
Preparation and characterization of SnO2-Li4Ti5O12 composite by sol-gel technique
XIONG Li-zhi(熊利芝)1, 2 , HE Ze-qiang(何则强)1, 2, YIN Zhou-lan(尹周澜)2, CHEN Qi-yuan(陈启元)2
1. College of Biology and Environmental Sciences, Jishou University, Jishou 416000, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 30 December 2009
_____________________________________________________________________________________________________
Abstract: SnO2-Li4Ti5O12 was prepared by sol-gel method using tin tetrachloride, lithium acetate, tetrabutylorthotitanate and aqueous ammonia as starting materials. The composite was characterized by thermogravimertric (TG) analysis and differential thermal analysis (DTA), X-ray diffractometry (XRD) and transmission electron microscopy (TEM) combined with electrochemical tests. The results show that SnO2-Li4Ti5O12 composite derived by sol-gel technique is a nanocomposite with core-shell structure, and the amorphous Li4Ti5O12 layer with 20-40 nm in thickness is coated on the surface of SnO2 particles. Electrochemical tests show that SnO2-Li4Ti5O12 composite delivers a reversible capacity of 688.7 mA?h/g at 0.1C and 93.4% of that is retained after 60 cycles at 0.2C. The amorphous Li4Ti5O12 in composite can accommodate the volume change of SnO2 electrode and prevent the small and active Sn particles from aggregating into larger and inactive Sn clusters during the cycling effectively, and enhance the cycling stability of SnO2 electrode significantly.
Key words: SnO2; Li4Ti5O12; composite; sol-gel method; lithium ion batteries
_____________________________________________________________________________________________________
1 Introduction
Since it was used as anode material for lithium-ion batteries in 1997[1], SnO2 has been thought as the most promising anode material for lithium ion batteries due to its high capacity[2-7]. While SnO2 is used as anode material for lithium ion batteries, Sn is the active component and the reversible capacity is delivered from the reversible formation and decomposition of Li-Sn alloys(LiSn, Li7Sn3, Li5Sn2, Li3Sn5, Li7Sn2 or Li22Sn5)[8-10]. In the first cycle, SnO2 acts with Li to form amorphous Li2O, which can be the buffer for tin aggregation. The formation of Li2O results in the irreversible capacity in the first cycle as high as 47.6%, which is a shortcoming of SnO2[2-3]. However, the most fatal problem to hinder the application of SnO2 in lithium ion batteries is volume mismatch, which results in a rapid drop in reversible capacity upon cycling[2-3]. To improve the volume mismatch, many methods were used. LI et al[11] reported that nano-SnO2 fiber may absorb the volume changes in cycling to keep good cyclability even at high current rate. SnO2 thin film was also used to decrease the volume change[9, 12]. Metal composite oxides MxSnyOz (M=Ni, Ca, Fe, Sb, Cu, Mg, Zn, etc) were also proposed to improve cycling stabililty of SnO2[13-16]. By means of surface modification, such as coating a layer conductive materials on the surface of SnO2 the cycling stabililty of SnO2 can be also improved[17-19]. So far, coating Li4Ti5O12 on surface of SnO2 to improve its cycling stabililty has not been reported yet. SnO2-Li4Ti5O12 composite was prepared by sol-gel method and the electrochemical properties were studied here.
2 Experimental
2.1 Preparation of SnO2-Li4Ti5O12 composite
A certain quantity of tetrabutyl titanate (AR) was solved in ethanol absolute (AR) with the volume ratio of tetrabutyl titanate to ethanol absolute of 1?5. The solution of tetrabutyl titanate was added to an aqueous ethanol solution of lithium acetate (AR) under stirring strongly according to the stoichiometric ratio of Li4Ti5O12 to get a
Foundation item: Project(20873054) supported by the National Natural Science Foundation of China; Project(2005037700) supported by Postdoctoral Science Foundation of China; Project(07JJ3014) supported by Hunan Provincial Natural Science Foundation of China; Project(07A058) supported by Scientific Research Fund of Hunan Provincial Education Department; Project(2004107) supported by Postdoctoral Science Foundation of Central South University
Corresponding author: HE Ze-qiang; Tel: +86-743-8564416; Fax: +86-743-8564416; E-mail: csuhzq@163.com
clear yellow sol. A certain quantity of SnO2 powder was dispersed uniformly in this sol with ultrasonic wave and stirrers. After stirring for 2-3 h, a gel was obtained. The gel was dried at 105 ℃ for 4 h in vacuum to get a precursor. The precursor was calcinated at 500 ℃ for 4 h to obtain SnO2-Li4Ti5O12 composite. In addition, Li4Ti5O12 without SnO2 was also prepared.
2.2 Characterization of SnO2-Li4Ti5O12 composite
The thermal behavior of the precursor was analyzed by thermal analysis apparatus (TGA/SDTA851e, METTLER TOLEDO). Phase identification studies of the samples were carried out by an X-ray diffractometer (Rigaku D/MAX-gA) with Cu Kα radiation. The surface morphology analyses of SnO2-Li4Ti5O12 composite were done by scanning electron microscope (JSM 5600LV).
A slurry containing 80% SnO2-Li4Ti5O12 composite, 10% acetylene black and 10% PVDF (polyvinylidene fluoride) was made using N-methylprrolidinone (NMP) as the solvent. Electrodes with an area of 1 cm2 for the measurements of electrochemical characterization were prepared by coating slurries (about 100 μm in thickness) on copper foils followed by drying in vacuum at 60 ℃ for 12 h. Electrochemical tests were conducted using a conventional coin-type cell, employing lithium foil as a counter electrode and utilizing 1.0 mol/L LiPF6 in ethylene carbonate/dimethyl carbonate (EC/DMC) (with an volume ratio of EC to DMC of 1?1) as the electrolyte. The assembly was carried out in an Ar-filled glove box. The electrochemical analyses were carried out with an electrochemical analysis system.
3 Results and discussion
3.1 Thermal analysis
Fig.1 shows the thermogravimertric (TG) analysis and differential thermal analysis (DTA) of dry gel precursor. From the TG curve, it can be seen that almost all mass loss takes place before 400 ℃. The mass loss is due to the loss of physical absorbed water on surface of the precursor and the decomposition of Li4Ti5O12. Specific mass loss stages can not be found from the TG curve, suggesting that the mass loss is a continuous process. Above 500 ℃, there is no mass loss at TG curve, indicating that the decomposition of the precursor is complete. Accordingly, an endothermic peak at 400 ℃ appears on the DTA curve, which may be caused by the crystal shape due to the decomposition of the precursor and formation of SnO2-Li4Ti5O12.
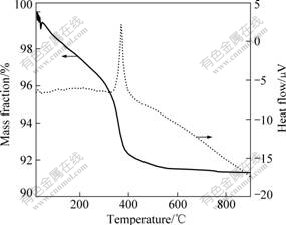
Fig.1 Thermal analysis curves of precursor of SnO2-Li4Ti5O12
3.2 XRD analysis
Fig.2 shows XRD patterns of SnO2-Li4Ti5O12 composites. All peaks in Fig.2(a) agree well with the standard peaks of JCPDS card No.26-1198, indicating that Li4Ti5O12 shares a face centered cubic structure with a space group of Fd3m[20]. The peaks in Fig.2(b) agree well with the standard peaks of JCPDS card No.21-1250, indicating that the sample is SnO2 with rutile structure. There are obvious peaks assigned to SnO2 and very weak peaks assigned to Li4Ti5O12 in Fig.2(c), suggesting that the amorphous Li4Ti5O12 layer is coated on the surface of SnO2 particles.
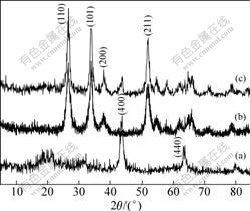
Fig.2 XRD patterns of samples: (a) Li4Ti5O12; (b) SnO2; (c) SnO2-Li4Ti5O12
3.3 TEM analysis
Fig.3 shows the TEM images of SnO2 and SnO2-Li4Ti5O12 composite. It is apparent that the morphology of SnO2 is regular spherical shape. The TEM image shows homogeneous appearance with particle size of 30-50 nm. As shown in Fig.3(a), the surface morphology of as-received SnO2 is smooth, but it becomes a little coarse after loading Li4Ti5O12 (Fig.3(b)). The transmission electron micrographs show that amorphous Li4Ti5O12 layer with 20-40 nm in thickness is uniformly distributed over the surface of SnO2 to form SnO2-Li4Ti5O12 composite with core-shell structure. Therefore, it is expected that nano-Li4Ti5O12 particle will act as bridge to result in good contact between SnO2 and Li4Ti5O12.
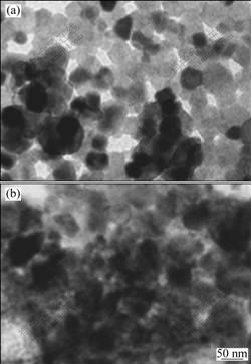
Fig.3 TEM images of SnO2(a) and SnO2-Li4Ti5O12(b)
3.4 Electrochemical properties
Fig.4 shows the initial charge-discharge curves of SnO2 and SnO2-Li4Ti5O12 as anodes for lithium-ion batteries at a constant current rate of 0.1C in the range of 0-1.0 V. The profiles of the charge and discharge curves were very similar, indicating that SnO2 and SnO2-Li4Ti5O12 have the same electrochemical mechanism. The first discharge capacities of SnO2 and SnO2-Li4Ti5O12 are 1 640.5 mA?h/g and 1 565.2 mA?h/g, and the first charge capacities of SnO2 and SnO2-Li4Ti5O12 are 689.8 mA?h/g and 688.7 mA?h/g, respectively. Obviously, after loading amorphous Li4Ti5O12 layer on SnO2, the first discharge capacity and charge capacity of SnO2 decrease. This may be due to two reasons: On the one hand, the theoretical capacity of Li4Ti5O12 (168 mA?h/g) is lower than that of SnO2 (782 mA?h/g), resulting in lower capacity of composite electrode after SnO2 being substituted by Li4Ti5O12. On the other hand, due to that the discharge-charge plateau is 1.5 V[21], the capacity can not be utilized when the charge-discharge voltage is chosen to be 0-1.0 V.
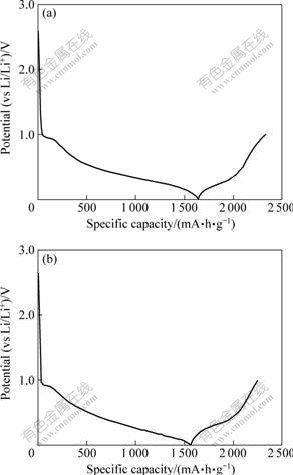
Fig.4 Initial charge-discharge curves of SnO2 (a) and SnO2-Li4Ti5O12 (b)
Fig.5 shows the comparison of cycling performance of SnO2 and SnO2-Li4Ti5O12 at current rate of 0.2C. From Fig.5, it can be seen that the discharge capacity of SnO2 at the second cycle is 688.1 mA?h/g, and the discharge capacity after 60 cycles is 587.8 mA?h/g. So, the capacity retention of SnO2 is 85.4%. Under the same conditions, the discharge capacity of SnO2-Li4Ti5O12 at the second cycle is 687.6 mA?h/g, and the discharge capacity after 60 cycles is 642.1 mA?h/g. So, the capacity retention of SnO2-Li4Ti5O12 is more than 94.3%. It is apparent that the cycling stability of SnO2 is improved effectively after being loaded a layer of amorphous Li4Ti5O12. As is known, Li4Ti5O12 is regarded as a “zero strain” insertion material[21]. The amorphous Li4Ti5O12 layer on SnO2 can avoid structural damage due to expansion and contraction of the electrode materials at the insertion material/solid electrolyte interfaces during the charge and discharge process and prevent the aggregation of Sn particles, resulting in the improvement of cycling performance of SnO2.
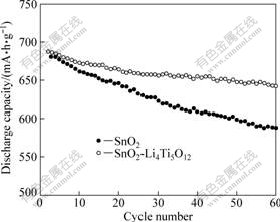
Fig.5 Cycling performance of SnO2 and SnO2-Li4Ti5O12
4 Conclusions
1) SnO2-Li4Ti5O12 composite was prepared by sol-gel method. The amorphous Li4Ti5O12 layer with 20-40 nm in thickness is coated on the surface of SnO2 particles to form SnO2-Li4Ti5O12 composite with core-shell structure.
2) SnO2-Li4Ti5O12 composite delivers a reversible capacity of 688.7 mA?h/g at 0.1C, and 93.4% of that is retained after 60 cycles at 0.2C. The amorphous Li4Ti5O12 in composite can accommodate the volume change of SnO2 electrode and prevent the small and active Sn particles from aggregating into larger and inactive Sn clusters during cycling effectively, and enhance the cycling stability of SnO2 electrode significantly.
References
[1] IDOTA Y, MATSUFUJI A, MAEKAWA Y, NIYASAKA T. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material [J]. Science, 1997, 276(5317): 1395-1397.
[2] COURTNEY A, DAHN J R. Electrochemical and in situ X-ray diffraction studies of the reaction of lithium with tin oxide composites [J]. J Electrochem Soc, 1997,144(6): 2045-2052.
[3] LIU W F, HUANG X J, WANG Z X,LI H,CHEN L Q. Studies of stannic oxide as an anode material for lithium-ion batteries [J]. J Electrochem Soc, 1998, 145(1): 59-62.
[4] COURTNEY A, DAHN J R. Key factors controlling the reversibility of the reaction of lithium with SnO2 and Sn2BPO6 glass [J]. J Electrochem Soc, 1997, 144(9): 2943-2948.
[5] HE Ze-qiang, LI Xin-hai, WU Xian-ming, HOU Zhao-hui, LIU En-hui, DENG Ling-feng, HU Chuan-yue, TIAN Hui-peng. Preparation and electrochemical properties of nanosized tin dioxide electrode material by sol-gel process [J]. Transaction of Nonferrous Metals Society of China, 2003, 13(4): 998-1002.
[6] HE Ze-qiang, LI Xin-hai, XIONG Li-zhi, LIU En-hui, HOU Zhao-hui. Preparation and electrochemical properties of tin-based composite oxide by high-energy ball-milling method [J]. Chinese Journal of Inorganic Chemistry, 2004, 20(1): 102-106. (in Chinese)
[7] HE Ze-qiang, LI Xin-hai, XIONG Li-zhi, WU Xian-ming, XIAO Zhuo-bing, MA Ming-you. Wet chemical synthesis of tin oxide-based material for lithium ion battery anodes [J]. Materials Research Bulletin, 2005, 40(5): 861-868.
[8] ANANI A, CROUCH-BAKER S, HUGGINS R A. Kinetic and thermodynamic parameters of several binary lithium alloy negative electrode materials at ambient temperature [J]. J Electrochem Soc, 1987, 134(12): 3098-3102.
[9] RETOUX R, BROUSSE T, SCHLEICH D M. High-resolution electron microscopy investigation of capacity fade in SnO2 electrodes for lithium-ion batteries [J]. J Electrochem Soc, 1999, 146(7): 2472-2476.
[10] BROUSSE T, RETOUX R, HERTERICH U, SCHLEICH D M. Thin-film crystalline SnO2-lithium electrodes [J]. J Electrochem Soc, 1998, 145(1): 1-4.
[11] LI N, MARTIN C R, SCROSATI B. A high-rate, high-capacity, nanostructured tin oxide electrode [J]. Electrochem Solid-State Lett, 2000, 3(7): 316-318.
[12] NAM S C, KIM Y H, CHO W I, CHO B W, CHUN H S, YUN K S. Charge-discharge performance of electron-beam-deposited tin oxide thin-film electrodes [J]. Electrochem Solid-State Lett, 1999, 2(1): 9-13.
[13] BESENHARD J O, YANG J, WINTER M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries? [J]. J Power Sources, 1997, 68(1): 87-90.
[14] CROSNIER O, BROUSSE T, DEVAUX X, FRAGNAUD P, SCHLEICH D M. New anode systems for lithium ion cells [J]. J Power Sources, 2001, 94(2): 169-171.
[15] TIRADO J L. Inorganic materials for the negative electrode of lithium-ion batteries: State-of-the-art and future prospects [J]. Mater Sci Eng R, 2003, 40(3): 103-115.
[16] YUAN Zheng-yong, YUAN Liang-jie, SUN Ju-tang. Synthesis and properties of nanosized tin-zinc composite oxides as lithium storage materials [J]. Chemical Journal of Chinese Universities, 2006, 27(12): 2252-2255. (in Chinese)
[17] MA Ming-you, HE Ze-qiang, XIONG Li-zhi, LI Xin-hai, XIAO Zhuo-bing, WU Xian-ming, LIU Wen-ping. Preparation and electrochemical properties of SnO2-graphite composites by homogeneous precipitation technique [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(5): 793-798. (in Chinese)
[18] BALAN L, SCHNEIDER R, WILLMANN P, BILLAUD D. Tin–graphite materials prepared by reduction of SnCl4 in organic medium: Synthesis, characterization and electrochemical lithiation [J]. J Power Sources, 2006, 161(1): 587-593.
[19] HE Ze-qiang, LIU Wen-ping, XIONG Li-zhi, SHU Hui, WU Xian-ming, CHEN Shang, HUANG Ke-long. Synthesis and characterization of SnO2-polyanine composite as anode for lithium ion batteries[J]. Chinese Journal of Inorganic Chemistry, 2007, 23(5): 813-816. (in Chinese)
[20] WANG G X, BRADHURST D H, DOU S X, LIU H K. Spinel Li[Li1/3Ti5/3]O4 as an anode material for lithium ion batteries [J]. J Power Sources, 1999, 83(1/2): 156-160.
[21] FU L J, LIU H, LI C, WU Y P, RAHM E, HOLZE R, WU H Q. Electrode materials for lithium secondary batteries prepared by sol-gel methods [J]. Prog Mater Sci, 2005, 50(7): 881-887.
(Edited by CHEN Ai-hua)