文章编号: 1004-0609(2006)07-1306-05
六方水合铁酸钙的合成及其脱硅
李来时, 翟玉春, 刘瑛瑛, 王佳东
(东北大学 材料与冶金学院, 沈阳 110004)
摘 要: 通过正交实验对六方水合铁酸钙的合成温度、 合成时间和合成体系中CaO与Fe2O3的摩尔比及合成体系的搅拌强度等因素对其脱硅效果的影响进行研究, 并给出六方水合铁酸钙的最佳合成条件。 结合X射线衍射分析讨论水合铁酸钙的脱硅机理, 提出水合铁酸钙是一种高度分散的含钙化合物, 其晶面间距较大, 脱硅活性较高, 并可大幅度降低铝损。 在最佳条件下添加水合铁酸钙进行深度脱硅时, 脱硅后铝酸钠溶液中的SiO2含量可降低到7mg/L, 硅量指数可达14000, 且脱硅产物中SiO2的饱和系数可达0.46。
关键词: 脱硅; 水合铁酸钙; 铝酸钠溶液; 合成 中图分类号: TF821
文献标识码: A
Synthesis of hexagonal calcium hydroferrite
and its desilication
LI Lai-shi, ZHAI Yu-chun, LIU Ying-ying, WANG Jia-dong
(School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China)
Abstract: The effects of temperature, time, mole ratio of CaO to Fe2O3 and agitation strength in the synthesis process of hexagonal calcium hydroferrite on desilication were studied by orthogonal experiments. The optimum synthesis conditions of hexagonal calcium hydroferrite were obtained. The reactive mechanisms on desilication of hexagonal calcium hydroferrite were analyzed by XRD. It is indicated that the hexagonal calcium hydroferrite is a kind of high scattered calciferous compound. The interplanar spacing of hexagonal calcium hydroferrite is wide. The desilication activity of hexagonal calcium hydroferrite is higher. And the loss of alumina can be reduced. The results show that the concentration of SiO2 can reduce to 7mg/L and the desilication index can reach 14000 in desilicated sodium aluminate solution with the addition of hexagonal calcium hydroferrite in deep desilication under the optimum conditions. The saturation coefficient in desilication product can reach 0.46.
Key words: desilication; hexagonal calcium hydroferrite; sodium aluminate solution; synthesis
在烧结法生产氧化铝的熟料溶出过程中, 由于原硅酸钙引起二次反应, 使溶出粗液中含有3.0~6.0g/L的二氧化硅, 硅量指数(A/S)仅为20左右, 因此在铝酸钠溶液分解之前必须设立专门的脱硅工艺[1-4]。 精液在碳酸分解过程中所能达到的分解率和生产出产品氢氧化铝的质量主要取决于精制液的硅量指数。 因此, 提高烧结法精制液的硅量指 [CM(22]数对于提高氧化铝产品的产量和质量起着关键的[CM)]作用。 深度脱硅是烧结法生产氧化铝实现优质高产的有效途径。 但目前烧结法中铝酸钠溶液深度脱硅的工艺不够完善, 添加剂所用的CaO与SiO2的摩尔比偏高[5], 且由于脱硅产物中含有大量的氧化铝, 造成大量氧化铝的损失, 脱硅产物返回烧结工序又会加大能耗和降低产能。 因此, 如何改进工艺来提高烧结法的氧化铝质量(硅量指数)是当前烧结法生产氧化铝必须认真解决的重要课题之一。 本文作者主要就脱硅剂水合铁酸钙的合成条件、 性质及其深度脱硅和降低铝损的机理进行了研究。
1 实验
实验用铝酸钠溶液是由工业纯氢氧化钠和氢氧化铝配制而成, 氯化钙和氯化铁均为分析纯。
C4FHn的合成与脱硅实验在1L的不锈钢杯并在带搅拌的超级恒温水浴中进行, 控温精度±0.5℃。 在搅拌的条件下, 将CaCl2和FeCl3按一定比例滴加到一定浓度的NaOH溶液中, 在70~80℃搅拌30~70min, 洗涤, 真空抽滤, 测定滤饼总量, 计算有效氧化钙的相对含量。 脱硅实验是以组成为Al2O3 104.5g/L, SiO2 0.33g/L, αk=1.56的铝酸钠溶液作为脱硅原液, 按有效CaO 1.65g/L添加C4FHn滤饼, 在85℃下脱硅1.5h。 脱硅实验结束后, 取样离心分离, 取上清液分析, 用光度法测定溶液中的SiO2浓度。 分离出的渣相用沸水洗涤、 过滤、 烘干, 进行化学分析、 X射线衍射分析, 并测定渣相中SiO2的饱和系数。 取部分最佳合成条件下合成的C4FHn滤饼, 冷风吹干。 对产物进行化学分析、 X射线衍射分析。 分析检测结果分别如表1和2所列及图1和2所示。
表1 水合铁酸钙的化学组成
Table 1 Composition of calcium hydroferrite(mass fraction, %)

表2 脱硅产物的化学组成
Table 2 Composition of desilication product (mass fraction, %)

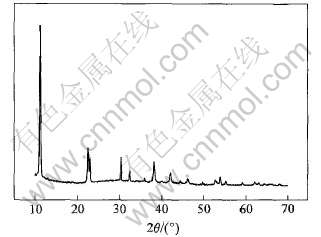
图1 最佳条件下合成六方水合铁酸钙的X射线衍射谱
Fig.1 XRD pattern of hexagonal calcium hydroferrite in optimum synthetic condition
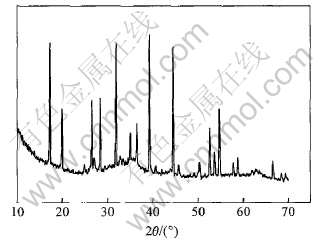
图2 脱硅产物的X射线衍射谱
Fig.2 XRD pattern of desilication product
2 结果与讨论
2.1 正交实验结果
在单因素实验基础上, 以脱硅后精制液中SiO2浓度为评价指标, 进行C4FHn合成的四因素三水平正交实验, 实验结果如表3所列。 从R值可以看出, 温度是C4FHn合成的主要影响因素, 各因素的[CM(22]影响顺序依次为合成温度>合成体系中CaO与Fe2O3的摩尔比>搅拌强度>合成时间。 通过分
表3 六方水合铁酸钙合成的正交实验结果
Table 3 Orthogonal experiment results of synthesis of C4FHn
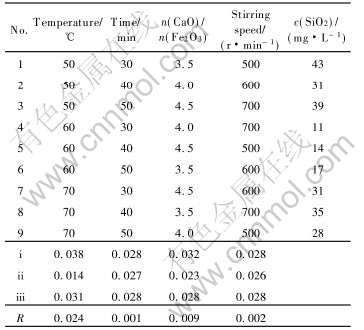
析, C4FHn的最佳合成条件为: 合成温度60℃, 合成时间50min, CaO与Fe2O3的摩尔比4.0, 合成体系搅拌速度600r/min。 在最佳条件下脱硅, 精制液中的SiO2浓度可降至7mg/L, 硅量指数可达14000。
2.2 合成温度对C4FHn脱硅效果的影响
由表2可知, 温度是影响铁酸钙合成的最主要因素。 在其他条件相同的情况下得出合成温度对C4FHn脱硅效果的影响曲线如图3所示。
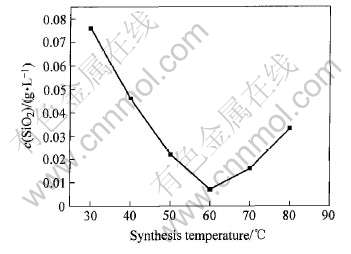
图3 合成温度对C4FHn脱硅效果的影响
Fig.3 Influence of synthesis temperature on desilication of C4FHn
由图3可看出, 温度升高有利于提高六方水合铁酸钙的合成速度, 但同时会降低六方水合铁酸钙的稳定性, 形成稳定的立方水合铁酸钙。 当温度达到80℃时, 完全形成立方水合铁酸钙, 使其脱硅能力下降。
2.3 合成时间C4FHn脱硅效果的影响
合成时间对C4FHn脱硅效果的影响如图4所示。 从图4可以看出, 在其他条件相同的情况下, 合成时间不同, 产物中C4FHn的脱硅效果不同。 合成时间过短, 合成体系中各物质反应不完全, 没有完全长成C4FHn六方结构, 影响脱硅效果。 合成时间过长, 产物脱硅效果也差, 这与C4FHn的稳定性有关。 由于C4FHn是一种介稳物质, 加热时间过长容易转变成较为致密的立方结构。
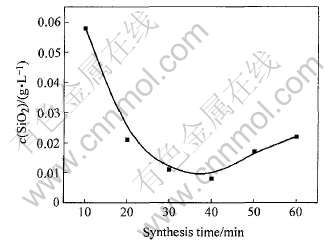
图4 合成时间对C4FHn脱硅效果的影响
Fig.4 Influence of synthesis time on desilication of C4FHn
2.4 CaO与Fe2O3的摩尔比对C4FHn脱硅效果的影响
合成C4FHn中CaO与Fe2O3的摩尔比对脱硅效果的影响如图5所示。 由图5可看出, CaO与Fe2O3的摩尔比为4.0时, 脱硅效果最好。 这是由于当摩尔比为4.0时, 合成产物几乎全部为C4FHn, 在其他比例的产物中均有不同含量的Ca(OH)2或Fe(OH)3存在, 从而影响脱硅效果。
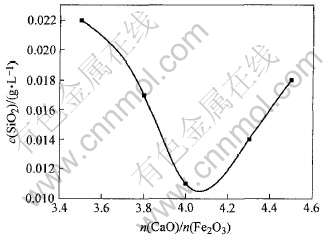
图5 合成C4FHn中CaO与Fe2O3的摩尔比对脱硅效果的影响
Fig.5 Influence of mole ratio of CaO to Fe2O3 in synthetic C4FHn on desilication
2.5 合成体系搅拌强度C4FHn脱硅效果的影响
合成体系搅拌强度对产物脱硅效果的影响如图6所示。 由图6可看出, 搅拌速度的提高可以促进C4FHn合成及其颗粒的细化, 这都对其脱硅效果产生正面影响, 但搅拌强度过大会降低C4FHn的稳定性, 使部分C4FHn转化为立方结构, 因此搅拌强度应适当。 实验证明, 在其他条件相同的情况下, 当搅拌速度为600r/min时, 合成的C4FHn脱硅效果最好。
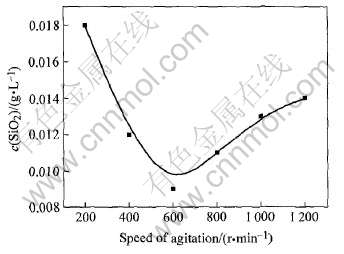
图6 搅拌速度对C4FHn脱硅效果的影响
Fig.6 Influence of speed of agitation on desilication of C4FHn
3 六方水合铁酸钙脱硅剂的脱硅机理
脱硅效果除了与合成条件有关外, 与该脱硅剂的使用条件也有很大关系。
本文作者考察了C4FHn脱硅温度和脱硅时间对脱硅效果的影响, 结果如图7所示。 由图7可看出, 温度是影响脱硅速度和脱硅深度的重要因素, 它直接关系到六方水合铁酸钙的反应活性。 由图7 可看出, 当温度为70~90℃时, 在相同的脱硅反应时间内, 温度越高, 铝酸钠溶液中SiO2的质量浓度越低, 脱硅速度越快, 从而在一定程度上提高了精制液的硅量指数。 随着反应温度的提高, 反应速度常数增大, 体系的粘度降低, 扩散层厚度变小, 脱 [CM(22]硅速度加快, 脱硅率提高。 由于C4FHn晶面间距
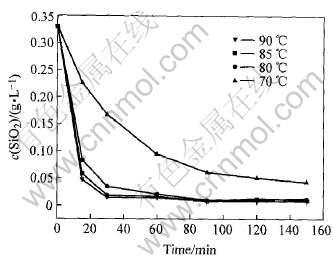
图7 脱硅温度和时间对脱硅深度的影响
Fig.7 Influence of temperature and time on desilication
大, 反应活性高, 因此当温度为80~90℃时, 开始阶段的脱硅速度较快, 绝大部分硅是在前30min脱除掉的。 30min后, 体系SiO2浓度低, 导致脱硅速度明显下降。 温度升高同时会增加铁水化石榴石的溶解度。 当脱硅温度为85℃时, 脱硅1.5h, 脱硅后铝酸钠溶液的硅量指数可达14000。
因此, C4FHn脱硅剂在达到相同脱硅效率时, 脱硅温度和脱硅时间均远低于传统石灰乳的[4, 6], 也低于水合铝酸钙[7]、 水合硫铝酸钙[8]和水合碳铝酸钙[9, 10]等新型脱硅剂。 在工业生产中降低铝损失的同时, 缩短了深度脱硅的时间。
鉴于脱硅产物含铝少, 硅饱和系数高, 在最佳条件下, 应用C4FHn脱硅剂进行一段脱硅(A/S=40)。 实验证明, 在85℃下脱硅1.5h, 硅量指数可达5000以上, 铝损失仅为2.3g/L, 与传统一段加压脱硅[4]的铝损失(2.1g/L)接近, 并可大幅度降低碱损失。 另外, 脱硅产物铁水化石榴石可作为一水硬铝石溶出的溶出添加剂[11]。 因此六方水合铁酸钙是一种很有前途的脱硅剂。
铝酸钠溶液结构研究表明[12-14], 在碳分条件下, Al主要是以Al(OH)-4离子存在, Si主要是以[SiO2(OH)2]2-离子存在。 合成产物C4FHn的X射线衍射谱表明, C4FHn具有六方晶系的晶体结构, 相对于立方结构的铝酸钙[15]来说, 其结构较为松散, 且其最大晶面间距可达0.799nm, 使铝酸钠溶液中[SiO2(OH)2]2-离子更容易进入晶体间与钙结合生成溶解度小的铁水化石榴石。 X射线衍射数据表明, C4FHn具有与六方水合铝酸钙[15]类似的六方层状结构, 其脱硅机理为[SiO2(OH)2]2-离子进入C4FHn晶体内部并替换其中OH-, 在脱硅温度下晶形发生转变, 由介稳的六方晶系转变成稳定的立方晶系。 六方水合铁酸钙脱硅反应为
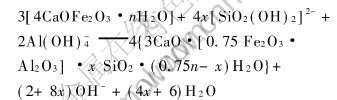
对脱硅产物的化学分析(表2)表明, 生成的铁水化石榴石含铝低, 含硅较高, 硅的饱和系数可达0.46, 高于Ca-Al系脱硅剂脱硅产物中硅的饱和系数(0.15~0.41)[4, 8, 15]。
4 结论
1) 六方水合铁酸钙是一种非常好的新型脱硅添加剂, 其脱硅产物铁水化石榴石可作为一水硬铝石的溶出添加剂。
2) 六方水合铁酸钙的最佳合成条件为: 合成温度60℃, 合成时间50min, CaO与Fe2O3的摩尔比4.0, 合成体系搅拌速度600r/min。
3) 在最佳条件下合成的六方水合铁酸钙进行深度脱硅, 脱硅后铝酸钠溶液中SiO2的含量可降至7mg/L, 硅量指数可达14000, 并得出该脱硅剂的使用条件。
4) 六方水合铁酸钙晶面间距大, 脱硅活性高, 脱硅产物中SiO2饱和系数可达0.46。
REFERENCES
[1]WANG Qing-wei, TIAN Geng-you, YANG Zhong-yu. Aspect of deep desilication with lime agent[A]. Light Metals[C]. Warrendale: TMS, 1997. 29-33.
[2]Rayzman V. More complete desilication of aluminate solution is the key-factor to radical improvement of alumina refining[A]. Light Metals[C]. San Francisco: TMS, 1996. 109-114.
[3]彭志宏. 铝酸钠溶液添加水合碳铝酸钙脱硅的研究[D]. 长沙: 中南工业大学, 1993.
PENG Zhi-hong. Study on desilication of adding the caliumhydroaluminocarbonate in aluminate solution[D]. Changsha: Central South University of Technology, 1993.
[4]杨重愚. 氧化铝生产工艺学[M]. 北京: 冶金工业出版社, 1993. 249-266.
YANG Zhong-yu. Alumina Production Technology[M] . Beijing: Metallurgical Industry Press, 1993. 249 -266.
[5]何良惠, 李自强, 李升章, 等. 铝酸钠溶液的脱硅及碳分过程[J] . 湿法冶金, 1993(3): 8-11.
HE Liang-hui, LI Zi-qiang, LI Sheng-zhang, et al. Process of carbonization and desilication of sodium aluminate solution[J]. Hydrometallurgy, 1993(3): 8-11.
[6]李小斌, 刘祥民, 刘桂华, 等. 强化烧结法生产氧化铝新工艺的研究与实践[J]. 中国有色金属学报, 2004, 14(6): 1031-1036.
LI Xiao-bin, LIU Xiang-min, LIU Gui-hua, et al. Study and application of intensified sintering process for alumina production[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 1031- 1036.
[7]元炯亮, 张懿. 铝酸钠溶液添加铝酸钙脱硅过程研究[J]. 有色金属, 2003, 55(1): 60-62.
YUAN Jiong-liang, ZHANG Yi. Study on desilication process of sodium aluminate solution by adding tricalcium hydroaluminate[J]. Nonferrous Metal, 2003, 55(1): 60-62.
[8]刘连利, 翟玉春, 田彦文, 等. 水合硫铝酸钙的合成[J]. 中国有色金属学报, 2003, 13(2): 506-509.
LIU Lian-li, ZHAI Yu-chun, TIAN Yan-wen, et al. Synthesis of calcium hydroaluminosulfate[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(2): 506-509.
[9]刘连利, 翟玉春, 刘玉静, 等. 水合碳铝酸钙的性质及脱硅反应研究[J]. 化学通报, 2004(5): 368-372.
LIU Lian-li, ZHAI Yu-chun, LIU Yu-jing, et al. Research on desilication reaction and property of calcium hydroaluminocarbonate[J]. Chemistry Report, 2004(5): 368-372.
[10]张用花. 水合碳铝酸钙深度脱硅的工业实践[J]. 轻金属, 2001, 8: 17-18.
ZHANG Yong-hua. The industrial application of HCAC in desilication[J]. Light Metals, 2001, 8: 17-18.
[11]陈万坤, 彭关才. 一水硬铝石型铝土矿的强化溶出技术[M]. 北京: 冶金工业出版社, 1997.
CHEN Wan-kun, PENG Guan-cai. Reinforced Digestion Technology of Diasporic Bauxite[M]. Beijing: Metallurgical Industry Press, 1997.
[12]刘桂华, 李小斌, 彭志宏, 等. 氧化钙和氢氧化钙与浓碱铝酸钠溶液的反应特性 [J] . 中国有色金属学报, 2000, 10(2): 266-269.
LIU Gui-hua, LI Xiao-bin, PENG Zhi-hong, et al. Reaction behaviour of calcium oxide or calcium hyoxide and aluminatesolution with heavy caustic soda[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(2): 266- 269.
[13]谢雁丽, 毕诗文, 杨毅宏, 等. 氧化铝生产中铝酸钠溶液结构的研究[J]. 有色金属, 2001, 52 (2): 59-76.
XIE Yan-li, BI Shi-wen, YANG Yi-hong, et al. Research on structure of sodium aluminate solution in alumina production[J]. Nonferrous Metal, 2001, 53(2): 59-76.
[14]王雅静, 翟玉春, 田彦文, 等. 铝酸钠和含硅铝酸钠溶液的红外光谱和拉曼光谱[J]. 中国有色金属学报, 2003, 13(1): 271-275.
WANG Ya-jing, ZHAI Yu-chun, TIAN Yan-wen, et al. Infrared and Raman spectra of aluminate and SiO2-containing sodium aluminate solutions[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(1): 271- 275.
[15]刘连利, 翟玉春, 田彦文, 等. 六方水合铝酸钙的性质研究[J]. 轻金属, 2003(3): 19-21.
LIU Lian-li, ZHAI Yu-chun, TIAN Yan-wen, et al. Property study of hexagonal calcium aluminate hydrate [J]. Light Metals, 2003(3): 19-21.
(编辑李艳红)
基金项目: 国家重点基础研究发展规划资助项目(G1999064901-4)
收稿日期: 2005-12-08; 修订日期: 2006-03-13
通讯作者: 翟玉春, 教授, 博士; 电话: 024-83687731; 传真: 024-83687731; E-mail: lilaishi@126.com