J. Cent. South Univ. Technol. (2008) 15: 188-192
DOI: 10.1007/s11771-008-0036-5
Synthesis of N, N-diethyl dodecyl amine and its flotation properties on bauxite
CAO Xue-feng(曹学锋), ZHANG Li-min(张丽敏),
HU Yue-hua(胡岳华), LIU Chang-miao(刘长淼), OUYANG Kui(欧阳魁)
(School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: N, N-diethyl dodecyl amine(DEN12) was synthesized from dodecyl amine, formic acid and acetic aldehyde. The collecting property of DEN12 on diaspore, kaolinite and illite was investigated by flotation test and infrared spectrum. The results show that in the presence of 2.0×10-4 mol/L DEN12 , the recoveries of kaolinite and illite are all higher than 78% and the recovery of diaspore is 50% in the pH range of 5.5-6.0. The mass ratio of Al2O3 to SiO2 in concentrate obtained from separation artificial mixture is higher than 10, suggesting that DEN12 can be used as a collector to separate the aluminosilicates from diaspore in bauxite ores at the pulp pH below 8. The measurements of the infrared spectrum approve that the action between aluminosilicates and tertiary amine collector is strong electrostatic adsorption and that of diaspore is weak electrostatic adsorption.
Key words: reverse flotation; electrostatic adsorption; infrared spectrum; kaolinite; illite; diaspore; N, N-diethyl-N-dodecyl amine (DEN12)
1 Introduction
Bauxite resources in China are abundant. However, most bauxite is diasporic bauxite with the typical character of high content of aluminum oxide and silica and the mass ratio of A12O3 to SiO2 of more than 80% bauxite is 4-6[1]. Main gangue minerals in diasporic bauxite are aluminosilicate minerals such as kaolinite, illite and pyrophyllite[2]. In China, aluminum oxide is mainly produced by sintering processing or a combination of sintering and Bayer process[3]. sintering processing is extremely energy-intensive and environmentally unfriendly, incurring a high alumina production cost. Therefore, it is very required to remove aluminosilicate minerals from bauxite to increase mass ratio of Al2O3 to SiO2 and to decrease the cost of the production. Flotation is known to be a highly versatile separation technology and has been widely used for industrial mineral processing. A notable character of bauxite is that major component is diaspore[4]. In the direct flotation a high collector consumption is necessary to float about 80% of feed materials, resulting in a high operating cost[5]. In the reverse flotation process, the minor aluminosilicate minerals are floated while diaspore is depressed[6]. It is evident that there is an economic and environmental incentive to consider reverse flotation an alternative.
The bauxite reverse flotation is required to collect
aluminosilicate minerals and depress diaspore[7-11]. Dodecyl amine(DDA) is common collector for reverse flotation, but it exhibits a weak collecting power for aluminosilicate minerals[12-14]. The aim of the present work is to synthesize N, N-diethyl dodecyl amine(DEN12) and to investigate its flotation behaviors on diaspore, kaolinite and illite and the reaction mechanism to find the possibility that DEN12 is used as a collector for bauxite reverse floatation.
2 Synthesis of N, N-diethyl dodecyl amine
2.1 Reaction principle
N, N-diethyl dodecyl amine(DEN12) is synthesized with dodecyl amine, formic acid and acetic aldehyde as raw material[15-17]. The reaction is shown as follows:
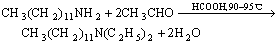 (1)
(1)
Firstly, DDA reacts with acetic aldehyde in an acid medium and Schiff base is formed, which subsequently translates to N=C compound by intramolecularly anhydration reaction.
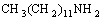 +
+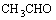
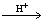 (2)
(2)
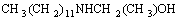
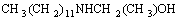
 CH3(CH2)11N=C2H4+
CH3(CH2)11N=C2H4+ (3)
(3)
After that, an oxidation-reduction reaction occurs
between formic acid and N=C compounds, and then secondary amine is obtained.
CH3(CH2)11N=C2H4+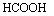

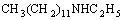 +CO2 (4)
+CO2 (4)
Secondary amine reacts with formic acid and acetic aldehyde and N, N-diethyl dodecyl amine is formed.
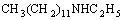 +
+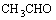
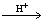
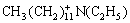 =C2H4+H2O (5)
=C2H4+H2O (5)
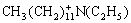 =C2H4+
=C2H4+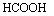

 +
+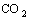 +H+ (6)
+H+ (6)
2.2 Operating procedure of synthesis reaction
50 g DDA was added in a round bottom flask and cooled in ice water, after that 46.3 mL formic acid was added slowly. After solid DDA totally solved, 70 mL acetic aldehyde was added into the round bottom flask. The reaction was kept at 90-95 ℃ for 6 h. The reaction product was purified by the methods of evaporation in rotary vacuum evaporator, extraction and crystallization. The final synthesized product is scarlet liquid.
2.3 Structure characterization of synthetic product
FTIR spectrum of synthetic product is presented in Fig.1. The DEN12 has —CH2 stretching bands at 2 924 cm-1 and 2 853 cm-1. The stretching of C—N is around 1 030 cm-1 to 1 120 cm-1. The peak around 3 400 cm-1
may be due to asymmetric and symmetric stretching of NH2. The peak in the range of 1 550 to 1 650 cm-1 may be the bending mode of —NH2 and —N(C2H5)2. It is indicated that synthetic product is DEN12 containing a little DDA.
3 Experimental
3.1 Materials
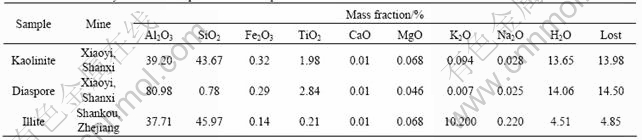
The pure mineral samples of diaspore, kaolinite and illite come from different mines of China. The samples were hand-picked, ground by porcelain mill and screened to less than 0.074 mm. The chemical compositions of these samples are listed in Table 1.
DEN12 synthesized in the laboratory was used as a collector. 0.1 mol/L analytical grade sodium hydroxide and hydrochloric acid were used to adjust the pH of the system. Distilled water was used in all tests.
3.2 Flotation test
The microflotation tests were performed in an XFG-type laboratory flotation machine with 40 mL cell. The impeller speed of flotation machine was fixed at 1 260 r/min. 40 mL distilled water and 3 g sample were used for each test. The pulp was stirred for 1 min with pH regulator, 3 min with collectors, and the flotation time was 4 min. The flotation products were filtrated, dried and weighed for recovery calculation.
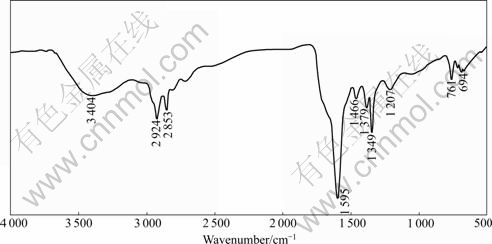
Fig.1 FTIR spectra of DEN12
Table 1 Chemical analytical results of pure mineral samples
3.3 Fourier transform infrared spectra
The FTIR spectra were obtained with PE system 2002 FTIR (Nicolet Corporation, USA) to characterize the nature of the interaction between the collector and minerals. The diffuse reflectance infrared spectra were measured on an air-dried powder with size less than 5 μm after treating with collector.
4 Results and discussion
4.1 Effects of DEN12 on flotation of pure minerals
Flotation recoveries of diaspore, kaolinite and illite as a function of pH are given in Fig.2. It can be seen that the aluminosilicate minerals exhibit a better floatability at pH<8, while the diaspore shows a good flotation response in an alkaline medium. Taking pH=6 for example, the recoveries of kaolinite and illite are 80% and 78%, respectively, while recovery of diaspore is only 50%. DEN12 shows stronger collecting power on aluminosilicate minerals. It suggests that reverse flotation separation of the aluminosilicates from diaspore in bauxite ores may be possible at the pulp pH below 8.
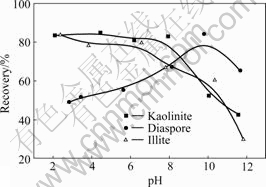
Fig.2 Flotation recoveries of diaspore, kaolinite and illite as function of pH at DEN12 concentration of 2×10-4 mol/L
4.2 Effects of DEN12 on flotation separation of artificial mixture
Effects of DEN12 on the flotation of artificial
mixture are listed in Table 2. It shows that floatation separation of aluminosilicates from diaspore in artificial mixture mineral can be completed in the range of pH 5.0-5.5 by using DEN12 as a collector.
For the mixture of diaspore and kaolinite (mass ratio 2.00?1.00), the mass ratio of Al2O3 to SiO2 in feed is 4.76, which is beneficial to obtaining a concentrate with the mass ratio of Al2O3 to SiO2 45.4, and a recovery 70.05%, in the presence of DEN12. For the mixture of diaspore and illite (2.50?1.00), the mass ratio of Al2O3 to SiO2 in feed is 4.09, after flotation separation using DEN12 as a collector, the mass ratio of Al2O3 to SiO2 in concentrate obtained reaches 46.62 with a recovery of 56.42%, which suggests that the synthesized DEN12 can be used as a collector for reverse flotation of diasporic bauxite.
4.3 FTIR spectra analysis of DEN12 adsorption by diaspore, kaolinite and illite
FTIR spectra of diaspore, kaolinite and illite in the absence and presence of DEN12 are presented in Fig.3 and Fig.4, respectively. It has been reported that the —OH bending vibrations of diaspore are at 973 and 1 082 cm-1 and the peak at 752 cm-1 is Al—O stretching vibration[18-19]. The aluminosilicate minerals spectra show the Si—O stretching vibrations around 1 030 cm-1, strong peak around 500 cm-1 and peaks followed contribute to vibration of Si—O—Si or Al—O—Si. The —OH stretching vibration is around 3 650 cm-1. It can be seen from Fig.1 that DEN12 has —CH3 stretching bands at 2 924 and 2 853 cm-1 and deformation vibration of —CH3 at 1 466 cm-1 and C—N stretching vibrations in the wavenumber range of 1 030-1 230 cm-1. The spectra of aluminosilicate minerals treated by DEN12 exhibit peaks at 2 924 and 2 854 cm-1. No band shift is observed, indicating that the adsorption of DEN12 on aluminosilicate minerals is dominated by physical absorption and that the alkyl chain is in a gauche conformation state. Because aluminosilicate minerals
Table 2 Results of reverse flotation for artificial mixture mineral with DEN12
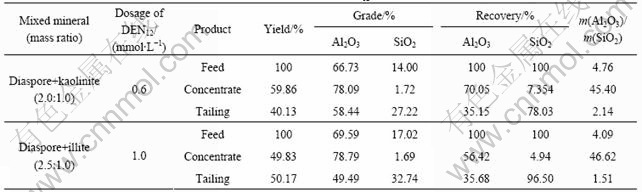
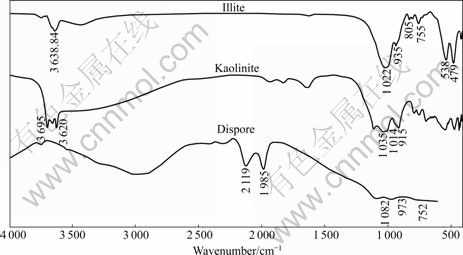
Fig.3 FTIR spectra of diaspore, kaolinite and illite
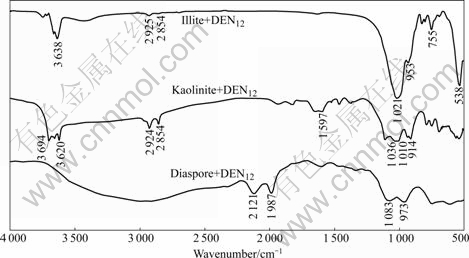
Fig.4 FTIR spectra of diaspore, kaolinite and illite treated by DEN12
are negatively charged in wide pH range, the physical adsorption of cation collector DEN12 may be due to the electrostatic interaction. The strength of the peaks at 2 924 and 2 854 cm-1 on kaolinite is stronger than that on illite, accounting for the stronger adsorption and collecting action of DEN12 on kaolinite than on illite. The spectrum of diaspore treated by DEN12 exhibits weak peaks from 1 300 to 1 600 cm-1. No band shift is observed, indicating the adsorption of DEN12 on diaspore is dominated by weak physical adsorption.
5 Conclusions
1) A new bauxite reverse flotation collector, N, N-diethyl dodecyl amine(DEN12), is synthesized. DEN12 exhibits stronger collecting action on the flotation of the aluminosilicate minerals and weaker collecting action on diaspore at pH<8. The mass ratio of Al2O3 to SiO2 in artificial mixture concentrates is higher than 40.
2) The action between aluminosilicates and DEN12 collector is strong electrostatic adsorption and that of diaspore is weak electrostatic adsorption, which can explain that DEN12 has different collecting power to aluminosilicate minerals and diaspore.
references
[1] GUO Jian, REN Ai-jun, FANG Qi-xue, HU Yong-ping. A study on separation diaspore and kaolinite by reverse flotation[J]. Nonferrous Metals, 2003(3): 1-5. (in Chinese)
[2] Gu Song-qing. Alumina production technology with high efficiency and low consumption from Chinese bauxite resource[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(1): 91-92. (in Chinese)
[3] HU Yue-hua, Liu Xiao-wen, XU Zheng-he. Role of crystal structure in flotation separation of diaspore from kaolinite, pyrophyllite and illite[J]. Minerals Engineering, 2003, 16: 219-227.
[4] Liu Guang-yi, LU Yi-ping, DAI Ta-gen. Reverse flotation with cationic polyacrylamide(CPAM) polymers to separate kaolinite from diaspore[J]. Metal Mine, 2003, 3(20): 48-51. (in Chinese)
[5] HU Yue-hua, JIANG Hao, QIU Guan-zhou. Solution chemistry of flotation separation of diasporic bauxite[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(1): 125-130. (in Chinese)
[6] XU Zheng-he, VERNE P, Liu Qi. Recent advances in reverse flotation of diasporic ores––A Chinese experience[J]. Minerals Engineering, 2004, 17: 1007-1014.
[7] CHEN Xiang-qing, HU Yue-hua, WANG Yu-hua, XIONG Dao-ling. Effects of sodium hexmetaphosphate on flotation separation of diaspore and kaolinite[J]. Journal of Central South University of Technology, 2005, 12(4): 420-424.
[8] WANG YU-hua, HU Yue-hua, HE Ping-bo, GU Guo-hua. Reverse flotation for removal of silicates from diasporic-bauxite[J]. Minerals Engineering, 2004, 17: 63-68.
[9] HANCER M, MILIER J D. The flotation chemistry of potassium double salts: Kaolinite and carnallite[J]. Minerals Engineering, 2000, 13: 1483-1493.
[10] HOUOT R. Beneficiation of iron ore by flotation[J]. Int J Min Process, 1983(10): 183-204.
[11] FENG Qi-ming, MU Xiao, ZHANG Guo-fan, L? Yi-ping, OU Le-ming, SHAO Yan-hai, CHEN Yun. Investigation on antifoaming of flotation bauxite [J]. Journal of Central South University: Science and Technology, 2005, 36(6): 955-959. (in Chinese)
[12] ZHAO Shi-min. Flotation of quartz using N-(2-aminopropyl)- octadecanamide as collector[J]. Journal of Central South University of Technology, 2003, 10(4): 329-332.
[13] Jiang Hao, HU Yue-hua, Qin Wen-qing, WANG Yu-hua, Wang Dian-zuo. Mechanism of flotation for diaspore and aluminum-silicate minerals with alkyl amine collectors[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 688-692. (in Chinese)
[14] DU Ping, CAO Xue-feng, HU Yue-hua, JIANG Yu-ren, LI Hai-pu. Study of structure and property of amine collectors[J]. Light Metal, 2003(1): 27-31. (in Chinese)
[15] ZHU guang-jun. Synthesis study of a new surfactant oxidated tertiary[J]. Fine Chemical Engineering, 1994(11): 1-3. (in Chinese)
[16] CAO Xue-feng, HU Yue-hua, XU Jing. Synthesis of γ-alkoxy- propylamines and their collecting properties on aluminium silicate minerals[J]. Journal of Central South University of Technology, 2004, 11(3): 281-285.
[17] DUAN Ming-feng, MEI Ping, XIONG Hong-lu. Research of synthesis reaction of N, N-2-dimethyl dodecylamine[J]. Chemical and Bioengineering, 2005(8): 28-30. (in Chinese)
[18] DONG qing-nian. Infra-red spectrum[M]. Beijing: Chemical Industry Press, 1979. (in Chinese)
[19] WEN Ge. Infra-red spectrum of mineral[M]. Chongqing: Chongqing University Press, 1988. (in Chinese)
(Edited by YANG Hua)
Foundation item: Project(2005CB623701) supported by the Major State Basic Research Development Program of China
Received date: 2007-09-15; Accepted date: 2007-11-18
Corresponding author: HU Yue-hua, Professor; Tel: +86-731-8879815; E-mail: hyh@csu.edu.cn