文章编号:1004-0609(2009)06-1155-07
Zn(Ⅱ)-Glu2?-CO32?-H2O体系热力学平衡分析
杨天足,任 晋,刘伟锋,窦爱春,刘 伟,张杜超
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:针对碱式碳酸锌(2ZnCO3·3Zn(OH)2)在L-谷氨酸一钠溶液中的热力学行为,根据配位化学理论绘制谷氨酸根(Glu2?)和碳酸根(CO32?)在溶液中的形态分布图;根据双平衡法理论研究Zn(Ⅱ)-Glu2?-CO32?-H2O体系中Zn(Ⅱ)的配合平衡热力学,并绘制L-谷氨酸一钠浓度在0~5 mol/L范围和pH在7~14范围内变化时的热力学平衡图,研究L-谷氨酸一钠浓度、pH值和游离碳酸根离子浓度对L-谷氨酸一钠浸出碱式碳酸锌的影响。同时对热力学计算结果进行实验验证。结果表明:在一定的L-谷氨酸一钠浓度下,当pH=10时,溶液中总锌浓度达到最大值;锌离子浓度理论计算值与实验值之间相对误差的绝对平均值为11.39%。
关键词:L-谷氨酸一钠;碱式碳酸锌;浸出;热力学
中图分类号:TF 813 文献标识码: A
Thermodynamics equilibrium analysis of
Zn(Ⅱ)-Glu2?-CO32?-H2O system
YANG Tian-zu, REN Jin, LIU Wei-feng, DOU Ai-chun, LIU Wei, ZHANG Du-chao
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Thermodynamic behaviors of basic zinc carbonate(2ZnCO3·3Zn(OH)2) with L-monosodium glutamate solution were studied. Species distribution diagrams of glutamate (Glu2?) and carbonate ion(CO32?) in the solution were obtained based on the coordination chemistry theory. Thermodynamics of Zn(Ⅱ) complex equilibrium in the system of Zn(Ⅱ)-Glu2?-CO32?-H2O were studied based on the double equilibrium theory. When the concentration of L-monosodium glutamate and pH value varied accordingly in ranges of 0?5 mol/L and 7?14, the equilibrium thermodynamic diagrams were constructed, and the effects of L-monosodium glutamate concentration, pH value and free carbonate ion concentration on leaching of basic zinc carbonate were also studied. The results show that the total zinc concentration reaches the maximum value when the pH value is 10 under a certain L-monosodium glutamate concentration. The absolute average value of relative error between the theoretical calculation and experimental results is 11.39%.
Key words: L-monosodium glutamate; basic zinc carbonate; leaching; thermodynamic
随着国内外锌矿资源的开发利用,高品位硫化锌矿资源日益枯竭,低品位氧化锌矿资源的利用变得尤为重要[1]。传统处理氧化锌的方法分为火法和湿法两种。火法主要是采用回转窑还原挥发法,但该法能耗高,污染环境[2]。湿法按浸出剂不同可分为硫酸浸出法、氨法等。硫酸浸出法[3?4]处理酸溶性杂质Ca、Mg含量较高的氧化锌矿石时,由于浸出过程需要消耗大量的硫酸,因此成本较高。氨法[5?6]采用NH3-(NH4)2CO3- H2O、NH3-(NH4)2SO4-H2O、NH3-NH4Cl-H2O体系浸出氧化锌矿石。氨法虽然可以避免硫酸浸出法的不足,但氨易挥发,氨损失量大,污染环境。
湿法主要是利用锌与无机配体的性质处理低品位氧化锌矿,如氨水等。随着有机化学学科的迅速发展,金属氨基酸配合物的研究日益受到人们的重视,这就为处理低品位氧化锌矿提供了研究方向。氨基酸锌是由锌离子与氨基酸中给电子的N原子和羧基形成的五圆环螯合物[7]。L-谷氨酸一钠(L-monosodium glutamate)是谷氨酸的单钠盐,分子式为NaC5H8O4N·H2O,结构式为NaOOC-(CH2)2-CH(NH2)-COOH·H2O。它为无 色或白色的八面柱状晶体,或白色结晶粉末,易溶于水[8]。
李尚德等[9]开展了以谷氨酸一钠与氧化锌反应合成固体谷氨酸锌的研究,并且得到了谷氨酸锌的最佳合成条件,但是试验中采用的原料为分析纯氧化锌。目前国内外还没有关于L-谷氨酸一钠浸出低品位氧化锌矿的报道。在L-谷氨酸一钠碱性配合物体系中,锌与谷氨酸根形成配合物而溶解,而碱性脉石不溶解,从而消除高碱性脉石对锌资源有效利用的影响。
低品位氧化锌矿中大量存在的氧化锌物相是碱式碳酸锌,碱式碳酸锌溶解后生成大量的CO32?,对体系平衡有重大影响。本文作者综合考虑Zn(Ⅱ)与Glu2?、OH?及CO32?等配位体之间的配合平衡问题,采用基于质量平衡和电荷平衡的双平衡原理,利用已有的配合物稳定常数等热力学数据,对Zn(Ⅱ)-Glu2?- CO32?-H2O碱性配合物冶金体系中Zn(Ⅱ)的配合平衡热力学进行研究,并以实验进行验证。
1 热力学数据和平衡方程
表1所列为T=298 K时L-谷氨酸一钠浸出过程中锌配合物及其它物种的稳定常数[10]。表2所列为T=298 K时相关物种的标准生成吉布斯自由能[11?12]。由于热力学数据的缺乏,锌与谷氨酸根形成配合物的积累稳定常数取T=293 K时的值。
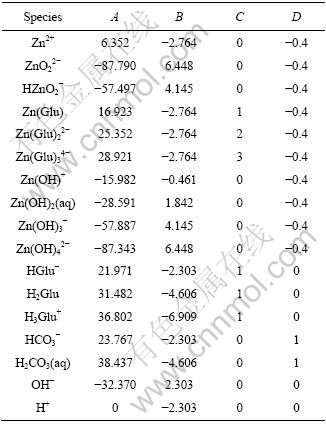
表1 锌配合物及其他物种的稳定常数
Table 1 Critical stability constants of zinc complexes and other species at 298 K

表2 在298 K时相关物种的标准生成吉布斯自由能
Table 2 Gibbs free energy of related species at 298 K

Zn(Ⅱ)-Glu2?-CO32?-H2O系是一类复杂的体系,在该体系中可能存在的固相有2ZnCO3·3Zn(OH)2、ZnCO3、Zn(OH)2。它们与液相的平衡就是与以下20个液相物种平衡:Zn2+、Zn(Glu)、Zn(Glu)22?、Zn(Glu)34?、Zn(OH)+、Zn(OH)2(aq)、Zn(OH)3?、Zn(OH)42?、ZnO22?、HZnO2?、Glu2?、HGlu?、H2Glu、H3Glu+、CO32?、HCO3?、H2CO3(aq)、Na+、H+、OH?。由文献[13?14]可知,在整个2ZnCO3·3Zn(OH)2的浸出过程中,只有2ZnCO3·3Zn(OH)2固相存在。
根据同时平衡原理,体系中只要存在2ZnCO3·3Zn(OH)2固相,每种锌配合离子或物种都分别与之平衡:

根据电算?指数方程法[15?16],这些离子或配合物的摩尔浓度可用下列通式来表示:

根据表1和表2中的数据,可以计算各物种浓度表达式的A、B、C和D值列于表3。
表3 与2ZnCO3·3Zn(OH)2平衡的各离子的电算指数常数
Table 3 Constants in exponential for calculating species concentration with 2ZnCO3·3Zn(OH)2
根据质量守恒定律可建立溶液中锌的总浓度c(Zn2+)T、谷氨酸根总浓度c(Glu2?)T、碳酸根总浓度c(CO32?)T的平衡方程。

根据溶液电中性原理,可建立电荷平衡方程:

在以上模型中,有4个平衡方程式(7)~(10),共有pH值、c(Glu2?)、c(CO32?)、c(Zn2+)T、c(Na+)、c(Glu2?)T、c(CO32?)T 7个未知数,由于体系中所有的锌及碳酸根全部由2ZnCO3·3Zn(OH)2分解产生,故c(Zn2+)T= 2.5 c(CO32?)T,同时c(Na+)=c(Glu2?)T。这样未知数变为5个。
在实际计算过程中,c(Glu2?)T给定,则未知数变为4个。因为浸出剂是L-谷氨酸一钠,所以c(Glu2?)T= c(NaC5H8O4N)。将以上数据和方程组输入由matlab6.5[17]编写的程序,求出了c(NaC5H8O4N)在0~5 mol/L和pH值在7~14范围内的其他未知数的浓度数值。
2 结果与讨论
2.1 谷氨酸根在溶液中的分布形态
L-谷氨酸分子中含有两个酸性的羧基和一个碱性的氨基,是一个既含有酸性基团,又有碱性基团的两性电解质,与酸或碱作用都可以生成盐。谷氨酸溶解于水后,呈离子状态存在,其分布形态取决于溶液的pH值[18]。在水溶液中Glu2?为弱酸根离子,它在溶液中可以与H+发生加质子反应,共有C5H7O4N2?、C5H8O4N?、C5H9O4N、C5H10O4N+ 这4个物种存在:
C5H7O4N2?:-COO-(CH2)2-CH(NH2)-COO?
C5H8O4N?:-COO-(CH2)2-CH(NH3+)-COO?
C5H9O4N:HCOO-(CH2)2-CH(NH3+)-COO?
C5H10O4N+:HCOO-(CH2)2-CH(NH3+)-COOH
本研究中分别用Glu2?、HGlu?、H2Glu和H3Glu+来表示C5H7O4N2?、C5H8O4N?、C5H9O4N和C5H10O4N+。
在水溶液中,不考虑体系中能与Glu2?形成配合物的Zn2+时,配体Glu2?与H+发生的加质子反应为:

根据离子平衡反应式(11)可得体系中谷氨酸根离子的总浓度c(Glu2?)′T为:


将表1中Glu2?与H+的积累加质子常数带入式(13)~(16),可得到α0、α1、α2、α3与pH值的关系,其结果如图1所示。

图1 α0、α1、α2和α3与pH值的关系
Fig.1 Relationships between α0, α1, α2 and α3 with pH value
由图1可以看出,当溶液的pH<2.19时,谷氨酸根主要以阳离子H3Glu+的形式存在;当2.19<pH<4.25时,主要以两性离子H2Glu的形式存在;当 4.25<pH<9.67时,主要以阴离子HGlu?的形式存在;而pH>9.67时,主要以Glu2?的形式存在。由于实验中是利用Glu2?与Zn2+的配合反应进行碱式碳酸锌的 浸出,因此要控制溶液有较高的pH值以确保有更多的Glu2?;当pH=11时,溶液中Glu2?存在率可达到99%。
2.2 碳酸根离子在溶液中的分布形态
在水溶液中,CO32?为弱酸根离子,它在溶液中与H+发生的加质子反应为:

由式(9)和(17)可知,c(CO32?)、c(HCO3?)、c(H2CO3)等所占c(CO32?)T的浓度分数[18] α′0、α′1、α′2可分别表示为:
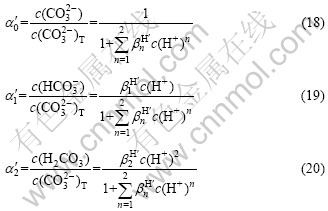
将表1中CO32?与H+的积累加质子常数带入式(18~20),可得到α′0、α′1、α′2与pH的关系,其结果如图2所示。

图2 α′0、α′1和α′2与pH值的关系
Fig.2 Relationships between α′0, α′1 and α′2 with pH value
由图2可以看出,当pH<6.2时,碳酸根主要以H2CO3的形式存在;当6.2<pH<10.8时,主要以HCO3?的形式存在;当pH>10.8时,主要以CO32?的形式存在;当溶液中的pH=12时,溶液中CO32?存在率可达到99%。
2.3 L-谷氨酸一钠浓度和pH值对游离谷氨酸根离子浓度的影响
游离谷氨酸根离子浓度c(Glu2?)随c(NaC5H8O4N)和pH值的变化情况如图3所示。
由图3可以看出,当pH<7时,游离谷氨酸根离子浓度几乎为零,这是因为由图1可知,含配体Glu2?

图3 c(Glu2?)与c(NaC5H8O4N)和pH值的曲面关系
Fig.3 Relationships between c(Glu2?) with c(NaC5H8O4N) and pH value
的溶液逐渐酸化时,Glu2?将逐渐与H+结合分别形成HGlu?、H2Glu、H3Glu+,故游离谷氨酸根离子本身的浓度很低;当pH>7时,配体Glu2?以未加合质子的形式存在,因此溶液中游离谷氨酸根离子浓度随着L-谷氨酸一钠浓度的增加而较快的增加;但当pH>11时,游离谷氨酸根离子浓度随L-谷氨酸一钠浓度增加而基本保持不变。
2.4 L-谷氨酸一钠浓度和pH值对总锌浓度的影响
总锌浓度c(Zn2+)T随着L-谷氨酸一钠浓度和pH值的变化情况如图4所示。
由图4可以看出,在一定的L-谷氨酸一钠浓度下,当pH=10时,溶液中的总锌浓度达到最大值;当 pH<10时,c(Zn2+)T随着L-谷氨酸一钠浓度的增加而直线增加。这是因为随着L-谷氨酸一钠浓度的增加,与Zn2+配合的游离谷氨酸根离子浓度也随之增大,导致总锌浓度增大。这说明在平衡过程中,适当增加L-谷
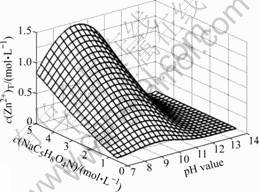
图4 c(Zn2+)T与c(NaC5H8O4N)和pH值的曲面关系
Fig.4 Relationships between c(Zn2+)T with c(NaC5H8O4N) and pH value
氨酸一钠浓度有利于提高浸出过程中的总锌浓度;但是当pH>10时,c(Zn2+)T随着pH的增加而迅速降低。这可能是因为由图2可知此时溶液中的游离碳酸根离子浓度迅速增大,根据同离子效应,总锌浓度将减小。
2.5 溶液中各种锌-谷氨酸根配离子的分布
根据理论计算可知,在pH=10、0<c(NaC5H8O4N)≤3 mol/L时,Zn2+、Zn(OH)+、Zn(OH)2(aq)、Zn(OH)3?、Zn(OH)42?、ZnO22?、HZnO2?的浓度占总锌浓度的比率均小于1×10?5,锌的这些物种可忽略不计。因此pH=10时,溶液中锌的主要物种浓度占总锌浓度的比率如图5所示。

图5 pH=10时溶液中锌的主要物种浓度占总锌浓度的比率
Fig.5 Ratio of main zinc complex ions at pH=10
由图5可知,Zn(Glu)的浓度几乎为零,锌主要以Zn(Glu)34?、Zn(Glu)22?配合物离子形式存在。随着体系中L-谷氨酸一钠浓度的增加,Zn(Glu)22?的浓度逐渐减少,而Zn(Glu)34?的浓度逐渐增加,这说明在L-谷氨酸一钠浓度较高的情况下,锌基本上以Zn(Glu)34?形式存在。
表4 锌在不同浓度的L-谷氨酸一钠溶液中和pH值下的平衡浓度计算值和实验值
Table 4 Experimental and calculated values of zinc equilibrium concentration under different L-monosodium concentrations and pH values

3 实验验证
将配好的相应浓度的L-谷氨酸一钠溶液和过量的化学纯2ZnCO3·3Zn(OH)2混合,用一定浓度的NaOH溶液调节好pH值,在25 ℃下磁力搅拌7 h,然后固液分离,分析溶液中的锌浓度。实验值和理论计算值见表4。
由表4可以看出,在不同的L-谷氨酸一钠浓度和pH值下,锌平衡浓度实验值和理论计算值相对误差的绝对平均值为11.39%,这说明该热力学模型是正确的,所选数据的准确性较好。产生大于5%误差的主要原因一是实验误差或分析误差或两者综合作用;二是在理论计算时用质量摩尔浓度代替活度也将导致理论计算和实验结果有偏差;三是某些实验有可能还未达到反应平衡,如实验4和12的误差分别达到25.81%和38.47%。所构建的热力学图可用来确定L-谷氨酸一钠的浓度和pH值,对低品位氧化锌矿的冶炼具有重要的理论指导意义。
4 结论
1) αn—pH图表明了各种谷氨酸根的加质子配离子与pH值的关系,当pH>9.67时,其主要以Glu2?的形式存在。α′n—pH图表明了各种碳酸根的加质子配离子与pH值的关系,当pH>10.8时,其主要以CO32?的形式存在。
2) 游离谷氨酸根离子浓度是随着溶液中L-谷氨酸一钠浓度的增加而增加,但当pH>11时,游离谷氨酸根离子浓度随L-谷氨酸一钠浓度增加而基本保持不变。
3) 总锌浓度随着L-谷氨酸一钠浓度的增加而增加,但当pH>10时,随着溶液中游离碳酸根离子浓度的增加而逐渐减小。
4) 锌主要以Zn(Glu)34?、Zn(Glu)22?配合物离子形式存在。随着体系中L-谷氨酸一钠浓度的增加,锌基本上以Zn(Glu)34?形式存在。
5) 在一定的L-谷氨酸一钠浓度下,当pH=10时,溶液中总锌浓度达到最大值;理论计算结果与实验数据符合的较好,锌平衡浓度相对误差的绝对平均值为11.39%。
REFERENCES
[1] 蒋继穆. 我国铅锌冶炼现状与持续发展[J]. 中国有色金属学报, 2004, 14(1): 52?62
JIANG Ji-mu. Status and sustainable development of lead zinc smelting industry in China[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(1): 52?62.
[2] 王成彦, 江培海. 云南中低品位氧化锌矿及元江镍矿的合理开发利用[J]. 中国工程科学, 2005, 7(4): 147?149.
WANG Cheng-yan, JIANG Pei-hai. The rational exploitation of middle & low grade zinc oxide ore and Yuanjiang nickel oxide ore in Yunnan[J]. Engineering Science, 2005, 7(4): 147?149.
[3] 蓝卓越, 胡岳华, 黎维中.低品位氧化锌矿硫酸浸出工艺研究[J]. 矿冶工程, 2002, 22(3): 63?65.
LAN Zhuo-yue, HU Yue-hua, LI Wei-zhong. Sulfuric acid leaching of low-grade zinc oxide ore-a study[J]. Mining and Metallurgical Engineering, 2002, 22(3): 63?65.
[4] ABDEL-AAL E A. Kinetic of sulfuric acid leaching of low-grade zinc silicate ore[J]. Hydrometallurgy, 2000, 55(3): 247?254.
[5] 唐谟堂, 鲁君乐, 袁延胜, 晏德生, 贺青蒲. Zn(II)- NH3-(NH4)2SO4-H2O系的氨络合平衡[J]. 中南矿冶学院学报, 1994, 25(6): 701?705.
TANG Mo-tang, LU Jun-le, YUAN Yan-sheng, YAN De-sheng, HE Qing-pu. On the ammoniation-complex equilibrium in the system of Zn(II)-NH3-(NH4)2SO4- H2O[J]. J Cent South Inst Min Metal, 1994, 25(6): 701?705.
[6] 张保平, 唐谟堂, 杨声海. 氨法处理氧化锌矿制取电锌[J]. 中南工业大学学报(自然科学版), 2003, 34(6): 619?623.
ZHANG Bao-ping, TANG Mo-tang, YANG Sheng-hai. Treating zinc oxide ore using ammonia-ammonium chloride to produce electrolysis zinc[J]. J Cent South Univ Technol: Natural Science, 2003, 34(6): 619?623.
[7] 胡立新, 占 稳, 张 婷. 微波固相合成赖氨酸锌配合物[J]. 化学与生物工程, 2007, 24(10): 25?26:
HU Li-xin, ZHAN Wen, ZHANG Ting. Microwave assisted solid state reaction synthesis of lysine complexes of zinc[J]. Chemistry & Bioengineering, 2007, 24(10): 25?26.
[8] POPULIN T, MORET S, TRUANT S, CONTE L S. A survey on the presence of free glutamic acid in foodstuffs, with and without added monosodium glutamate[J]. Food Chemistry, 2007, 104: 1712?1717.
[9] 李尚德, 李 移, 莫丽儿, 程荷凤, 关雄泰, 东野广智. 谷氨酸锌的合成最佳条件研究[J]. 广东微量元素科学, 2001, 8(12): 54?57.
LI Shang-de, LI Yi, MO Li-er, CHENG He-feng, GUAN Xiong-tai, DONGYE Guang-zhi. Study on the best conditions for preparation of zinc glutamate[J]. Guangdong Trace Element Science, 2001, 8(12): 54?57.
[10] DEAN J A. Langes handbook of chemistry[M]. 15th edition. Beijing: Science Press, 2003: 8.80?8.82.
[11] MARTELL E, SMITH R M. Critical stability constants[M]. New York: Plenum Press, 1974: 27?28.
[12] ZIEMER S P, WOOLLEY E M. Thermodynamics of the first and second proton dissociations from aqueous L-aspartic acid and L-glutamic acid at temperatures from(278.15 to 393.15) K and at the pressure 0.35 MPa: Apparent molar heat capacities and apparent molar volumes of zwitterionic, protonated cationic, and deprotonated anionic forms at molalities from (0.002 to 1.0)mol/kg[J]. J Chem Thermodynamics, 2007, 39: 645?666.
[13] 张保平. 锰锌软磁铁氧体前躯体碳酸盐共沉淀过程基础理论及工艺研究[D]. 长沙: 中南大学, 2004: 27?32.
ZHANG Bao-ping. The basic and technical research on the coprecipitation by carbonate process of Mn-Zn soft magnetic ferrite precursor[D]. Changsha: Central South University, 2004: 27?32.
[14] 李 波. 金属碳酸盐沉淀过程的热力学分析[J]. 稀有金属与硬质合金, 2005, 33(2): 4?8.
LI Bo. The thermodynamics analysis on the deposition process of metal carbonates[J]. Rare Metals and Cemented Carbides, 2005, 33(2): 4?8.
[15] TANG Mo-tang, ZHAO Tian-cong. A thermodynamic study on the basic and negative potential fields of systems of Sb-S-H2O and Sb-Na-S-H2O[J]. J Cent South Inst Min Metall, 1988, 19(1): 35?43.
[16] TANG Mo-tang, ZHAO Tian-cong. Principle and application of the new chlorination-hydrolization process[J]. J Cent South Inst Min Metall, 1992, 23(4): 405?411.
[17] 宋兆基, 徐流美. Matlab 6.5在科学计算中的应用[M]. 北京: 清华大学出版社, 2005: 118?275.
SONG Zhao-ji, XU Liu-mei. Application of Matlab 6.5 in the scientific computation[M]. Beijing: Tsinghua University Press, 2005: 118?275.
[18] 张克旭. 氨基酸发酵工艺学[M]. 北京: 中国轻工业出版社, 1992: 205?210.
ZHANG Ke-xu. Study on the amino acid ferment[M]. Beijing: Light Industry Press, 1992: 205?210.
[19] 张祥麟, 康 衡. 配位化学[M]. 长沙: 中南工业大学出版社, 1986: 42?93.
ZHANG Xiang-lin, KANG Heng. Coordination chemistry[M]. Changsha: Central South University of Technology Press, 1986: 42?93.
基金项目:国家重点基础研究发展计划资助项目(2007CB613604)
收稿日期:2008-03-19;修订日期:2008-06-10
通讯作者:杨天足,教授,博士;电话:0731-8836791;E-mail: tianzuyang@163.com
(编辑 何学锋)