DOI: 10.11817/j.ysxb.1004.0609.2020-35731
CO气基还原MoO3制备MoC
宋成民,张国华,周国治
(北京科技大学 钢铁冶金新技术国家重点实验室,北京 100083)
摘 要:MoC由于其独特的物理化学性能,是一种广泛应用于催化和电化学领域的材料。本文以高纯MoO3为原料在CO气氛中合成MoC。通过扫描电子显微镜(SEM)、X射线衍射仪(XRD)和动力学曲线分析其反应过程。结果表明,MoO3向MoC的转变过程包含3个阶段:首先,MoO3被还原为MoO2;之后,MoO2继续与CO反应生成Mo2C。最后,Mo2C继续被碳化生成MoC,在过程中始终没有钼生成。MoO3与CO反应的终产物与反应温度有关,随着反应温度升高,MoO3与CO的反应速度加快;当温度上升到1043 K,最终的反应产物不是MoC而是Mo2C。
关键词:碳化钼;气基还原;一氧化碳;三氧化钼
文章编号:1004-0609(2020)-04-0906-06 中图分类号:TF8 文献标志码:A
钼的碳化物具有一般过渡金属碳化物的性质,如高的硬度、优异的耐高温性能和热稳定等特性[1-4]。在1973年,LEVY和BOUDART[5]报道了α-WC在一系列的反应中展现了类贵金属铂的优异催化性能。此后,过渡金属碳化物由于其优异的表面理化性能受到研究者们的广泛关注。许多研究表明,过渡金属碳化物在烷烃异构化及水煤气合成等反应中具备优异的性能[6-10],而过渡金属碳化物的类贵金属催化性能与其结构有关。通常过渡金属碳化物被称为“间充型合金”,是由体积较小的碳原子进入过渡金属的间隙位置而形成的,其基本结构有面心立方结构(FCC)、六方密堆结构(HCP)和简单六方结构(HEX)。目前有4种结构的碳化钼,分别为δ-MoC、β-Mo2C、γ-MoC和η-MoC。其中由于Mo2C具有ABAB的原子堆垛结构,所以最为稳定,因此被研究的也较多[11-12]。尽管η-MoC和δ-MoC均是温度高于1700 ℃时能稳定存在的物相,但是VELIKANOVA等[13]发现在室温下γ-MoC是可以稳定存在的物相。
MoC的应用主要集中于催化和电化学领域,由于其特殊的应用环境,所以对其性能、尺寸和形貌有着较高的要求。目前,其制备过程具有制备工艺复杂和反应周期较长的特点。例如,郭文显等[14-15]以三氧化钼悬浮于过氧化氢溶液中,之后加入蔗糖溶液,最后把得到的前驱体在惰性气氛中900 ℃下焙烧处理得到MoC;AN和LIU等[16]采用机械球磨法,在CO气氛下室温球磨30 h制备了平均尺寸为100 nm的MoC。BOUCHY等[17]在以MoO3和甲烷为原料制备Mo2C的反应中发现有MoC生成。王璐等[18]以其自制的超细MoO3为原料与CO制备Mo2C过程中发现有MoC生成。在上述的研究中MoC要么是作为副产物出现,要么是经过较长的制备流程,尚难以低成本制备纯MoC。本文以MoO3和CO为原料制备纯相MoC,确定了其合适的反应温度区间并分析了其反应过程。
1 实验
本实验以一氧化碳(CO)和金堆城钼业公司提供的工业级三氧化钼(MoO3)为原料制备碳化钼(MoC)。采用热重分析设备(HJ-2,北京恒久仪器公司生产)研究MoC制备过程及机理,图1所示为热重设备简图。具体制备工艺如下:在进行实验之前,称取大约100 mg的MoO3放置在实验坩埚中,之后通入CO并保持60 mL/min的流量排出热重反应室中的空气,随后以10 ℃/min的升温速率升到指定温度(923、953、983、1013和1043 K)并保温一段时间。待反应完成后,使热重设备自然冷却,当温度降至室温后将CO气体切为氩气。对所得到的样品进行X射线扫描分析,扫
描角度为10°~90°,X衍射仪的型号为Model TTRIII,Rigaku。采用场发射电子扫描电镜观测试样的形貌,电镜型号为Zeiss SUPRA 55,Oberkochen。

图1 TG热分析实验装置简图
Fig. 1 Schematic diagram of experimental apparatus for TG analysis
2 结果与讨论
2.1 热重分析
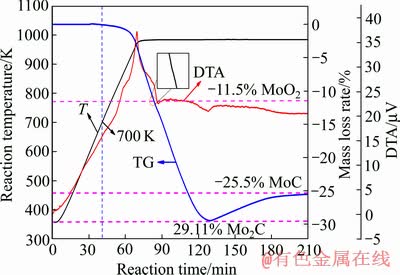
图2 CO还原碳化工业级MoO3的TG-DTA曲线
Fig. 2 TG-DTA reduction carburization kinetic curves of industrial grade MoO3 powders with pure CO gas from room temperature to 983 K
MoO3与CO气体在升温至983 K并保温的动力学曲线如图2所示。从图2中可以看到,随着反应温度升高到指定温度983 K之前,在700 K左右开始有失重现象,表明MoO3与CO的起始温度约为700 K。随着温度继续上升并到达指定温度并保温一段时间,可以看到失重率经历了3个阶段。首先,当反应的失重率在-11.5%左右时,TG(热重)曲线的斜率发生变化,与此同时DTA曲线出现吸热峰,表明此时有物相的转变,根据失重分析,此时为MoO3被还原为MoO2(从MoO3到MoO2的理论失重率为-11.1%)。之后,随着反应的继续进行,样品质量不断失重并到达一极值(约-29.1%),随后样品又缓慢增重,其趋势逐渐平缓,直到失重率进入平台阶段(约-25.5%),反应过程中的两个失重数据分别与MoO3→Mo2C和MoO3→MoC的理论失重率-29.2%和-25.03%基本一致。这说明MoO3与CO反应生成MoC的过程依次进行了化学反应(1)、(2)和(3)。
MoO3+CO=MoO2+CO2 (1)
2MoO2+6CO=Mo2C+5CO2 (2)
Mo2C+2CO=2MoC+CO2 (3)
图3所示为CO与MoO3反应制备MoC过程中相关反应的吉布斯自由能变与温度的关系,图4所示为CO-MoO3二元系相图。图3和4的数据均来自Factsage 7.0。从图3中可以看到,从热力学角度上,反应温度在1075 K之前对制备MoC是有利的,但温度继续升高后,热力学稳定相始终为Mo2C。当温度超过1155 K后,CO与MoO3的反应产物将为MoO2。结合图4可以发现,MoC的热力学稳定区域面积小且狭窄(区域3),制备难度较高[19]。为了确定制备MoC的最佳条 件,分别进行了目标温度为923、953、983、1013和1043 K的实验,其动力学曲线如图5所示。从图5中可以看到,除了1043 K外,其余温度下的产物均为MoC,并且随着温度的升高,不但在到达失重极值(29%)前的失重速率逐渐增大,而且进入失重平台阶段的时间也缩短。通过比较可以发现,1043 K与1013 K的失重基本一致,表明1013 K是本文中最佳的反应温度,在满足热力学的前提下,也为反应提供了较好的动力学条件。

图3 反应的吉布斯自由能变与温度的关系
Fig. 3 Relationship between temperature and change of standard Gibbs free energy

图4 MoO3和CO反应的稳定产物
Fig. 4 Stable products after reaction between MoO3 and CO at 1×105 MPa

图5 CO还原碳化工业级MoO3的恒温动力学曲线
Fig. 5 Isothermal reduction-carburization kinetic curves of industrial grade MoO3 with pure CO gas from room temperature to different desired temperatures at heating rate of 10 K/min with holding time
2.2 X射线衍射分析
图6所示为不同温度下以CO气体还原MoO3后的产物的XRD谱。从图6中可以看到,在反应温度为923 K、953 K、983 K和1013 K时,根据XRD谱并结合失重曲线判断,最终的产物均为MoC,衍射峰低矮且宽。这种X射线衍射峰的形状与王璐等的发现一致[16-17, 20-22]。随着温度的升高,衍射峰逐渐变得高而窄,表明结晶度随着温度升高而提高。当温度升到1043 K时,可以看到其物相转变为Mo2C,但与Mo2C的标准卡片比对,其结晶度依然不足。

图6 不同反应温度下的产物的XRD谱
Fig. 6 XRD patterns of products at different reaction temperatures
2.3 扫描电镜分析
图7所示为原料MoO3和不同反应温度下CO气体还原MoO3制备的产物的形貌图。由图7可以看到,MoO3由许多小的片型颗粒构成,其粒径范围为0.5~2 μm;通过对比产物的形貌,可以发现MoO3与CO在不同温度下反应制备的产物形貌基本一致;在1043 K制备的Mo2C与953 K和983 K制备的MoC都呈现为亚微米厚度的片状结构,并且大小相似。但是通过比较原料与产物形貌可以看到,圆滑的片状MoO3与生成物的形貌有较大的不同,这与反应过程的物相转变机理有关。Lü等[23]在以CH3OH还原并碳化MoO2制备Mo2C的过程中发现,在较低的反应温度下,不经过钼而直接制备Mo2C的形貌变化机理为假晶转变(Pseudomorphic transformation mechanism),产物的形貌基本与原料基本保持一致;而在较高温度下则经历了化学气相转移的过程(Chemical vapor transport mechanism),在该过程中由于产生了热力学不稳定物相(MoO2(OH)2),其极易在传输过程中分解、沉积,从而使产物的形貌发生大的变化[24]。在本课题组以前的研究中,党杰等[2]以CO与片状MoO2反应,不经历中间相钼直接制备Mo2C的实验中,发现产物Mo2C与原料MoO2的形貌基本保持一致;同样的情况在王璐等[18]以CO还原并碳化MoO3制备Mo2C的过程中也出现,产物的形貌变化符合假晶转变机理。本文中的MoC是由CO继续碳化Mo2C而制备的,反应过程中不存在不稳定的可迁移物相(MoO2(OH)2),因此转变过程形貌由假晶转变机理控制。而王璐等[18]在以CO还原球形MoO3反应制备MoO2的过程中,发现球形颗粒上有片状颗粒形成。本文在以MoO3为原料制备MoC的过程中,MoO3被还原为MoO2是第一个物相转变阶段,之后的MoO2被继续碳化为MoC的过程为假晶转变机理控制,样品的形貌基本保持不变。因此,MoO3向MoO2的转变是形貌变化的最关键阶段。

图7 原料MoO3和不同反应温度下CO气体还原MoO3制备产物的形貌图
Fig. 7 FE-SEM micrographs of MoO3 and samples reduced by pure CO gas from room temperature to different desired temperatures at heating rate of 10 K/min
3 结论
1) 随着反应温度升高,MoO3与CO的反应速度加快,制备MoC的最佳反应温度为1013K,而当温度为1043 K时,产物为Mo2C。
2) 由MoO3制备MoC的过程中包含3个子过程,过程中没有钼生成:首先,MoO3被还原为MoO2;之后,MoO2被还原碳化为Mo2C;最后,Mo2C被继续碳化为MoC。
3) 不同温度下制备的MoC的形貌基本一致,都是厚度为亚微米级的片状结构。
REFERENCES
[1] PATEL M, SUBRAHMANYAM J. Synthesis of nanocrystalline molybdenum carbide (Mo2C) by solution route[J]. Materials Research Bulletin, 2008, 43: 2036-2041.
[2] DANG J, ZHANG G, WANG L, CHOU K, PISTORIUS P C, SMITH D. Study on reduction of MoO2 powders with CO to produce Mo2C[J]. Journal of the American Ceramic Society, 2016, 99: 819-824.
[3] 吴燕明, 赵 坚, 陈小明, 伏 利, 毛鹏展, 周夏凉. 高温及氧化对 WC-10Co4Cr 涂层微观结构及性能的影响[J].中国有色金属学报, 2017, 27(7): 1395-1402.
WU Yan-ming, ZHAO Jian, CHEN Xiao-ming, FU Li, MAO Peng-zhan, ZHOU Xia-liang. Effect of high temperature and oxidation on microstructure and properties of WC-10Co4Cr coatings[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(7): 1395-1402.
[4] 朱 斌, 柏振海, 高 阳, 罗兵辉. WC粒度对 WC-15Fe-5Ni 硬质合金组织与性能的影响[J]. 中国有色金属学报, 2016, 26(5): 1065-1074.
ZHU Bin, BAI Zhen-hai, GAO Yang, LUO Bing-hui. Effects of WC particle size on microstructure and properties of WC-15Fe-5Ni cemented carbi5des[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(5): 1065-1074.
[5] LEVY R B, BOUDART M. Platinum-like behavior of tungsten carbide in surface catalysis[J]. Science, 1973, 181: 547-549.
[6] CHEN Y, CHOI S, THOMPSON L T. Ethyl formate hydrogenolysis over Mo2C-based catalysts: Towards low temperature CO and CO2 hydrogenation to methanol[J]. Catalysis Today, 2016, 259: 285-291.
[7] BARTHOS R, SZECHENYI A, SOLYMOSI F. Efficient H2 production from ethanol over Mo2C/C nanotube catalyst[J]. Catalysis Letters, 2007, 120: 161-165.
[8] ALABA P A, ABBAS A, HUANG J, DAUD W M A W. Molybdenum carbide nanoparticle: Understanding the surface properties and reaction mechanism for energy production towards a sustainable future[J]. Renewable & Sustainable Energy Reviews, 2018, 91: 287-300.
[9] SHI C, ZHANG A, LI X, ZHANG S, ZHU A, MA Y, AU C. Ni-modified Mo2C catalysts for methane dry reforming[J]. Applied Catalysis A—General, 2012, 431: 164-170.
[10] 周燕强, 陈 萌, 徐立军, ABUDULA A, 马宇飞, 方岩雄. Pt/MoC的制备及其在电解水析氢反应中的催化性能[J]. 精细化工, 2018, 35: 1921-1927.
ZHOU Yan-qiang, CHEN Meng, ABUDULA A, MA Yu-fei, FANG Yan-xiong. Preparation of Pt/MoC and its performance in electrolysis hydrogen evolution[J]. Fine Chem, 2018, 35: 1921-1927.
[11] HUGOSSON H W, ERIKSSON O, NORDSTROM L, JANSSON U, FAST L, DELIN A, WILLS J M, JOHANSSON B. Theory of phase stabilities and bonding mechanisms in stoichiometric and substoichiometric molybdenum carbide[J]. Journal of Applied Physics, 1999, 86: 3758-3767.
[12] 梁 婷, 所艳华, 马守涛, 汪颖军. 碳化钼催化剂的制备及应用研究进展[J]. 化学与粘合, 2017, 39(4): 286-291.
LIANG Ting, SUO Yan-hua, MA shou-tao. Research progress in preparation and application of molybdenum carbide catalyst[J]. Chem Adhesion, 2017, 39(4): 286-291.
[13] VELIKANOVA T Y, KUBLII V Z, KHAENKO B V. Solid state transformations and phase equilibria in the molybdenum-carbon system[J]. Soviet Powder Metallurgy and Metal Ceramics, 1988, 27: 891-896.
[14] 郭文显, 陈妹琼, 张 敏, 柳 鹏, 张 燕, 蔡志泉. α-MoC/石墨烯复合材料的氧还原性能及其在微生物燃料电池中的应用[J]. 化工进展, 2016, 35(11): 3558-3562.
GUO Wen-xian, CHEN Mei-qiong, ZHANG Min, LIU Peng, ZHANG Yan, CAI Zhi-quan. Research on the oxygen reduction performance of α-MoC/graphene and its application in microbial fuel cells[J]. Chem Ind Eng Prog, 2016, 35(11): 3558-3562.
[15] 郭文显, 陈妹琼, 程发良. α-MoC在无介体微生物燃料电池阳极的应用研究[J]. 化工新型材料, 2016, 44(7): 258-260.
GUO Wen-xian, CHEN Mei-qiong, CHENG Fa-liang. Study of α-MoC on the anode of mediato-less microbial fual cells[J]. New Chem Mater, 2016, 44(7): 258-260.
[16] AN G, LIU G. Preparation of ultramicro molybdenum carbide powders and study on wear properties of their coating[J]. Rare Metals, 2011, 30(3): 262-266.
[17] BOUCHY C, HAMID D A, DEROUANE E G. A new route to the metastable FCC molybdenum carbide α-MoC[J]. Chemical Communications, 2000(2): 125-126.
[18] WANG L, ZHANG G H, CHOU K C. Preparation of Mo2C by reducing ultrafine spherical beta-MoO3 powders with CO or CO-CO2 gases[J]. Journal of the Australian Ceramic Society, 2018, 54: 97-107.
[19] PIERSON H O. Handbook of refractory carbides and nitrides[M]. Weatwood: William Andrew Publishing, 1996: 110-113.
[20] ZHOU S, CHEN H, MA L. Novel carbon-based nc-MoC/a-C(Al) nanocomposite coating towards low internal stress and low-friction[J]. Surface & Coatings Technology, 2014, 242: 177-182.
[21] DENG M, QI J, LI X, XIAO Y, YANG L, YU X. MoC/C nanowires as high-rate and long cyclic life anode for lithium ion batteries[J]. Electrochimica Acta, 2018, 277: 205-210.
[22] VITALE G, GUZMAN H, FRAUWALLNER M L, SCOTT C E, PEREIRA-ALMAO P. Synthesis of nanocrystalline molybdenum carbide materials and their characterization[J]. Catalysis Today, 2015, 250: 123-133.
[23] Lü Z, DANG J, WU Y, Lü X, ZHANG S. Preparation of Mo2C by reduction and carbonization of MoO2 with CH3OH[J]. Journal of Materials Science, 2018, 53: 10059-10070.
[24] WANG D H, SUN G D, ZHANG G H. Preparation of ultrafine Mo powders via carbothermic pre-reduction of molybdenum oxide and deep reduction by hydrogen[J]. International Journal of Refractory Metals and Hard Materials, 2018, 75: 70-77.
Preparation of MoC by reducing MoO3 with CO gas
SONG Cheng-min, ZHANG Guo-hua, ZHOU Guo-zhi
(State Key Laboratory of Advanced Metallurgy, University of Science and Technology, Beijing 100083, China)
Abstract: Due to its unique physical and chemical properties, MoC is an important material widely used in the fields of catalysis and electrochemistry. The reaction between high purity MoO3 powder and CO was investigated for purpose of producing MoC. From the scanning electron microscopy (SEM), X-ray diffractometer (XRD) and dynamic curve analysis. The results show that the reaction process between MoO3 and CO is composed of three stages: the reduction of MoO3 to MoO2, the carburization from MoO2 to Mo2C and further carburization of Mo2C to MoC, while molybdenum never forms in the reaction process. In addition, it is found that the final product is related to the reaction temperature, with the increase of reaction temperature, the reaction speed of MoO3 and CO accelerates, and as the temperature rises to 1043 K, MoC will be transformed to Mo2C.
Key words: molybdenum carbide; gas-based reduction; carbon monoxide; molybdenum trioxide
Received date: 2019-04-02; Accepted date: 2019-09-16
Corresponding author: ZHANG Guo-hua; Tel: +86-10-82376882; E-mail: ghzhang0914@ustb.edu.cn
(编辑 王 超)
收稿日期:2019-04-02;修订日期:2019-09-16
通信作者:张国华,研究员,博士;电话:010-82376882;E-mail:ghzhang0914@ustb.edu.cn