. Besides, in terms of terminal acceptor groups of Y6, fluorination, a clear-cut distinction and also an effective strategy, consolidates the intramolecular charge transfer (ICT) and redshifts as well as broadening the absorption band, which has been proven on many occasions about NFAs [21-28]. Except for this, there is more dominance to fluorinate the end groups. First of all, the energy level can be downshifted to an appropriate level to make the electron acceptor preferably match with the electron donor without striking steric hindrance, due to the small atomic radius of fluorine [29]. More importantly, a tight and stable nano-network can be formed and the morphology of active layer can be improved, benefitting from the strong noncovalent interactions between fluorine atom and hydrogen atom or sulfur atom [30-32]. Since the advent of DFIC, a fluorinated terminal, those reported NFAs with excellent performance have tended to contain multiple fluorine atoms in the end groups [33].
Although many benefits can be obtained by fluorination, it is easier and low-cost to introduce chlorine atom into the end groups of acceptors. Generally, the quoted price of 2Cl-IC is estimated to be lower than that of DFIC in the market, which will directly determine the reduction of material cost [34, 35]. Besides, chlorination has also similar ability to redshift the absorption band and enhance the ICT, even more prominently, due to the fact that the idle 3d orbitals around chlorine atom can hold electronics from the fused ring [35-37]. In terms of the performance, a good example is the recent report of BDT-4Cl based on Y6 [38]. Photovoltaic devices based on PM6:BDT-4Cl achieve a high PCE of 16.5%, which is obviously improved, compared with Y6 under the same processing conditions. More interesting than the above, BDT-4Cl showed relatively high Voc without the decrease of the Jsc, as the result of the further reduction on non-radiative energy loss. Thus, chlorinating NFAs may also be a potentially outstanding strategy to improve device performance. However, only a few reports focus on the chlorination, to date, and among these, relatively few parts can attract scholar’s attention. From the whole, the strategy to chlorinate the end groups remains to be studied a lot, in order to reflect a common scientific law.
Here, a new non-fullerene acceptor named Y19 (the structure is shown in synthetic route of Y19, Figure 1) with benzotriazole as the electron- deficient core and 2Cl-ICs as the strong electron- withdrawing end group was designed and synthesized was designed and synthesized on account of the chlorination strategy, to which 2Cl-ICs as the strong electron-withdrawing end groups were introduced. Then, the photoelectric properties of Y19 were explored. First of all, the ultraviolet-visible (UV-Vis) absorption and cyclic voltammetry (CV) curves of Y19 were scanned in order to choose the appropriate donor material. As expected, Y19 shows the strong effect of ICT. The polymer PM6 is considered the electron donor of active layer to measure and analyze photovoltaic performance of Y19. An original efficiency of 10.46% is achieved in the as-cast device while the best PCE of 12.76 % is achieved after optimizing morphology of the PM6:Y19 film with 1, 8-diiodooctane (DIO) additive and thermal annealing (TA) treatment. Subsequently, the as-cast and optimal films were fully characterized via a series of measurements and testing techniques, including their external quantum efficiency (EQE) and J-V curves in terms of their intrinsic performance, their light intensity dependence of the Jsc and photocurrent density versus effective voltage reflecting the excition separation and charge recombination processes, and their J1/2-V curves to explore the electron and the hole mobilities. Moreover, the TEM and AFM height images were probed to analyze the surface morphology of the optimal PM6:Y19 film.

Figure 1 Synthetic route of Y19
2 Results and discussion
2.1 Synthesis and thermal properties
The synthetic route of Y19 is shown in Figure 1 and more detailed procedures are documented in the supporting information. All raw materials were purchased from the website of energy chemical without further purification. The compound 5 was synthesized with high yield based on our previous work [17]. The NFA Y19 was obtained through the Knoevenagel condensation reaction between compound 5 and 2Cl-IC. This target structure was proven via 1H NMR, 13C NMR and the high-resolution mass spectrum (MALDI-TOF) from Figures S1-S5. As to thermogravimetric analysis (TGA), as shown in Figure 2, Y19 nearly has no mass loss before 300 °C (Td=335 °C, the temperature with mass loss of 5%), which confirms its thermal stability in the process of fabricating photovoltaic devices.

Figure 2 Thermogravimetric analysis (TGA) results of Y19 with a heating rate of 10 °C/min under nitrogen purge
2.2 Optical and electrochemical properties
As depicted in Figure 3(a), the recorded normalized UV-Vis absorption curves are broad and strong. In chloroform, Y19 shows an absorption band at 600-845 nm, with a peak at 776 nm. Compared with the solution absorption, the corresponding maximum absorption in the film state exhibits a large redshift of 86 nm due to the strong intermolecular π-π interaction. The film absorption onset (λonset) of Y19 is measured at 948 nm, corresponding to an Egopt of 1.308 eV calculated by the formula: Egopt=1240/λonset. Y19 can be classified as one of the narrow band gap material. The electrochemical properties were measured by cyclic voltammetry (CV) and the test results are shown in Figure 3(b). Based on the general equation EHOMO/LUMO=-(4.80-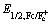 +Eonest,ox/red), the energy levels of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are estimated at -3.95 eV and -5.68 eV with the standardization of the Fc/Fc+ (-4.8 eV), respectively. After fully considering complementary absorption and suitable energy levels, PM6 is chosen as the electron donor materi1al to study the photovoltaic properties of the PM6: Y19-based devices. Complementary absorption is beneficial for harvesting as more photons as possible, which is beneficial for obtaining a high Jsc. The matched energy level can ensure a sufficient driving force for efficient excitons dissociation. The relevant optical parameters are listed in Table 1 and the energy level diagrams of PM6 and Y19 are shown in Figure 3(c).
+Eonest,ox/red), the energy levels of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are estimated at -3.95 eV and -5.68 eV with the standardization of the Fc/Fc+ (-4.8 eV), respectively. After fully considering complementary absorption and suitable energy levels, PM6 is chosen as the electron donor materi1al to study the photovoltaic properties of the PM6: Y19-based devices. Complementary absorption is beneficial for harvesting as more photons as possible, which is beneficial for obtaining a high Jsc. The matched energy level can ensure a sufficient driving force for efficient excitons dissociation. The relevant optical parameters are listed in Table 1 and the energy level diagrams of PM6 and Y19 are shown in Figure 3(c).

Figure 3 (a) Normalized UV-Vis absorption spectra of Y19 in CF solution and in film; (b) Electrochemical curves of Y19 in CH3CN+0.1 mol/L Bu4NPF6; (c) Molecular energy levels of PM6 and Y19; (d) Molecular structure of PM6
Table 1 Thermal and optical parameters of Y19

2.3 Photovoltaic properties
Inverted OPV cells with a structure of ITO/ZnO/PM6:Y19/MoO3/Ag were made to evaluate the performance of Y19. The related optimizing processes are recorded in Tables S1-S4 and the ultimate results are shown in Table 2. In the as-cast device, an original efficiency of 10.46 % is achieved with Voc of 0.88 V, Jsc of 20.11 mA/cm2 and FF of 59.76%. Through keeping 0.5 % DIO and thermal annealing (TA at 130°C for 5 min) invariant to adjust the mass ratios of D/A from 1.2:1 to 1:1.5, the efficiencies are all over 11.4 % with the high Jsc of 21-24 mA/cm2. When the D/A ratio is precisely 1:1, a better PCE of 12.33% is obtained. Subsequently, the content of DIO and temperature of TA are also regulated to improve the morphology of the blend (PM6/Y19=1:1, w/w) film. While employing the optimal condition of 0.3% DIO and TA at 110 °C for 10 min to process, a best PCE of 12.76% is achieved with Voc of 0.84 V, Jsc of 22.38 mA/cm2 and FF of 68.18 %. These device parameters are consistent with J-V characterizations of the as-cast and optimal devices existing in Figure 4(a), so their authenticity and reliability can be guaranteed. To further investigate the optimal device, the energy loss is roughly calculated as 0.466 eV according to the equation: Eloss=Egopt –eVoc, where e is the elementary charge. This small value means the low radiation and non-radiation loss in the optimal devices.
In order to further verify the high Jsc, the external quantum efficiency (EQE) curves were tested as shown in Figure 4(b). For all the PM6:Y19-based devices, the broad photocurrent response covers the range from 300 to 950 nm, corresponding to the union of the absorption region of PM6 and Y19. All the EQE values are more than 60% in a wide range of 480-860 nm, indicating that the active layer composed of PM6 and Y19 can efficiently capture photons and generate carriers. However, an obvious and representative difference occurs in their maximum value of EQE where the as-cast device is 68% at 608 nm and the optimal device is up to 74% at 497 nm. Generally, relatively high EQE comes from the more collected charges after absorbing equal photons. Consequently, it can be justified that the optimal devices achieve a higher Jsc compared to the as-cast. And the calculated integrated current densities are 20.11 and 22.55 mA/cm2 for as-cast and optimized based devices, respectively, which are in good agreement with the Jsc values obtained from corresponding J-V curves.

Figure 4 (a) J-V characterizations; (b) EQE plots of as-cast and optimal devices based on PM6:Y19
Table 2 As-cast and optimal devices data based on PM6: Y19

To analyze the excitons separating and then electrons/holes collecting processes separately, the correlation curves between photocurrent (Jph) and effective voltage (Veff) were fitted for the as-cast and optimal devices (Figure 5(a)). Through utilizing the defined formula ηdiss=Jph/Jsat, where Jsat means the saturation photocurrent density, the exciton dissociation probabilities (ηdiss) were reckoned. At Veff=2.50 V, the value of Jsat is 22.92 mA/cm2 for the as-cast devices and 24.30 mA/cm2 for the optimal devices. In the as-cast devices, the ηdiss value is calculated to be 90.2 %. After optimizing these processing conditions, this value increases to 92.1%, arguing a more high-efficient process of exciton dissociation and charge collection in optimal devices. With regard to the charge recombination process, the positive correlation between the Jph and light intensity (Plight ) was measured as shown in Figure 5(b). For the optimal device, the α value existing in the defined formula: reaches 0.997, which is close to 1,indicating the relatively few charge recombination in this process. To summarize these two opposite processes, it is reasonable that the matching extent of Y19 and PM6 is enough and the morphological control of photoactive layer is effective to acquire a big proportion of the conversed energy in the absorbed photon energy.
reaches 0.997, which is close to 1,indicating the relatively few charge recombination in this process. To summarize these two opposite processes, it is reasonable that the matching extent of Y19 and PM6 is enough and the morphological control of photoactive layer is effective to acquire a big proportion of the conversed energy in the absorbed photon energy.
As shown in Figure 5(c) and Figure 5(d), J1/2-V curves of the as-cast and optimal PM6:Y19 film were probed, with a purpose to analyze charge mobilities. In the as-cast device, the electron mobilities (μe) and the hole mobilities (μh) are calculated to be 4.14×10-4 cm2/(V·s) and 9.53×10-5 cm2/(V·s), respectively, and accordingly, the ratio of μe/μh is estimated to be 4.34. In contrast, a very meaningful raise appears in the optimal devices where μe is 6.52×10-4 cm2/(V·s) and μh is 3.75×10-4 cm2/(V·s). Meanwhile, it is more balanced that the ratio of μe/μh reduces to 1.74, standing for a more excellent charge transfer characteristic in the optimal devices. Nevertheless, a great inconsistency between the electron and hole mobilities is hard to be ignored. The hole mobilities are always significantly less than the electron mobilities, meaning an existence of some trapped holes by defect in the D/A film, which is related to the great FF loss of 31.82%-40.24%.
2.4 Morphology analysis
In order to further study the morphology of the optimal PM6:Y19 film, AFM and TEM were characterized as shown in the Figure 6. From the AFM image, a smooth surface can be observed with root mean square (RMS) value of 1.32 nm. This moderate value makes it clear that there is less existence of the over-aggregation and large phase separation in this optimal film, which is consistent with the appearance of the TEM image under 200 nm with the uniform size and staggered brightness distributions. Thus, the optimal active layer exhibits some desired morphological characteristics to make an excellent photovoltaic performance appear in the PM6:Y19-based devices.

Figure 5 (a) Light intensity dependence of Jsc; (b) Photocurrent density versus effective voltage characteristics; (c) Electron mobilities; (d) Hole mobilities of PM6: Y19-based devices

Figure 6 (a) AFM height image and (b) TEM image of optimal PM6:Y19 film
3 Conclusions
In conclusion, a new NFA named Y19 with 2Cl-ICs as the terminal groups was synthesized and adequately characterized. Y19 exhibits the prominent optical and electrochemical properties. The corresponding film absorption covers the absorption of 600-948 nm with Egopt of 1.31 eV and the LUMO and HOMO energy levels are -3.95 eV and -5.68 eV, respectively. As-cast devices based on PM6:Y19 achieve an efficiency of 10.46 %. After optimizing morphology of the active layer, the best PCE of 12.76 % is obtained with Voc of 0.84 V, Jsc of 22.38 mA/cm2 and FF of 68.18%. Further tests and analysis indicate that Y19 can form a desired morphology with PM6 and demonstrate a high electron mobility of 6.52×10-4 cm2/(V·s) and a broad EQE response of over 60% in the range of 480-860 nm. Our study shows that this low-cost strategy for chlorinating NFAs can improve photovoltaic performance.
Supporting information
1 Instruments
Unless otherwise indicated, all 1H NMR and 13C NMR spectra were obtained by using a Bruker AV-400 spectrometer in deuterated chloroform solution at 298 K and reporting chemical shift values with tetramethylsilane as an internal reference. UV-Vis absorption spectra were measured on the SHIMADZU UV-2600 spectrophotometer. Donor and acceptor in chloroform were spin-coated on quartz plates to measure the absorption of the thin-films. The electrochemical curves were estimated in tetrabutylammonium hexafluorophosphate (Bu4NPF6, 0.1 mol/L) acetonitrile solutions with electrochemical workstation (CHI660E), in which Pt plate is as working electrode, Pt slice is as counter electrode, and Ag/AgCl electrode is as reference electrode. Use Perkin-Elmer TGA-7 to conduct thermogravimetric analysis (TGA) with a heating rate of 20 K/min under nitrogen. The morphologies of the blend films were investigated by atomic force microscopy (AFM) and transmission electron microscopy (TEM).
2 Material synthesis
2-(5,6-dichloro-2,3-dihydro-3-oxo-1H-inden-1-ylidene)malononitrile was purchased from Derthon OPV Co Ltd; 4, 7-dibromo-2-(2-ethylhexyl)-5, 6-dinitro-2H-benzo[d] [1, 2, 3] triazole and 3-hexylthieno [3, 2-b] thiophene were synthesized by referring to the previous work [1].
1) Synthesis of tributyl (6-hexylthieno [3, 2-b] thiophen-2-yl) stannane (2) is shown in Route 1.

To a solution of compound 1 (4.5 g, 20 mmol) in ultra-dry tetrahydrofuran (93 mL), 2.5 mol/L n-butyllithium (8 mL, 20 mmol) was added dropwise at -78 °C under argon, and stirred for 1 h. Further, Sn(Bu)3Cl (5.4 mL, 20 mmol) was added to the mixture at -78 °C, and stirred for another 2 h, then transferred to room temperature and stirring overnight. The reaction was quenched with water (100 mL). The mixture was extracted with dichloromethane. Removing the solvent under reduced pressure gave the crude compound 2. The product was used into the following reaction without any further purification.
2) Synthesis of 2-(2-ethylhexyl)-4, 7-bis (6-hexylthieno [3, 2-b] thiophen-2-yl)-5, 6- dinitro-2H-benzo [d] [1, 2, 3] triazole (3) is shown in Route 2.
Compound 2 (7.6 g, 14.8 mmol), compound 3 (2.3 g, 4.7 mmol) and the Pd(PPh3)2Cl2 (0.14 g, 0.42 mmol) as catalyst were added to 100 mL ultra- dry tetrahydro furan under argon and stirred at 70 °C for 48 h. The reaction mixture was allowed to cool to room temperature and then mixed with cold water. The mixture was extracted with chloroform (CHCl3). The compound 4 as orange solid was

obtained by chromatographically on a silica gel column eluting with dichloromethane/petroleum ether (1/1, v/v) after removing the solvent from the crude product 3 (9.1 g, 80% yield).
1H NMR (compound 3) (400 MHz, chloroform-d) δ 7.72 (s, 2H), 7.12 (s, 2H), 4.75 (d, J =6.7 Hz, 2H), 2.76 (d, J=7.6 Hz, 4H), 1.36 (dd, J= 14.4, 7.0 Hz, 26H), 0.95-0.87 (m, 12H).
3) Synthesis of 6,12,13-tris(2-ethylhexyl)- 3,9-dihexyl-12,13-dihydro-6H-thieno[2'',3'':4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b][1,2,3]triazolo[4,5-e]indole (4) is shown in Route 3.
Under argon, compound 3 (1.5 g, 2 mmol) and triethyl phosphate (10 mL) were dissolved in the o-dichlorobenzene (o-DCB, 4.5 mL). Being heated at 180 °C overnight, the aqueous phase was extracted with dichloromethane and the organic layer was dried over Na2SO4 and filtered. After removing the solvent with a rotary evaporator, the residue was added into a reaction flask without further purification. Potassium hydroxide (1.3 g, 24 mmol), potassium iodide (0.08 g, 0.48 mmol), 1-bromo-2-ethylhexane (2.3 g, 12 mmol) and DMF (20 mL) were added and the mixture was evacuated and deoxygenated with argon three times. The mixture was refluxed at 90 °C overnight,and then was extracted with ethyl acetate and H2O. The organic layers were dried over MgSO4, filtered and purified with column chromatography on silica gel using dichloromethane/petroleum ether (1/5, v/v) as the eluent to obtain a yellow solid 4 (0.32 g, 17% yield).
1H NMR (compound 4) (400 MHz, chloroform-d) δ 6.98 (s, 2H), 4.72 (d, J=7.2 Hz, 2H), 4.58 (d, J=7.7 Hz, 4H), 2.82 (t, J=7.6 Hz, 4H), 2.00-1.93 (m, 2H), 1.89-1.82 (m, 4H), 1.36 (t, J=8.8 Hz, 22H), 0.95 (dd, J=29.1, 7.1 Hz, 26H), 0.58 (dd, J=20.3, 5.9 Hz, 13H).
4) Synthesis of 6,12,13-tris(2-ethylhexyl)-3,9- dihexyl-12,13-dihydro-6H-thieno [2'',3'':4',5']thieno [2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b][1,2,3]triazolo[4,5-e]indole-2,10-dicarbaldehyde (5) is shown in Route 4.
Under the protection of argon, 30 mL of ultra-dry N,N-dimethylformamide (DMF) was added to the compound 4 (0.32 g, 0.34 mmol), and the mixture was evacuated and deoxygenated with argon three times. Stirring at 0°C for 30 min, POCl3 (0.47 mL, 5.1 mmol) was added dropwise, and the mixture was stirred at the same temperature for 2 h. The solution was then transferred to 90°C and stirred under reflux for 24 h. The reaction mixture was poured into cold water and then extracted with dichloromethane, then removing the solvent from the organic layer with a rotary evaporator. The crude product was purified with column chromatography on silica gel using dichloromethane petroleum ether (1/1, v/v) as the eluent to give a yellow solid 5 (0.22 g, 65% yield).
1H NMR (compound 5) (400 MHz, Chloroform-d) δ 10.13 (s, 2H), 4.73 (d, J=7.0 Hz, 2H), 4.61 (d, J=7.5 Hz, 4H), 3.20 (t, J=7.7 Hz, 4H), 1.96-1.90 (m, 6H), 1.50-1.32 (m, 22H), 0.95 (dt, J=36.3, 7.5 Hz, 26H), 0.66-0.53 (m, 13H).
5) Synthesis of 6,12,13-tris(2-ethylhexyl)-3, 9-dihexyl-12,13-dihydro-6H-thieno[2'',3'':4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b][1,2,3]triazolo[4,5-e]indole-2,10-diyl-bis(methanylylidene)-bis(5,6-dichloro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene)dimalononitrile (Y19) is shown in Route 5.
Compound 5 (0.19 g, 0.2 mmol), 2-(5,6- dichloro-2,3-dihydro-3-oxo-1 H-inden-1-ylidene) malononitrile (0.21 g, 0.8 mmol) and chloroform (30 mL) were added to a round bottom flask under argon, and then pyridine (1 mL) was added with stirring. The mixture was stirred at 65 °C overnight. After cooling to room temperature, the mixture was poured into methanol and filtered. The residue was purified with column chromatography on silica gel using dichloromethane/petroleum ether (1/1, v/v) as the eluent. 0.15 g of solid product Y19 (50% yield) was obtained.



1H NMR (compound Y19) (400 MHz, Chloroform-d) δ 9.17 (s, 2H), 8.79 (s, 2H), 7.96 (s, 2H), 4.74 (d, J=7.1 Hz, 6H), 3.26-3.22 (m, 4H), 1.92-1.84 (m, 6H), 1.46-1.33 (m, 22H), 1.01-0.89 (m, 26H), 0.75-0.71 (m, 7H), 0.60 (d, J=4.0 Hz, 6H).
13C NMR (compound Y19) (101 MHz, Chloroform-d) δ 158.77, 154.16, 139.29, 138.93, 138.74, 137.99, 136.91, 136.03, 135.87, 135.61, 133.54, 130.00, 126.77, 124.87, 119.36, 115.23, 114.75, 112.09, 77.22, 68.11, 59.72, 55.46, 40.34, 31.62, 31.26, 30.41, 29.86, 29.60, 29.52, 28.37, 27.58, 23.93, 23.31, 22.94, 22.74, 22.54, 14.08, 14.03, 13.69, 10.52, 10.27.
3 Figures of 1H NMR and 13C NMR

Figure S1 1H NMR spectrum of compound 4 in CDCl3

Figure S2 1H NMR spectrum of compound 5 in CDCl3

Figure S3 1H NMR spectrum of compound 6 in CDCl3

Figure S4 1H NMR spectrum of Y19 in CDCl3

Figure S5 13C NMR spectrum of Y19 in CDCl3
4 Additional tables
Table S1 Device process aimed at optimizing mass ratio of PM6 and Y19

Table S2 Device process aimed at optimizing temperature of TA

Table S3 Device process aimed at optimizing additive content

Table S4 Change carrier mobilities and Jph-Veff characteristics of PM6:Y19-based devices

Contributors
The overarching research goals were developed by CAO Rui, CHEN Yu and ZOU Ying-ping. CAO Rui, CHEN Yu and CAI Fang-fang performed the experiments. CHEN Hong-gang, LIU Wei and GUAN Hui-lan collected the data. The initial draft of the manuscript was written by CAO Rui. WEI Qing-ya, LI Jing, CHANG Qin and LI Zhe reviewed and edited the manuscript.
Conflict of interest
CAO Rui, CHEN Yu, CAI Fang-fang, CHEN Hong-gang, LIU Wei, GUAN Hui-lan, WEI Qing-ya, LI Jing, CHANG Qin and LI Zhe declare that they have no conflict of interest.
References
[1] INGANAS O. Organic photovoltaics over three decades [J]. Advanced Materials, 2018, 30(35): 1800388. DOI: 10.1002/ adma.201800388.
[2] LI Gang, ZHU Rui, YANG Yang. Polymer solar cells [J]. Nature Photonics, 2012, 6(3): 153-161. DOI: 10.1038/ nphoton.2012.11.
[3] WADSWORTH A, MOSER M, MARKS A, LITTLE M S, GASPARINI N, BRABEC C J, BARAN D, MCCULLOCH I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells [J]. Chemical Society Reviews, 2019, 48(6): 1596-1625. DOI: 10.1039/c7cs00892a.
[4] HONG Ling, YAO Hui-feng, WU Zi-ang, CUI Yong, ZHANG Tao, XU Ye, YU Run-nan, LIAO Qing, GAO Bo-wei, XIAN Kai-hu. Eco-compatible solvent-processed organic photovoltaic cells with over 16% efficiency [J]. Advanced Materials, 2019, 31(39): 1903441. DOI: 10.1002/ adma.201903441.
[5] MENG Ling-xian, ZHANG Ya-min, WAN Xiang-jian, LI Chen-xi, ZHANG Xin, WANG Yan-bo, KE Xin, XIAO Zuo, DING Li-ming, XIA Ruo-xi. Organic and solution-processed tandem solar cells with 17.3% efficiency [J]. Science, 2018, 361(6407): 1094-1098. DOI: 10.1126/science.aat2612.
[6] TANG C W. Two-layer organic photovoltaic cell [J]. Applied Physics Letters, 1986, 48(2): 183-185. DOI: 10.1063/ 1.96937.
[7] CHENG Pei, LI Gang, ZHAN Xiao-wei, YANG Yang. Next-generation organic photovoltaics based on non-fullerene acceptors [J]. Nature Photonics, 2018, 12(3): 131-142. DOI: 10.1038/s41566-018-0104-9.
[8] FU Hui-ting, WANG Zhao-hui, SUN Yan-ming. Polymer donors for high-performance non-fullerene organic solar cells [J]. Angewandte Chemie International Edition, 2019, 58(14): 4442-4453. DOI: 10.1002/anie.201806291.
[9] LIU Ya-hui, LU Hao, LI Miao, ZHANG Zhe, FENG Shi-yu, XU Xin-jun, WU You-zhi, BO Zhi-shan. Enhancing the performance of non-fullerene organic solar cells using regioregular wide-bandgap polymers [J]. Macromolecules, 2018, 51(21): 8646-8651. DOI: 10.1021/acs.macromol. 8b01677.
[10] NIELSEN C B, HOLLIDAY S, CHEN H Y, CRYER S J, MCCULLOCH I. Non-fullerene electron acceptors for use in organic solar cells [J]. Accounts of Chemical Research, 2015, 48(11): 2803-2812. DOI: 10.1021/acs.accounts.5b00199.
[11] XU Xiao-peng, BI Zhao-zhao, MA Wei, WANG Zi-shuai, CHOY W C, WU Wen-lin, ZHANG Guang-jun, LI Ying, PENG Qiang. Highly efficient ternary-blend polymer solar cells enabled by a nonfullerene acceptor and two polymer donors with a broad composition tolerance [J]. Advanced Materials, 2017, 29(46): 1704271. DOI: 10.1002/adma. 201704271.
[12] DONG Yi-fan, CHA H, ZHANG Jiang-bin, PASTOR E, TULADHAR P S, MCCULLOCH I, DURRANT J R, BAKULIN A A. The binding energy and dynamics of charge-transfer states in organic photovoltaics with low driving force for charge separation [J]. The Journal of Chemical Physics, 2019, 150(10): 104704. DOI: 10.1063/ 1.5079285.
[13] NIU Meng-si, WANG Kang-wei, YANG Xiao-yu, BI Peng-qing, ZHANG Kang-ning, FENG Xian-jin, CHEN Fei, QIN Wei, XIA Jian-long, HAO Xiao-tao. Hole transfer originating from weakly bound exciton dissociation in acceptor–donor–acceptor nonfullerene organic solar cells [J]. The Journal of Physical Chemistry Letters, 2019, 10(22): 7100-7106. DOI: 10.1021/acs.jpclett.9b02837.
[14] WANG Jia-liang, PEURIFOY S R, BENDER M T, NG F, CHOI K S, NUCKOLLS C, ARNOLD M S. Non-fullerene acceptors for harvesting excitons from semiconducting carbon nanotubes [J]. The Journal of Physical Chemistry C, 2019, 123(35): 21395-21402. DOI: 10.1021/acs.jpcc. 9b06381.
[15] YOUNTS R, DANILOV E O, GUNDOGDU K, GAUTAM B R. Charge generation dynamics in polymer nonfullerene solar cells with low energy loss [J]. Journal of Photonics for Energy, 2018, 8(3): 032209. DOI: 10.1117/1.JPE.8.032209.
[16] YUAN Jun, HUANG Tian-yi, CHENG Pei, ZOU Ying-ping, ZHANG Huo-tian, YANG J L, CHANG S Y, ZHANG Zhen-zhen, HUANG Wen-chao, WANG Rui. Enabling low voltage losses and high photocurrent in fullerene-free organic photovoltaics [J]. Nature Communications, 2019, 10(1): 1-8. DOI: 10.1038/s41467-019-08386-9.
[17] YUAN Jun, ZHANG Yun-qiang, ZHOU Liu-yang, ZHANG Gui-chuan, YIP H L, LAU T K, LU Xin-hui, ZHU Can, PENG Hong-jian, JOHNSON P A. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core [J]. Joule, 2019, 3(4): 1140-1151. DOI: 10.1016/j.joule.2019.01.004.
[18] YANG Yan-kang, ZHANG Zhi-Guo, BIN Hai-jun, CHEN Shan-shan, GAO Liang, XUE Ling-wei, YANG C, LI Yong-fang. Side-chain isomerization on an n-type organic semiconductor ITIC acceptor makes 11.77% high efficiency polymer solar cells [J]. Journal of the American Chemical Society, 2016, 138(45): 15011-15018. DOI: 10.1021/jacs. 6b09110.
[19] LIU Qi-shi, JIANG Yu-fan, JIN Ke, QIN Jian-qiang, XU Jin-gui, LI Wen-ting, XIONG Ji, LIU Jin-feng, XIAO Zuo, SUN Kuan. 18% efficiency organic solar cells [J]. Science Bulletin, 2020, 65(4): 272-275. DOI:10.1016/j.scib.2020.01. 001.
[20] WANG Rui, YUAN Jun, WANG Rui, HAN Guang-chao, HUANG Tian-yi, HUANG Wen-chao, XUE Jing-jing, WANG Hao-cheng, ZHANG Chun-feng, ZHU Chen-hui. Rational tuning of molecular interaction and energy level alignment enables high-performance organic photovoltaics [J]. Advanced Materials, 2019, 31(43): 1904215. DOI: 10.1002/ adma.201904215.
[21] ALDRICH T J, MATTA M, ZHU Wei-gang, SWICK S M, STERN C L, SCHATZ G C, FACCHETTI A, MELKONYAN F S, MARKS T J. Fluorination effects on indacenodithienothiophene acceptor packing and electronic structure, end-group redistribution, and solar cell photovoltaic response [J]. Journal of the American Chemical Society, 2019, 141(7): 3274-3287. DOI: 10.1021/jacs. 8b13653.
[22] BAUER N, ZHANG Qian-qian, RECH J J, DAI Shui-xing, PENG Zheng-xing, ADE H, WANG Jia-yu, ZHAN Xiao-wei, YOU Wei. The impact of fluorination on both donor polymer and non-fullerene acceptor: The more fluorine, the merrier [J]. Nano Research, 2019, 12(9): 2400-2405. DOI: 10.1007/s12274-019-2362-3.
[23] CHEN Chih-Ping, CHEN Yi-Chun, YU Chao-ying. Increased open circuit voltage in a fluorinated quinoxaline- based alternating conjugated polymer [J]. Polymer Chemistry, 2013, 4(4): 1161-1166. DOI: 10.1039/C2PY20798B.
[24] CHEN Fang-xiao, XU Jing-qi, LIU Zhi-xi, CHEN Ming, XIA Ruo-xi, YANG Yong-chao, LAU T K, ZHANG Ying-zhu, LU Xin-hui, YIP H L. Near-infrared electron acceptors with fluorinated regioisomeric backbone for highly efficient polymer solar cells [J]. Advanced Materials, 2018, 30(52): 1803769. DOI: 10.1002/adma.201803769.
[25] FEI Zhu-ping, SHAHID M, YAACOBI G N, ROSSBAUER S, ZHONG Hong-liang, WATKINS S E, ANTHOPOULOS T D, HEENEY M. Thiophene fluorination to enhance photovoltaic performance in low band gap donor–acceptor polymers [J]. Chemical Communications, 2012, 48(90): 11130-11132. DOI: 10.1039/C2CC35079C.
[26] LECLERC N, CHAVEZ P, IBRAIKULOV O A, HEISER T, LEVEQUE P. Impact of backbone fluorination on π-conjugated polymers in organic photovoltaic devices: A review [J]. Polymers, 2016, 8(1): 11. DOI: 10.3390/ polym8010011.
[27] MORI T, MA Xiao-ling, FURUHASHI H, NISHIKAWA T. Control of open voltage of organic photovoltaic cells using fluorinated self-assembled monolayer [J]. Journal of Photopolymer Science and Technology, 2013, 26(3): 377-381. DOI: 10.2494/photopolymer.26.377.
[28] TAKAO Y, MASUOKA T, YAMAMOTO K, MIZUTANI T, MATSUMOTO F, MORIWAKI K, HIDA K, IWAI T, ITO T, MIZUNO T. Synthesis and properties of novel fluorinated subnaphthalocyanines for organic photovoltaic cells [J]. Tetrahedron Letters, 2014, 55(33): 4564-4567. DOI: 10.1016/j.tetlet.2014.06.069.
[29] TANG Dong-sheng, WAN Jia-hui, XU Xiao-peng, LEE Y W, WOO H Y, FENG Kui, PENG Qiang. Naphthobistriazole- based wide bandgap donor polymers for efficient non- fullerene organic solar cells: Significant fine-tuning absorption and energy level by backbone fluorination [J]. Nano Energy, 2018, 53: 258-269. DOI: 10.1016/j.nanoen. 2018.08.059.
[30] CHEN Meng-xue, ZHANG Zhuo-han, LI Wei, CAI Jin-long, YU Jiang-sheng, SPOONER E L, KILBRIDE R C, LI Dong-hui, DU Bao-cai, GURNEY R S. Regulating the morphology of fluorinated non-fullerene acceptor and polymer donor via binary solvent mixture for high efficiency polymer solar cells [J]. Science China Chemistry, 2019, 62(9): 1221-1229. DOI: 10.1007/s11426-019-9484-8.
[31] LIN Zhi-jing, HUANG Kun-xiang, WANG Zhao-long, CHEN Xian-jie, SUN Jing, XU Zheng, HE Tian, YIN Shou-chun, LI Miao-miao, ZHANG Qian. Alkyl side-chain and fluorination engineering in the indeno [1, 2-b] fluorene-based small-molecule acceptors for efficient non-fullerene organic solar cells [J]. Dyes and Pigments, 2019, 160: 432-438. DOI: 10.1016/j.dyepig.2018.08.039.
[32] ZHANG Guang-jun, XU Xiao-peng, BI Zhao-zhao, MA Wei, TANG Dong-sheng, LI Ying, PENG Qiang. Fluorinated and alkylthiolated polymeric donors enable both efficient fullerene and nonfullerene polymer solar cells [J]. Advanced Functional Materials, 2018, 28(10): 1706404. DOI: 10.1002/ adfm.201706404.
[33] YAN Cen-qi, BARLOW S, WANG Zhao-hui, YAN He, JEN K Y, MARDER S R, ZHAN Xiao-wei. Non-fullerene acceptors for organic solar cells [J]. Nature Reviews Materials, 2018, 3(3): 18003. DOI: 10.1038/natrevmats. 2018.3.
[34] WU Ya-nan, AN Cun-bin, SHI Lan-lan, YANG Li-yan, QIN Yun-peng, LIANG Ning-ning, HE Chang, WANG Zhao-hui, HOU Jian-hui. The crucial role of chlorinated thiophene orientation in conjugated polymers for photovoltaic devices [J]. Angewandte Chemie International Edition, 2018, 57(39): 12911-12915. DOI: 10.1002/anie.201807865.
[35] ZHANG Shao-qing, QIN Yun-peng, ZHU Jie, HOU Jian-hui. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor [J]. Advanced Materials, 2018, 30(20): 1800868. DOI: 10.1002/adma.201800868.
[36] FAN Qun-ping, ZHU Qing-lian, XU Zhuo, SU Wen-yan, CHEN Juan, WU Jing-nan, GUO Xia, MA Wei, ZHANG Mao-jie, LI Yong-fang. Chlorine substituted 2D-conjugated polymer for high-performance polymer solar cells with 13.1% efficiency via toluene processing [J]. Nano Energy, 2018, 48: 413-420. DOI: 10.1016/j.nanoen.2018.04.002.
[37] LUO Mei, ZHU Can, YUAN Jun, ZHOU Liu-yang, KESHTOV M, GODOVSKY D Y, ZOU Ying-ping. A chlorinated non-fullerene acceptor for efficient polymer solar cells [J]. Chinese Chemical Letters, 2019, 30(12): 2343-2346. DOI: 10.1016/j.cclet.2019.07.023.
[38] CUI Yong, YAO Hui-feng, ZHANG Jian-qi, ZHANG Tao, WANG Yu-ming, HONG Ling, XIAN Kai-hu, XU Bo-wei, ZHANG Shao-qing, PENG Jing. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages [J]. Nature Communications, 2019, 10(1): 1-8. DOI: 10.1038/s41467- 019-10351-5.
(Edited by YANG Hua)
中文导读
光电转换率超过12%的含氯非富勒烯受体基有机光伏器件
摘要:末端基团的氟化能显著改善有机受体材料的光电性能并已实现了广泛的应用。相比之下,氯化策略被关注得较少且对有机光伏电池的影响仍不明确。这里, 我们报道了一个以苯并三唑为缺电子核及2Cl-IC为末端基团的新型非富勒烯受体Y19。Y19表现了优异的光学及电化学性质。Y19的薄膜吸收边带为948 nm,LUMO能级水平为-3.95 eV。基于PM6:Y19的光伏器件实现了12.76%的能量转换效率,其开路电压为0.84 V,短路电流密度为22.38 mA/cm2,填充因子为0.68。优化后的活性层有理想的形貌并且电子迁移率达到6.52×10-4 cm2/(V·s)。EQE测试表明外部量子效率在480~860 nm的范围内超过了60%。这一研究表明氯化作为一种低成本的分子设计策略在一定程度上也能改善光伏性能。
关键词:非富勒烯受体;氯化;缺电子核;器件性能
Foundation item: Project(21875286) supported by the National Natural Science Foundation of China
Received date: 2020-03-22; Accepted date: 2020-07-22
Corresponding author: ZOU Ying-ping, PhD, Professor; Tel: +86-18711140102; E-mail: yingpingzou@csu.edu.cn; ORCID: https:// orcid.org/0000-0003-1901-7243

