J. Cent. South Univ. Technol. (2008) 15: 786-790
DOI: 10.1007/s11771-008-0145-1
Effects of temperature and initial molar ratio of Na2O to Al2O3 on
agglomeration of fine Al(OH)3 seed in synthetic Bayer solution
ZHANG Bin(张 斌)1, 2, CHEN Qi-yuan(陈启元)2, LI Jie(李 洁)2, YIN Zhou-lan(尹周澜)2
(1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: Fine Al(OH)3 crystals were aggregated from supersaturated aluminate solution in the batch reaction tanks. By means of laser particle size analyzer and scanning electron microscopy, the influences of temperature and initial molar ratio of Na2O to Al2O3 (αK) on agglomeration of fine seed in Bayer process were investigated. The results show that agglomeration is almost finished in 8 h, and seeds with size less than 2 μm are easily aggregated together, and almost disappear in 8 h under the optimal process conditions. In the aluminate solution with the same moderate initial αK, when the reaction temperature reaches 75 ℃, the secondary nucleation does not occur, and the effect of agglomeration is better. And at the same reaction temperature, when the initial αK is 1.62, the initial supersaturation of aluminate solution is moderate, the binders on the surfaces of the seed are enough to maintain the agglomeration process, and the agglomeration degree is better. From SEM images, agglomeration mainly occurs in the fine particles, the combinations among the fine particles are loose and the new formed coarse crystal shapes are irregular.
Key words: sodium aluminate; Bayer process; screen separation; agglomeration; initial molar ratio of Na2O to Al2O3
1 Introduction
Seeded precipitation of sodium aluminate solution is an important step in Bayer process for the production of alumina. It is well known that the seeded precipitation of sodium aluminate solution is a chemical crystallization process, which involves several main steps as follows: the secondary nucleation, the agglomeration of fine particles, the seed or nuclei growth, and the breakage and the attrition of crystal. The seed growth and agglomeration of fine particles can make the size of product larger, but the second nucleation, the breakage and attrition of crystal can make the size of product smaller. Because the growth rates are very low, the agglomeration of gibbsite crystals is an important size enlargement process. And it not only makes the crystal size rapidly enlarged, but also determines the product characteristics such as its strength, crystal size distribution (CSD) or crystal shape[1-2]. So over the last 40 years, researchers have made many studies on the process parameters and kinetics of the agglomeration process of gibbsite particles[3-6]. To better understand the whole agglomeration process of gibbsite crystals, the effects of the crystallization temperature, supersaturation, seed mass, stirring rate and seed size were investigated[7-9]. However, because of the complexity of the combined processes (nucleation, crystal growth and crystal breakdown), the mechanism of agglomeration of crystal particles is still poorly understood, especially on a microscopic scale. The investigation on the evolution of the size of the agglomerates has been focused on the crystals with medium diameter, and the agglomeration of the fine seed in the aluminate solution has not individually and detailedly been studied yet at present.
In the present work, the fine seed of gibbsite was firstly screened by electromagnetic shale shaker. Then the effects of temperature and initial molar ratio of Na2O to Al2O3 (αK) on agglomeration of fine seed in the Bayer process were studied by Malvern laser particle size analyzer. And the particle shape and size of gibbsite crystals were observed by scanning electron microscopy.
2 Experimental
2.1 Screening of fine seed
Gibbsite seeds were put in a mesh screen which was clipped and vibrated by electromagnetic shale shaker while water run down. The sieve pore of the used standard mesh screen was 45 μm.
2.2 Experimental process
The concentrated caustic aluminate solutions with the initial αK in sodium aluminate solution were prepared by dissolving industrial hydroxide aluminum(from Changcheng Aluminum Ltd Co, Zhengzhou, China) in NaOH (chemically pure) solutions with enough heating. After completely dissolving, the solutions were carefully filtered twice so that they were optically clear (no solid nuclei present).
The concentrated and boiled sodium hydroxide solution was put into a 2.5 L reaction tank (d 10 cm) for 2 h, the adhered Al(OH)3 and other impurities on the internal wall of tank were carefully washed, and the ethyl alcohol was also used to dissolve organic substances. The concentrated sodium aluminate solutions were diluted to contain alkali concentration (Na2O) of (150±5)g/L, and put into the reaction tank with a stainless propeller of three-blade, pitch of 45? and 7 cm in diameter. The distance of the propeller to the bottom was 1.5 cm, and there was no baffle within the tanks. The agitation rate was (200±2) r/min. The initial seed of 35 g/L was added in the tank for each experiment. 10 mL slurry sample was extracted from the reaction tank at a fixed interval during the seeded precipitation. Each sample was washed and diluted repeatedly by distilled water three times. And the distribution size of solid particles was determined by Malvern Mastersizer 2000, and their SEM images were obtained by JSM-5600LV scanning electronic microscope (JEOL, Japan).
3 Results and discussion
3.1 Influence of time on agglomeration of fine seeds
The experiment conditions were as follows: the initial content of seed was 35 g/L, the seed size was less than 45 μm, reaction temperature was 75 ℃, the initial αK was 1.62, and time intervals were 0, 0.5, 2.0, 4.0, 8.0, 16.0 h, respectively. The experimental results are shown in Fig.1. In Fig.1, the percentage is the ratio of the number of those particles with size in certain range to the total number of particles, not volume ratio or mass ratio. The transform among volume ratio, mass ratio and number ratio can be performed on the measuring software of Malvern Mastersizer 2000. From Fig.1, it can be known that the seeds with particle size less than 5 μm, especially less than 2 μm are predominant in the primary seed, so the number of seed particles in unit volume in the sodium aluminate solution is great. Agglomeration also mainly happens among seeds with size less than 5 μm, especially less than 2 μm. With the development of agglomeration process, the percentages of particle less than 10 μm all change rapidly, the particles with size less than 2 μm are easily aggregated into coarse particles and the percentage of particles with size less than 2 μm falls down from 76.67 % to 0 in 8 h, but the percentage of particles in size range of 2-5 μm increases from 20.53% to 48.32%, and the percentage of particles in size range of 5-10 μm is increased from 2.29% to 43.56%, showing obviously that the agglomeration of the seeds with size less than 2 μm is complete, and the agglomeration degree in the seeds with size larger than 2 μm is lower than that of the seeds with size less than 2 μm. The reason may be that the coarse seeds in the initial seeds are less than fine seeds, the collision probability among coarse particles or between coarse particles and fine particles is less than that among fine seeds, and the defects on the surfaces of coarse crystals are not enough, and therefore, the active sites on the surfaces of coarse particles are also not adequate[10-12]. And with the disappearance of seeds with size less than 2 μm in 8 h and the decrease of supersaturation of aluminate solution, the agglomeration slows down and then stops.

Fig.1 Relationship between percentage in seed particles and time at 75 ℃ and initial αK of 1.62
3.2 Influence of temperature on agglomeration of fine seed
The experimental conditions were as follows: reaction temperatures were 75, 65, 85 and 95 ℃, respectively, the initial αK was 1.62. The experimental results are shown in Fig.1, Figs.2(a), (b), and (c), respectively. At present, a mechanism about agglomeration[13-15] has already been recognized by the researchers that in the agglomeration process, when fine particles are adhered together into new coarse particles after stirring, some very small crystal particles (i.e. binders) on the surfaces of seeds or intervals among seeds are firstly formed because of nucleation, and then filled or adhered among the seeds particles. These fine particles in the new coarse particles can be tightly aggregated. From Fig.2(a), the percentage of particles with size less than 2 μm is reduced from 76.67% to 25.71% in 8 h. This shows that when the reaction temperature is 65 ℃, although initial supersaturation of aluminate solution is improved, and those binders formed among the seeds are enough to maintain the agglomeration process, the secondary nucleation also happens on the surfaces of seeds, and many new fine crystal particles are formed. Therefore, the whole agglomeration degree is weaker than that at 75 ℃. From Figs.2(b) and (c), when the reaction temperature is 85℃, the percentage of particles with size less than 2 μm is reduced from 76.67% to 11.04% in 8 h, and when the reaction temperature is 95 ℃, the percentage of particles with size less than 2 μm reduces from 76.67% to 35.44% in 8 h. When the reaction temperature is 85 ℃, although the initial αK is the same as that in Fig.1, the initial supersaturation of aluminate solution is lowered with the increase of temperature, the binders among the seeds are not enough to maintain the whole agglomeration process, the effect of the agglomeration is a little weaker than that at 75 ℃ (Fig.1), but higher than that at 65 ℃ (Fig.2(a)). When the reaction temperature is 95 ℃, the initial supersaturation of aluminate solution is too low, so the effect of the agglomeration is the weakest.

Fig.2 Relationship between percentage in seed particles and time at initial αK of 1.62 and different temperatures: (a) 65 ℃; (b) 85 ℃; (c) 95 ℃
3.3 Influence of initial molar ratio of Na2O to Al2O3 on agglomeration of fine seed
The experimental conditions were as follows: initial αK values were 1.62, 1.41, 1.78 and 1.92, reaction temperature was 75 ℃. The experimental results are shown in Fig.1, Figs.3(a), (b) and (c), respectively. From Fig.3(a), when the initial αK is 1.41, the percentage of particles with size less than 2 μm falls down from 76.67% to 35.71% in 8 h, the agglomeration degree of the seeds is weaker than that with the initial αK of 1.62 (Fig.1). When the initial αK is 1.41, although the reaction temperature is still 75 ℃, the agglomeration speed is very fast, but the initial supersaturation of aluminate solution is too high, the secondary nucleation is also fast, many new fine crystal particles are formed from the surfaces of the seeds. Therefore, the agglomeration degree of the particles becomes weaker. From Fig.3(b), it can be seen that the percentage of particles with size less than 2 μm falls down from 76.67% to 45.61% in 8 h, and the agglomeration degree of the seed is also weaker than that of the initial αK of 1.62 (Fig.1). When the initial αK is 1.78, the initial supersaturation of aluminate solution is lower than that with the initial αK of 1.62, so the agglomeration degree of the particles is also weaker. From Fig.3(c), it can be seen that the percentage of particles with size less than 2 μm falls down from 76.67% to 63.59% in 8 h, and the agglomeration among the seed particles almost does not happen. When the initial αK is 1.92, the initial supersaturation of aluminate solution is too low, although fine particles can be adhered together into coarse particles for a time after stirring, and the binders among the seeds are also too few to maintain the agglomeration process, and the coarse particles are still broken into fine particles due to stirring, so the agglomeration degree of the seeds is the weakest.
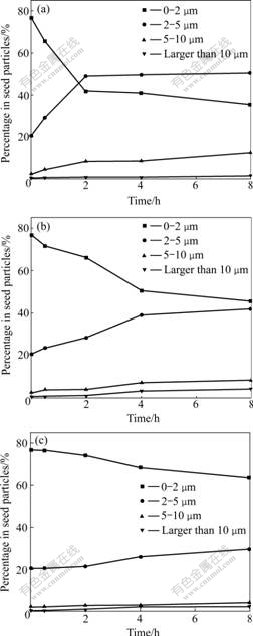
Fig.3 Relationship between percentage in seed particles and time at 75 ℃ and different initial αK values: (a) 1.41; (b) 1.78; (c) 1.92
4 Scanning electron microscopy research
The scanning electron microscopy (SEM) images of samples obtained at time intervals of 0.5, 4.0 and 8.0 h, are shown in Fig.4. From Fig.4(a), in the early agglomeration stage, it is obvious that the fine particles are in predominant, the number of coarse particles is less, and the coarse particles are scattered and surrounded by the fine particles. The fine particles are not adhered on the surfaces of the coarse particles, but are easily adhered with each other loosely. From Figs.4(b) and (c), many fine particles are aggregated into one or more coarse particles, and the size of the particles is also enlarged because of the agglomeration of the fine particles. From Fig.4(c), the new coarse particles formed from the fine particles are still loose in 8 h and the newly formed coarse crystal shapes from fine particles are irregular because the crystal growth is too slow to offer the strength of the agglomeration particles.
5 Conclusions
1) The agglomeration process of fine seed particles is almost finished in 8 h. The percentage of particles with

Fig.4 SEM images of Al(OH)3 seeds obtained at different intervals: (a) 0.5 h; (b) 4.0 h; (c) 8.0 h
size less than 2 μm falls down from 76.67% to 0 in 8 h, and the percentages of particles with size larger than 2 μm are increased step by step, which indicates that continuous agglomeration happens among the fine particles and the coarse particles are formed gradually, and particles sizes with the agglomeration abilities from strong to weak are less than 2 μm, 2-5 μm, 5-10 μm, larger than 10 μm.
2) Because of active sites on the surfaces of the seeds, some very fine new crystal particles can be formed for the secondary nucleation, and are adhered or adsorbed among the seeds. Two or more seeds are therefore adhered each other, and some larger new crystal particles are formed. This is mechanism of agglomeration. But some coarse seeds are lack of active sites for regular surfaces and difficult to be aggregated. On the condition of the adequate initial αK and temperature (the initial αK of 1.62, 75 ℃), the formed fine new crystals are the most, the binders among seeds are enough to maintain the whole agglomeration, and the effect of agglomeration is the strongest.
3) From SEM images, during the agglomeration process, the fine seed particles, especially the particles with size less than 2 μm are easy to be aggregated into coarse particles, and even used as binders to be adhered among the coarse seeds. Two or more seeds are aggregated and the sizes of the particles are also enlarged. But for crystal growth is too slow, the new coarse particles aggregated from the fine particles are still loose and irregular.
References
[1] ZHANG Jiang-feng, YIN Zhou-lan, CHEN Qi-yuan. Study of agglomeration during the precipitation of sodium aluminate solution [J]. JOM, 2004, 56(11): 282-286.
[2] CHEN Guo-hui, CHEN Qi-yuan, YIN Zhou-lan, YIN Zhi-min. Characterization of irregular seeds on gibbsites precipitated from caustic aluminate solutions [J]. Trans Nonferrous Met Soc China, 2006, 16(2): 483-487.
[3] CHEN Qi-yuan, WU Zheng-ping, YIN Zhou-lan, LI Jie. Bond population analysis on combination of favorable growth unit of Al(OH)3 crystals [J]. Trans Nonferrous Met Soc China, 2006, 16(1): 191-197.
[4] ZHANG Bin, ZHOU Ke-chao, CHEN Qi-yuan. Influences of seed size and number on agglomeration in synthetic Bayer liquors [J]. J Cent South Univ Technol, 2006, 13(5): 511-514.
[5] ADDAI-MENSAH J, LI J, PRESTIDGE C A. Aggregation behaviour of gibbsite crystals in supersaturated sodium and potassium aluminate liquors [J]. Developments in Chemical Engineering and Mineral Processing, 2002, 10(5/6): 539-551.
[6] LIU Chang-qing, ZHANG Ping-min, YIN Zhou-lan, CHEN Qi-yuan. Mathematic models of the seed precipitation process of sodium aluminate solution [J]. JOM, 2004, 56(11): 333-336.
[7] IIIEVSKI D, LIVK I. An agglomeration efficiency model for gibbsite precipitation in a turbulently stirred vessel [J]. Chemical Engineering Science, 2006, 61(6): 2010-2022.
[8] LI H X, ADDAI-MENSAH J, THOMAS J C, GERSON A R. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions [J]. Journal of Crystal Growth, 2005, 279(3/4): 508-520.
[9] LIVK I, IIIEVSKI D. A macroscopic agglomeration kernel model for gibbsite precipitation in turbulent and laminar flows [J]. Chemical Engineering Science, 2007, 62(14): 3787-3797.
[10] PRESTIDGE C A, AMETOV L, ADDAI-MENSAH J. Rheological investigations of gibbsite particles in synthetic Bayer liquors [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 157(1/3): 137-145.
[11] ADDAI-MENSAH J, RALSTON J. The influence of interfacial structuring on gibbsite interactions in synthetic Bayer liquors [J]. Journal of Colloid and Interface Science, 1999, 215(1): 124-130.
[12] IIIEVSKI D, AUSTIN P, WHITTINQTON B. Studies into the internal structure of gibbsite agglomerates [J]. Chemical Engineering and Technology, 2003, 26(3): 363-368.
[13] WANG Zhi, BI Shi-wen, YANG Yi-hong, YUAN Zhang-fu. Evolution of particle size and strength of hydrargillite from carbonization in seeded sodium aluminate liquors [J]. Journal of Crystal Growth, 2005, 274(1/2): 218-225.
[14] ADDAI-MENSAH J, PRESTIDGE C A, PALSTON J. Interparticle forces, interfacial structure development and agglomeration of gibbsite particles in synthetic Bayer liquors [J]. Minerals Engineering, 1999, 12(6): 655-669.
[15] SEYSSIECQ I, VEESLER S, BOISTELLE R, LAMERANT J M. Agglomeration of gibbsite Al(OH)3 crystals in Bayer liquors, influence of the process parameters [J]. Chemical Engineering Science, 1998, 53(12): 2177-2185.
Foundation item: Project(2005CB623702)supported by the Major State Basic Research and Development Program of China; Project(20476107) supported by the National Natural Science Foundation of China
Received date: 2008-04-05; Accepted date: 2008-05-28
Corresponding author: ZHANG Bin, PhD; Tel: +86-731-8877364; E-mail: zbparrik2002@hotmail.com
(Edited by CHEN Wei-ping)