从盐湖卤水中萃取锂
孙锡良1, 2,陈白珍1,徐 徽1,石西昌1
(1. 中南大学 冶金科学与工程学院,湖南 长沙,410083;
2. 中南大学 物理科学与技术学院,湖南 长沙,410083)
摘 要:选取磷酸三丁酯(TBP)为萃取剂,200号溶剂汽油为稀释剂,氯化铁(FeCl3?6H2O)为共萃取剂,从青海盐湖含锂卤水中萃取锂,并对TBP质量分数对萃取率的影响,相比对萃取率及分配比的影响进行研究。研究结果表明:共萃剂FeCl3在萃取过程中作用明显,同时,水相氢离子浓度是非常重要的影响因素,适当的酸度既可以保证锂离子进入有机相,减少氢离子与有机溶剂络合的机会,又可以保证铁离子在溶液中不发生水解;最佳萃取工艺条件如下:TBP质量分数为60%,萃取相比(O/A)为1.5,n(Fe3+)/n(Li+)为1.3,水相氢离子浓度为0.05 mol/L。在此条件下,锂的萃取率可达到80%,锂、镁分离效果较好,萃取液经洗涤、反萃取和深度除镁后,可制备高纯度碳酸锂。
关键词:盐湖卤水;锂;萃取;分离
中图分类号:O614.111; O652.62 文献标识码:A 文章编号:1672-7207(2007)02-0262-05
Extraction of lithium from bittern
SUN Xi-liang1, 2, CHEN Bai-zhen1, XU Hui1, SHI Xi-chang1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Physics Science and Technology, Central South University, Changsha 410083, China)
Abstract: With tri-butyl-phosphate (TBP) as extraction reagent, mineral spirit (200#) as dilution reagent and FeCl3?6H2O as co-extraction reagent, the extraction of lithium from Qinghai salt lake was studied. The effect of TBP concentration on extraction rate, and the effect of phase ratio on extraction rate and distribution ratio were analysed. The results show that the concentration of hydrogen ion in water phase is a very important factor, and a proper concentration of hydrogen ion not only ensures that lithium ion enters into organic phase and decreases the complexing of hydrogen ion and organic solvent, but also assures iron ion against hydrolyzing. The optimum parameters in the solvent extraction process are as follows: the phase ratio is 1.5, the molar ratio of Fe3+ to Li+ is 1.3, TBP concentration is 60%, the concentration of hydrogen ion in water phase is 0.05 mol/L. Under the above conditions, the extraction rate of lithium can reach 80%, the separating effect of lithium with magnesium is good, and a high pure Li2CO3 can be made from the extracting solution after washing and demagnesium deeply.
Key words: bittern; lithium; extraction; separation
锂及锂盐广泛应用于陶瓷、玻璃、原子能、航空航天、军事工业、制冷、焊接、锂合金、锂电池、冶金和医药等领域[1-4]。目前,我国的锂盐生产仍以锂辉石、锂云母等含锂矿石为主。盐湖卤水中蕴藏丰富的锂资源,占我国已探明的锂总储量的87%,储量居世界第2位,仅次于玻利维亚。其中,青海和西藏盐湖卤水锂的储量与世界其他国家已探明的总储量相当,是全球的重要锂资源,也是我国今后发展锂盐工业的重要资源基础[5-7]。盐湖锂的提取方法有沉淀法、蒸发结晶法、离子交换法、盐析法等[8]。采用沉淀法时,在碳化过程中,碳化液及焙烧浸取过程蒸发大量水,耗能高,工艺流程复杂;采用蒸发结晶法时耗碱量大,成本高;采用离子交换法回收率低,采用盐析法难以解决除硼、脱色等问题,回收率也很低,无法实现工业化。鉴于上述原因,有必要探索新方法,以加强我国卤水锂资源的开发,促进我国锂盐工业的进一步发展[9-10]。溶剂萃取法[11-12]是目前国内外比较关注的一种新方法,也是比较适用于我国青海盐湖锂的萃取。为此,本文作者采用溶剂萃取法对从青海盐湖卤水萃取锂进行研究。
1 实 验
1.1 实验原料及试剂
a. 实验中所用卤水来自青海察尔汗盐湖,其主要成分为:Mg2+ 104 g/L,Li+ 0.253 g/L,Ca2+ 0.087 g/L, Na+ 2.357 g/L; K+ 0.945 g/L。
b. 实验中所用试剂主要有:磷酸三丁酯(工业纯);200号溶剂汽油(工业纯);FeCl3?6H2O(化学纯);盐酸(化学纯);EDTA;络黑T等。
1.2 设备及检测方法
a. 主要仪器有:WXY-402C原子吸收分光光度计(沈阳分析仪器厂);pH酸度计;分析天平;康氏振荡器。
b. 检测方法:用原子吸收分光光度法分析铁离子和锂离子的含量;采用EDTA滴定法测定镁离子含量。
1.3 实验流程
溶剂萃取法提取锂的总流程如图1所示,在此只对其中的萃取过程进行研究。
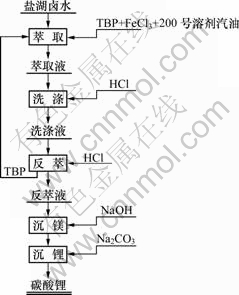
图1 从盐湖卤水中萃取锂的流程图
Fig.1 Flowchart of extraction of lithium from bittern
2 实验结果与讨论
2.1 热力学计算
采用TBP+FeCl3+200号溶剂汽油作为萃取体系的萃取反应为:
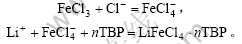
2.1.1 萃取焓变?H
温度(T)、分配比D(Li+)及焓变(?H)关系式为[13]:
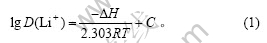
将不同温度下的分配比D(Li+),根据式(1)的lgD(Li+)对1/T作图,结果如图2所示。
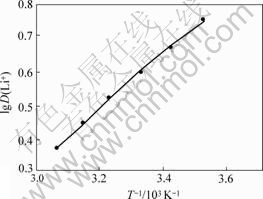
图2 TBP+FeCl3+200号溶剂汽油萃取温的lgD(Li+)与1/T关系曲线
Fig.2 lgD(Li+) and 1/T curves during extration of lithium in TBP+FeCl3+200# sulfacted kerosene system
根据图2中的直线斜率,求得焓变?H=-14.32 kJ/mol,表明体系对锂的萃取过程是放热反应,故在常温或者低温下萃取较为有利,萃取能力较强。本实验采用常温萃取。
2.1.2 萃取自由能变化?G
温度与自由能变化的关系可由下式表示:
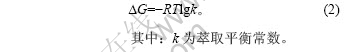
根据萃取机理可知[13-14]:
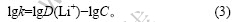
由式(3)可以求出不同条件下的平衡常数k,再按照式(2)求出?G。
2.1.3 萃取熵变?S
熵变的关系式为:
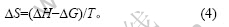
将由式(1)和(2)求得的值代入式(4)即可得到不同条件下的熵变值。
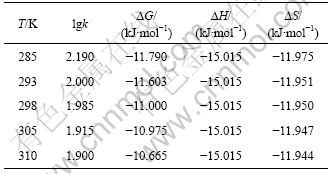
根据式(1),(2)和(4)确定不同温度条件下萃取锂的热力学函数值,如表1所示。根据表1中的计算结果可知,萃取过程的熵变是一个负值,温度对其影响较小,但对?G的影响相对明显。
表1 TBP+FeCl3+200号溶剂汽油体系萃取锂的热力学数据
Table 1 Thermodynamic data of TBP+FeCl3+200# mineral spirit system for extration of lithium
2.2 磷酸三丁酯质量分数对锂萃取率的影响
TBP质量分数分别取30%,50%,60%和80%,其他条件保持固定:相比为1.5,铁锂的量比为 1.3,pH值为1.5。将水相与有机相混合,倒入锥形瓶中盖紧,均匀摇动8 min后,将混合液倒入分液漏斗中静置分层,水相在下层,有机相在上层,取出水相。条件不变,继续萃取2次, 最后用原子吸收分光光度计检测水相中锂离子的浓度,从而计算进入有机相中锂离子的总量,磷酸三丁酯浓度对锂萃取率的影响如图3所示。
由图3可知,当TBP质量分数为30%时,萃取率低,随着TBP质量分数的升高,萃取率随之提高,但当TBP质量分数达到60%时,萃取率达到最大值,再进一步增大TBP质量分数时,萃取率有下降趋势。因此,TBP质量分数控制在60%。
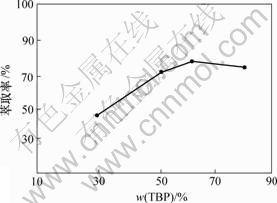
图3 TBP质量分数对锂萃取率的影响
Fig.3 Relation of TBP concentration and extraction rate
根据萃取动力学的基本理论,由于TBP质量分数增加,有机分子与锂离子之间的接触机会增多,在其他条件相同的情况下形成配合物的机会增大,从而使更多的锂离子进入有机相。但当TBP质量分数增大到一定程度后再继续增加时,稀释剂相对减少,降低了萃取剂的稀释效果,从而使萃取效率反而下降。
2.3 相比对锂萃取率的影响
改变有机相和水相的体积比,取V(油)/V(水)=0.8,1.0,1.5,2.0,其他条件保持不变:TBP质量分数为60%,铁锂的量比为1.3,pH值为1.5,将萃取液稀释后用分光光度计检测其中的主要离子的含量,从而进一步得出有机溶剂中各种离子的含量。根据检测和计算数据,可以得到有机相和水相中各离子的平衡浓度,结果如表2所示。
表2 萃取相比试验两相平衡时各物质的浓度
Table 2 Concentration of ions at two phase balance during extraction at different phase ratios ρ/(g?L-1)
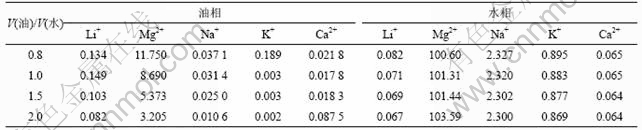
由表2可知:Ca2+,Na+和Mg2+的浓度在萃取过程中随相比增加变化不大。离子的萃取率随相比提高而迅速提高,其萃取能力从高到低依次为:Li+, Ca2+, Mg2+, Na+。当相比在1.0~1.5时,Ma2+和Na+的萃取率低于3%,而Ca2+的萃取率则有40%,表明Ca2+的存在对Li+的萃取不利。但是,经洗涤、反萃取后,Li+和Ca2+的分离系数比较大,在最后的反萃液中Ca2+含量非常低。而Mg2+在有机相中的含量始终都比较低,并随着相比增加有下降的趋势,可以很好地实现Mg2+与Li+分离。当相比超过1.5以后,再增加相比,锂的萃取率变化不大。因为Li+及其他离子的总量是一定的,增加有机溶剂,除了增加有机分子与水相中阳离子的碰撞几率外,并不能成为决定萃取率的控制步骤。所以,将相比控制在1.5既可以保证Li+有较高的萃取率,还可以降低萃取成本。
相比对Li+和Mg2+分配比的影响如图4所示。由图4可以看出:Li+的分配比随相比的增加而升高,但当相比超过1.5以后,Li+的分配比开始缓慢下降;相比低于1.0时,Mg2+的分配比随相比的增加而降低,当相比大于1.0以后,Mg2+的分配比变化很小。为了更好地实现Mg2+与Li+分离,同时尽可能地减少有机溶剂的用量,并结合相比对萃取率的影响,选择最佳相比为1.5。
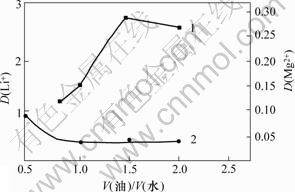
图4 萃取相比V(油)/V(水)对Li+分配比D(Li+)的影响
Fig.4 Effect of phase ratio on distribution ratio during extration
2.4 n(Fe3+)/n(Li+)对萃取过程的影响
在萃取分离过程中,共萃剂起着非常重要的作用。本研究选择FeCl3?6H2O作为共萃取剂,在TBP质量分数为60%,相比为1.5,pH值为1.5的条件下,研究n(Fe3+)/n(Li+)对萃取液中的Li+浓度的影响,可以得到n(Fe3+)/n(Li+)与Li+分配比的关系及与萃取率的关系, 结果如图5和图6所示。
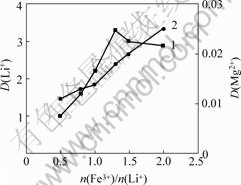
1—Li+; 2—Mg2+
图5 n(Fe3+)/n(Li+)与Li+分配比的关系
Fig.5 Relation of n(Fe3+)/n(Li+) and distribution ratio of lithium
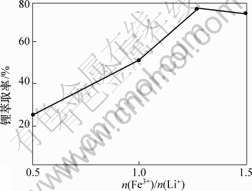
图6 n(Fe3+)/n(Li+)与锂萃取率的关系
Fig.6 Relation of n(Fe3+)/n(Li+) and extraction rate of lithium
由图5可以看出:当n(Fe3+)/n(Li+)升高时,Li+的分配比也提高,但是,当n(Fe3+)/n(Li+)超过1.3以后,Li+的分配比反而呈下降趋势。这可能是由于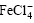 浓度升高,增加了Mg2+与
浓度升高,增加了Mg2+与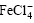 结合的机会,而每个Mg2+要消耗2个
结合的机会,而每个Mg2+要消耗2个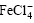 ,所以,导致锂的分配比降低。
,所以,导致锂的分配比降低。
由图6可以看出:随着n(Fe3+)/n(Li+)的增加,锂的萃取率也逐步提高,当n(Fe3+)/n(Li+)超过1.3时,再增加n(Fe3+)/n(Li+)对锂萃取率并无明显影响。综合萃取率与分配比的共同效果,最终选择最佳n(Fe3+)/n(Li+)为1.3。
2.5 水相酸度对萃取过程的影响
水相酸度也是萃取过程中的一个重影响因素,一方面,要求Li+尽可能地进入有机相,减少氢离子与有机溶剂络合;另一方面,要求Fe3+在溶液中不容易发生水解,所以,保证酸性条件是必要的。改变水相酸度,固定其他条件:n(Fe3+)/n(Li+)为1.3,相比为1.5,60% TBP+40%溶剂汽油,时间为8 min,得出不同酸度条件下的H+和Li+的分配比曲线如图7所示。
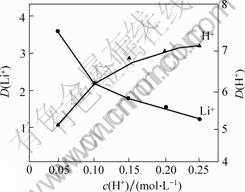
图7 H+浓度与Li+分配比的关系
Fig.7 Relation of pH and distribution ratio of lithium during extration
由图7可以看出:当H+浓度低于0.05 mol/L时,Li+的分配比很高,而H+的分配比很低;当H+浓度大于0.05 mol/L时,Li+的分配比明显降低,而H+的分配比则显著升高;当H+浓度超过0.10 mol/L时,H+的分配比大于Li+的分配比,这样将对锂的萃取极为不利,所以,将酸度控制在0.05 mol/L(pH=1.5以下),既有利于锂的萃取,又可以保证Fe3+不会发生水解,从而保证萃取相的稳定与萃取效率。
3 结 论
a. 随着TBP浓度的升高,Li+萃取率随之提高,当TBP质量分数达到60%时,Li+萃取率达到最大,再进一步增大TBP的含量,Li+萃取率反而有下降趋势。所以,选取TBP质量分数为60%。
b. Li+的萃取率随相比提高而提高,将相比控制在1.5既可以保证较高的萃取率,又可以降低萃取成本。
c. 随着n(Fe3+)/n(Li+)升高,Li+的分配比提高,当n(Fe3+)/n(Li+)超过1.3以后,Li+萃取率反而下降,所以,最佳n(Fe3+)/n(Li+)为1.3。
d. 酸度控制在0.05 mol/L(pH=1.5)以下,既有利于锂的萃取,又可以保证铁离子不发生水解,从而保证萃取相的稳定与萃取效率。
参考文献:
[1] 蒋 明. 锂及锂化合物的应用[J]. 无机盐工业, 1983(9): 32-36.
JIANG Ming. The application of lithium and lithium compounds[J]. Inorganic Chemical Industry, 1983(9): 32-36.
[2] 游清治. 锂及其化合物在医药中的应用[J]. 世界有色金属, 1997(12): 41-43.
YOU Qing-zhi. The application of lithium and lithium compounds in medicine field[J]. The World Journal of Nonferrous Metals, 1997(12): 41-43.
[3] Johonson F N. The early history of lithium therapy[J]. Lithium Current Application in Medicine Science and Technology, 1985(1): 337-338.
[4] 李海英,翟秀静,符 岩. 锂离子电池正极材料 LiCoO2的微波合成及其结构表征[J]. 分子科学学报, 2002, 18(4): 199-203.
LI Hai-ying, ZHAI Xiu-jing, FU Yan. Microwave processing and constructive character of LiCoO2 cathode materials for lithium ion batteries[J]. Journal of Molecular Science, 2002, 18(4): 199-203.
[5] Alexanden J H. Lithium[J]. Mining Engineering, 1986(1): 362-363.
[6] 马欣华. 苦卤化工[M]. 北京: 化学工业出版社,1995.
MA Xing-hua. Bittern chemical industry[M]. Beijing: Chemical Industry Press, 1995.
[7] 任凤莲, 邱昌桂, 连 琰. 百合总皂甙的提取工艺[J]. 中南大学学报: 自然科学版, 2005, 36(1): 69-72.
REN Feng-lian, QIU Chang-gui, LIAN Yan. Extraction process of total saponins from lilium brownii[J]. Journal of Central South University: Science and Technology, 2005, 36(1): 69-72.
[8] 于明臻. 我国锂盐工业的现状及技术进展[J]. 无机盐工业, 1999, 31(1): 17-19.
YU Ming-zhen. Present condition and technology progress of lithium industry in China[J]. Inorganic Chemical Industry, 1999, 31(1): 17-19.
[9] 高世杨. 青海盐湖锂盐开发与环境[J]. 盐湖研究, 2000, 8(1): 17-23.
GAO Shi-yang. Exploring and envierment of lithium of Qinghai salt lake[J]. Journal of Salt Lake Research, 2000, 8(1): 17-23.
[10] Barrett W T. Recovery of lithium from saline brines using solar evaporation[J]. Journal of Applied Chemistry and Biotechnology, 1983, 28(4): 317-325.
[11] 刘晓荣,邱冠周,胡岳华. 萃取界面乳液的固体微粒稳定机理[J]. 中南大学学报:自然科学版,2004,35(1):5-10.
LIU Xiao-rong, QIU Guan-zhou, HU Yue-hua. Soild particles stabilizing interfacial emulsion in copper-SX[J]. Journal of Central South University: Science and Technology, 2004, 35(1): 5-10.
[12] 刘晓荣,邱冠周,胡岳华. 铜溶液萃取O/W型乳化液的结构稳定性[J]. 中南大学学报: 自然科学版, 2005, 36(6): 929-932.
LIU Xiao-rong, QIU Guan-zhou, HU Yue-hua. Framework- dependent stability of oil-in-water emulsions[J]. Journal of Central South University: Science and Technology, 2005, 36(6): 929-932.
[13] Hamzaoui A H, M’nif A N. Rokbani R. Medicine and technology[J]. Asian Journal of Chemistry, 2001, 13(1): 27-34.
[14] Hamzaoui A H, M’nif A N, Hammi H. Contribution to the lithium recovery from brine[J]. Desalination, 2003, 158(3): 221-224.
收稿日期:2006-10-15
基金项目:国家科技攻关项目(2005BA639C)
作者简介:孙锡良(1971-),男,湖北武穴人,博士研究生,从事盐湖卤水开发与利用研究
通讯作者:孙锡良,男,博士研究生;电话:13786199946(手机);E-mail: sunxl1971@163.com