Laccase biosensor using magnetic multiwalled carbon nanotubes and chitosan/silica hybrid membrane modified magnetic carbon paste electrode
来源期刊:中南大学学报(英文版)2011年第6期
论文作者:庞娅 曾光明 汤琳 章毅 李贞 陈丽娟
文章页码:1849 - 1856
Key words:magnetic multiwalled carbon nanotubes; paramagnetism; chitosan/silica sol; laccase biosensor; catechol
Abstract: A simple and rapid strategy to construct laccase biosensor for determination of catechol was investigated. Magnetic multiwalled carbon nanotubes (MMCNT) which possess excellent capability of electron transfer were prepared by chemical coprecipitation method. Scanning electron microscope (SEM) and vibrating sample magnetometer (VSM) were used to identify its surfacetopography and magnetization, respectively. Laccase was immobilized on the MMCNT modified magnetic carbon paste electrode by the aid of chitosan/silica (CS) hybrid membrane. Using current-time detection method, the biosensor shows a linear response related to the concentration of catechol in the range from 10-7 to 0.165×10-3 mol/L. The corresponding detection limit is 3.34×10-8 mol/L based on signal-to-noise ratios (S/N) ≥3 under the optimized conditions. In addition, its response current retains 90% of the original after being stored for 45 d. The results indicate that this proposed strategy can be expected to develop other enzyme-based biosensors.
J. Cent. South Univ. Technol. (2011) 18: 1849-1856
DOI: 10.1007/s11771-011-0913-1![]()
PANG Ya(庞娅)1, 2, ZENG Guang-ming(曾光明)1, 2, TANG Lin(汤琳)1, 2,
ZHANG Yi(章毅)1, 2, LI Zhen(李贞)1, 2, CHEN Li-juan(陈丽娟)1, 2
1. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China;
2. Key Laboratory of Environmental Biology and Pollution Control of Ministry of Education, Hunan University, Changsha 410082, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: A simple and rapid strategy to construct laccase biosensor for determination of catechol was investigated. Magnetic multiwalled carbon nanotubes (MMCNT) which possess excellent capability of electron transfer were prepared by chemical coprecipitation method. Scanning electron microscope (SEM) and vibrating sample magnetometer (VSM) were used to identify its surfacetopography and magnetization, respectively. Laccase was immobilized on the MMCNT modified magnetic carbon paste electrode by the aid of chitosan/silica (CS) hybrid membrane. Using current-time detection method, the biosensor shows a linear response related to the concentration of catechol in the range from 10-7 to 0.165×10-3 mol/L. The corresponding detection limit is 3.34×10-8 mol/L based on signal-to-noise ratios (S/N) ≥3 under the optimized conditions. In addition, its response current retains 90% of the original after being stored for 45 d. The results indicate that this proposed strategy can be expected to develop other enzyme-based biosensors.
Key words: magnetic multiwalled carbon nanotubes; paramagnetism; chitosan/silica sol; laccase biosensor; catechol
1 Introduction
Biosensors are widely used in medicine, environment and food processing in recent years [1-4], due to the advantages of high specificity, fast response and low cost. Effective immobilization of enzyme is a key step in fabrication of enzyme biosensor, as it affects the activity of enzyme, and more importantly, determines the lifetime of the biosensor [5-6]. Adsorption method, polymer entrapment, covalent immobilization and cross-linking are the common methods applied in enzyme immobilization [7-10]. However, some of these methods are relatively complex, requiring the existence of solvent that may cause instability and poor bioactivity to the enzymes. Therefore, at present, researchers are committed to develop new materials such as organic/ inorganic hybrid composite, nanocomposite and porous materials to immobilize bioactive components of biosensor [11-13]. Among them, the multiwalled carbon nanotube (MCNT) is a kind of promising material for biosensor due to its excellent properties of large specific surface area and remarkable electrochemical reactivity. For example, novel MCNT based biosensor was fabricated for quick detection of glucose [3, 8]. LUAIS et al [14] reported a biosensor using MCNT, which was treated with a microwave plasma system (CO2 and N2/H2) to functionalize its surface with oxygenated and aminated groups. DENG et al [15] noted that MCNT modified glassy carbon electrode could be used to immobilize glucose oxidase by layer-by-layer self- assemble, which provided a favorable microenvironment to keep the bioactivity and stability of the glucose oxidase. Besides, MCNT integrated on a microfluidic chip for chemical and biological analyses has been reported recently [16].
Magnetic nanoparticles, which are characterized by their magnetic properties, low toxicity and biocompatibility, have gained increasing interests and been served as a superior support for biomolecule. Various functionalized magnetic nanoparticles were used in fabrication of biosensor. SiO2@Fe3O4 shell-core magnetic shell nanoparticles were successfully prepared and applied in immobilization of laccase with high bioactivity and capacity [17]. It was found that compared with the native papain, covalent conjugation of papain on functionalized Fe3O4 nanoparticles exhibited enhanced enzyme activity and better tolerance to the variations of medium pH and temperature [18]. Immobilization of single-stranded biotinylated oligonucleotides onto gold-coated magnetic nanoparticles was applied on fabrication of highly sensitive DNA sensor [19]. Furthermore, in identification of microorganism species, IgG decorated magnetic particles were exploited to generate affinity probes, which concentrated the traces of target bacteria from the sample solution [20].
In order to exploit the good properties of MCNT and magnetic nanoparticles simultaneously, in the present work, magnetic multiwalled carbon nanotubes (MMCNT) were easily synthesized and spread on magnetic carbon paste electrode by paramagnetism. During the process of preparing MMCNT, the poor solubility of MCNT was also solved by treating MCNT with toluidine blue (TB) instead of concentrated acid. For fabrication of laccase biosensor, the enzyme was immobilized on MMCNT modified magnetic carbon paste electrode, then the prepared chitosan/silica (CS) hybrid membrane, which had excellent biocompatibility and mechanical strength, was used to coat the electrode surface. The MMCNT/laccase/CS hybrid membrane compositive multilayer provided a good micro- environment for retaining the bioactivity of laccase and was successfully applied to determine the catechol with a wide range. Parameters such as pH value of supporting electrolyte and working potential that governed the analytical performance of the biosensor were optimized. In addition, the performances of the biosensor in terms of repeatability, stability and selectivity were investigated. The biosensor is easy to make and convenient to operate, and the whole assay time from immobilizing laccase to finishing a test is approximately 2 h.
2 Experimental
2.1 Reagents and apparatus
Multiwalled carbon nanotubes were purchased from Applied Nanotechnologies, Inc. (purity≥97.7%, mass fraction). Laccase (EC=1.10.3.2, from Trametes versicolor, 23.3 U/mg) was bought from Sigma Aldrich Fluka. Chitosan (degree of deacetylation ≥90%), tetraethoxysilane (TEOS), toluidine blue (TB), catechol, acetic acid, (NH4)2Fe(SO4)2·6H2O, NH4Fe(SO4)2·12H2O, NaOH, Na2HPO4 and KH2PO4 were of analytical grade and used as received. The supporting electrolyte was phosphate buffered saline (PBS) prepared with 67 mmol/L KH2PO4 and 67 mmol/L Na2HPO4. All solutions were prepared with double distilled water.
Cyclic voltammetry (CV) and amperometric measurements were carried out on CHI660B electrochemistry system (Chenhua Instrument, Shanghai, China). The three-electrode system consisted of a magnetic carbon paste working electrode, a saturated calomel reference electrode (SCE) and Pt auxiliary electrode. Unless otherwise mentioned, all the work was done at room temperature. Scanning electron micrographs of MMCNT were obtained with JSM-6700F field emission scanning electron microscope (SEM, JEOL Ltd., Japan). Magnetic field intensity of MMCNT was measured with vibrating sample magnetometer (VSM) (Nanjing University Instrument Plant). pH value was tested with Model PHSJ-3F laboratory pH meter (Leici Instrument, Shanghai, China). Ultrasonicator, vacuum freezing dryer and mechanical vibrator were exploited in the experiment. A magnetic stirrer was used to stir the solution in amperometric measurement.
2.2 Preparation of MMCNT
Toluidine blue was utilized to increase the solubility of MCNT [21]. 0.12 g of pristine MCNT was mixed with 60 mL of 0.5 mmol/L TB solution and ultrasonicated for 1 h at room temperature. The resulting suspension was filtered with a millipore porous filter (0.3 μm). Then, the TB modified MCNT was washed thoroughly with distilled water and dried in a vacuum freezing dryer. Subsequently, the experiment was performed as previously reported, with some modifications [22]. 0.1 g of the treated MCNT was dissolved in 40 mL of solution, which contained 0.17 g (NH4)2Fe(SO4)2·6H2O and 0.25 g NH4Fe(SO4)2·12H2O, under continuous ultrasonic stirring. A volume of 1 mL of 8 mol/L NH4OH solution was slowly added into the suspension in the presence of nitrogen atmosphere, and continuously reacted for 30 min in a magnetic stirrer (300 r/min) at 50 °C water bath. The pH value of the final mixture was controlled at 11. After the reaction was completed, the suspension was cooled at room temperature and the products were collected by a permanent magnet separation and then washed with distilled water until the solution approached to be neutral and dried with a vacuum freezing dryer.
2.3 Preparation of chitosan/silica sol
An amount of 0.05 g chitosan was dissolved in 10 mL of 1% (volume fraction) acetic acid by the aid of 30 min ultrasonic stirring. Soon, it was stored in 4 °C environment until the chitosan solution looked transparent. A volume of 15 μL TEOS, 60 μL ethanol, and 450 μL chitosan solution were mixed orderly, followed by treatment with ultrasonication for 30 min. Then, the prepared chitosan/silica sol was placed in a refrigerator of 4 °C. Before use, the pH value was adjusted to 7.0 with NaOH solution.
2.4 Construction of laccase biosensor
The working electrode was prepared according to the procedure reported in a previous work with some modifications [17]. The mass ratio of paraffin to graphite was 1:3 (w/w). The magnet and kryptol came from the discarded earphones and batteries, respectively. This electrode was kept in a dust-free hood for 2 d to harden. Prior to use, it was polished thoroughly with No.6 diamond paper, and then sonicated in diluted nitric acid, acetone and water successively. A PBS (pH=5.6) was used to dissolve the laccase for preparation of a final concentration of 10 mg/mL. The procedures of constructing biosensor were as follows: First, 40 μL of 5 mg/mL MMCNT was evenly cast on the working electrode. After 35 to 50 min, 20 μL laccase solution was spread uniformly on the MMCNT modified electrode. When the surface was dry, 20 μL of CS sol was employed to cover the laccase, and the whole time for immobilizing laccase was less than 2 h. For the detection of catechol, the operation was carried out by amperometric measurement on CHI660B electrochemistry system, with a beaker filled with 30 mL PBS electrolyte. Working electrode was washed by PBS for three times before next detection; when not in use, it was kept in moist environment of 4 °C. The sketch map of biosensor is shown in Fig.1.
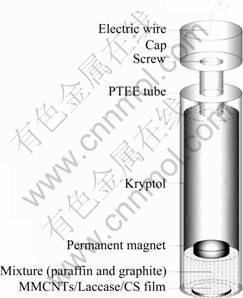
Fig.1 Sketch map of self-made laccase biosensor
3 Results and discussion
3.1 Characterization of MMCNT
Pristine MCNT is an insoluble substance. Routine acid treatment, temporarily dispersing in organic solvent [23], and polymer wrapping or grafting [24] are the common ways to solve the problem. However, these methods need either large amount of strong acid or complicated operation procedure. Researches showed that MCNT has a unique sidewall curvature and possesses super hydrophobic surface conjugated π electronic structure, which allowed it to interact with some aromatic compounds [25]. As an aromatic compound, TB can assemble on the MCNT surface by interacting with MCNT by π-π electron stacking and van der Waals’ force. The solubility of MCNT took great change after being treated by TB, and this solubility state can be kept for 4 h.
As can be seen from the micrographs of SEM, the pristine MCNT is desultory (Fig.2(a)). After being treated by TB and fully reacted with ferrous/ferric solution, magnetic nanoparticles are formed (arrow shown in Fig.2(b)) and deposited on the surface of MCNT irregularly. By comparing Fig.2(a) with Fig.2(b), the configuration of MCNT does not take obvious changes except that the latter has better dispersion, indicating that the nano-structure of MCNT is unchanged. The magnetic field strength of MMCNT was tested by VSM, as shown in Fig.2(c). It presents a typical magnetic hysteresis loop of soft magnetic material. The magnetism is 30.07 emu/g, which was analyzed by Origin7.5 software. This value accords well with the magnetism of magnetic nanoparticle made by coprecipitation [26]. It is indicated that magnetic iron oxide nanoparticle is successfully deposited on MCNT and the method for preparing the MMCNT is feasible. In the following experiments, MMCNT can be tightly immobilized on the working electrode by paramagnetism.
3.2 Optimization of experimental conditions
The pH value is an important parameter for enzyme biosensor as it significantly influences the activity of enzyme. Laccase is a multicopper oxidase. For different sources, the optimal pH values are different. Figure 3 presents the amperometric response of 100 μmol/L catechol in the pH range 4.0-6.8 (each value is obtained from 3 repeated tests, with error bars shown in the figure). In the pH range of 4.0-5.6, the current response keeps increasing with the rising of pH value until it reaches the highest point, and then it decreases sharply when the pH value continues to increase. At pH 6.8, the current response is only 22% of that at pH 5.6. Before the current response reaches the top, the increase is attributed to the enhancement of the enzyme activity. When pH>5.6, its decrease is owned to the involvement of protons in the reduction reaction of o-quinone. The optimum biosensor response is achieved at pH 5.6, which is consistent with the optimum pH range of 5.0- 6.0 for laccase [17]. This suggests that the immobilization process does not influence the activity of laccase significantly. Therefore, a 67 mmol/L PBS with pH of 5.6 is selected as the supporting electrolyte in the present work.
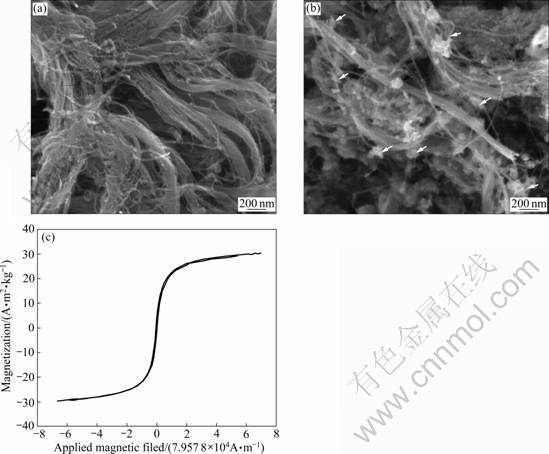
Fig.2 Scanning electron micrographs of pristine MCNTs (a) and MMCNT (b) and magnetic hysteresis loop of MMCNT (c)
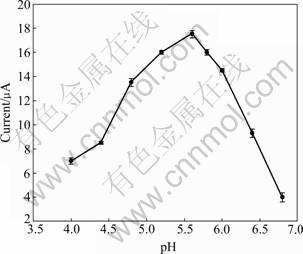
Fig.3 Effect of pH on current response of 100 μmol/L catechol in 67 mmol/L PBS (Working potential: -0.052 V vs SCE)
Working potential has significant effect on the performance of electrochemical biosensor. In this work, the principle of the laccase biosensor is based on the amperometric detection of the enzymatic product o-quinone, which is generated during the laccase catalyzed oxidation of catechol in the presence of dissolved oxygen. However, the oxidation and deoxidation peaks are not distinct in the pictures given by CV. To confirm the optimum working potential for the biosensor operation, current-time measurement is carried out with 100 μmol/L catechol detected at working potential ranging from 0.4 V to -0.4 V. The highest ratio of signal-to-background current is obtained at -0.052 V and the current response is steady (data are not shown). Therefore, -0.052 V is chosen as the working potential for the amperometric measurements. The reason for getting such a low working potential may be attributed to the excellent electrical catalytic capability of the MMCNT, along with the fact that TB/MCNT plays the role of electron mediators and electrocatalyst by forming a special three-dimensional network structure on the surface of the electrode [27], which is good for the transfer of substrates. Thus, under this working potential, the analytical system is less exposed to the interference from several other components, mainly in complex matrixes.
3.3 Characterizations of biosensor under CV
Figure 4 shows the typical cyclic voltammograms of naked magnetic carbon paste electrode (Curve 1), MMCNT modified electrode (Curve 2) and MMCNT/ laccase/CS electrode (Curve 3). As can be seen, the current response range of naked electrode is very narrow while the electrode modified by MMCNT presents a wider range (Curve 1, nearly from -1 500 μA to 1 500 μA). The results demonstrate that the MMCNT dramatically enhances the speed of electron transfer between reagents and electrode, resulting in a larger response range. The phenomenon can be attributed to the cooperative effect of MCNT and magnetic nanoparticles, for MCNT owns excellent electrical conductivity and Fe3O4 nanoparticles have remarkable properties such as conductivity and biocompatibility. When laccase solution and CS sol are cast on the MMCNT modified electrode, the current response range is narrowed down, which suggests a slight decline of conductivity of the biosensor, as shown on Curve 3. The main reasons come from the poor electrical conductibility of laccase and the resistance of chitosan/silica composite, which would hinder the transfer of electron.
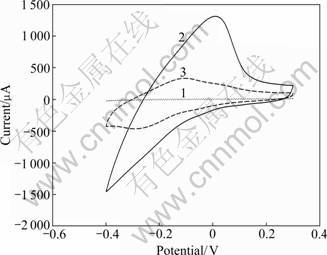
Fig.4 Cyclic voltammograms of naked electrode (Curve 1), MMCNT modified electrode (Curve 2) and MMCNT/laccase/ CS coated electrode (Curve 3) in 67 mmol/L PBS (pH 5.6) (Working potential range: from 0.4 V to -0.4 V vs SCE; Scanning rate: 50 mV/s)
3.4 Biosensor used for determination catechol
Catechol is a toxic organic compound characterized by mutagenicity [28] and is often produced in industry. Because of its non-polar structure, it can be easily absorbed by cells but difficult to be discharged, resulting in accumulation in the body. Additionally, its oxidised product is o-quinone, which is able to react with some important biomolecules, such as protein, DNA and liposome [29]. Many biosensors have already been developed to detect catechol. Analytical characteristics of some catechol biosensors are summarized in Table 1.
In this work, the principles of the enzymatic reaction are as follows:
catechol+O2![]() o-quinone+H2O (1)
o-quinone+H2O (1)
o-quinone+2H++2e-→catechol (at electrode) (2)
By determinating the reduction current of o-quinone, the concentration of catechol can be obtained. The result is presented in Fig.5. Compared with some recently reported biosensors for catechol (Table 1), the linear range of this biosensor is wider. The behavior may be explained by the higher loading of laccase in the MMCNT that has large surface area. Although the sensitivity is lower than that in the previous report [33], its stability is better. This is possible due to the chitosan/silica hybrid membrane, which could effectively prevent the leaking of laccase from the biosensor.
As shown on chronoamperometric curve, the biosensor responds rapidly and 98% of the steady state current is reached within 50 s. The fast response is ascribed to the rapid diffusion of catechol from bulk solution to the immobilized laccase. The response current is linear with the catechol concentration from 10-7 to 0.165×10-3 mol/L with the correlation coefficient of 0.999 and detection limit of 3.34×10-8 mol/L:
I=0.279 1 c+0.257 4 (3)
where I is the response current (μA) and c is the catechol concentration (μmol/L). The wide linear range is able to meet the phenol wastewater detection, suggesting its potential application in environment.
Table 1 Comparison of analytical characteristics of some catechol biosensors reported in literature in recent years
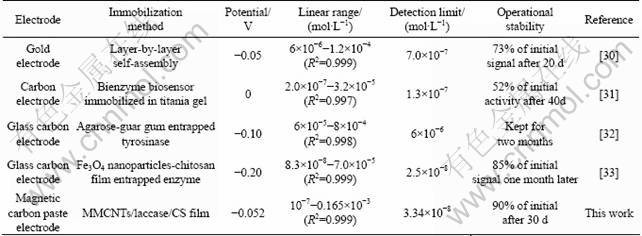
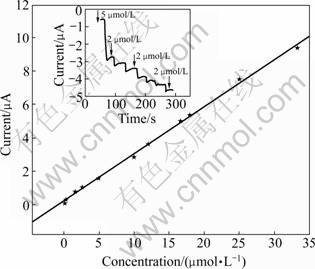
Fig.5 Calibration curve of biosensor for catechol determined in PBS (67 mmol/L, pH 5.6) with working potential of -0.052 V vs SCE (Scanning rate: 50 mV/s) (Inset shows chronoamperometric curve)
3.5 Repeatability and stability of biosensor
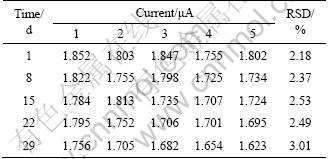
The repeatability and stability of the biosensor are checked by determination of 4 μmol/L catechol for a month. As exhibited in Table 2, at an interval of every 7 d, 4 μmol/L catechol is determined for five times. The relative standard deviation (RSD≤3.01%) of the results indicates the reliable repeatability of the biosensor.
Table 2 Response current of 4 μmol/L catechol at different time
When not in use, the working electrode is stored in a moist environment of 4 °C. The response current is around 95% of its original after 29 d; moreover, the enzyme electrode keeps 90% of its original response current ![]() later. This demonstrates that the immobilization method is able to retard the denaturation of laccase. The good repeatability and stability mainly owe to the microenvironment of biosensor, which has good biocompatibility for laccase existing. Besides, the MMCNT has a function of absorbing protein [34]. During the constructing of the biosensor, the laccase is immobilized without any chemical modifications, avoiding the adverse influences on its conformation. As a biomaterial, chitosan is easy to form film; but in this work, the electrolyte is PBS of pH 5.6, which is not fit for chitosan to keep steady film under acid condition. Therefore, TEOS is used to form organic/inorganic hybrid membrane with chitosan by hydrolysis and polycondensation reaction. The organic/inorganic film possesses good flexibility of chitosan and stability of TEOS, making it suitable for fabrication of biosensor [35-36]. Besides, the chitosan/silica hybrid membrane allows small molecular compounds (such as O2, H2O, catechol) to get through, but prevents laccase from leak, resulting in the good repeatability and stability.
later. This demonstrates that the immobilization method is able to retard the denaturation of laccase. The good repeatability and stability mainly owe to the microenvironment of biosensor, which has good biocompatibility for laccase existing. Besides, the MMCNT has a function of absorbing protein [34]. During the constructing of the biosensor, the laccase is immobilized without any chemical modifications, avoiding the adverse influences on its conformation. As a biomaterial, chitosan is easy to form film; but in this work, the electrolyte is PBS of pH 5.6, which is not fit for chitosan to keep steady film under acid condition. Therefore, TEOS is used to form organic/inorganic hybrid membrane with chitosan by hydrolysis and polycondensation reaction. The organic/inorganic film possesses good flexibility of chitosan and stability of TEOS, making it suitable for fabrication of biosensor [35-36]. Besides, the chitosan/silica hybrid membrane allows small molecular compounds (such as O2, H2O, catechol) to get through, but prevents laccase from leak, resulting in the good repeatability and stability.
3.6 Interference studies
Figure 6 shows the effect of interferents on the laccase biosensor for catechol. The first bar shows the current change gotten from 10 μmol/L catechol. The remaining bars show the current change for the mixture of catechol (10 μmol/L) and interferent (10 μmol/L). Cate and inter 1, 2, 3, 4 stand for catechol, phenol, guaiacol, vanillin and N,N-dimethylaniline, respectively. The degree of interference is calculated according to the following equation:
i=|I0-In|/In (4)
where i is the percent of interference; I0 and In stand for the response currents of 10 μmol/L catechol and interferent, respectively. The maximum relative response current change increases to 1.19% for guaiacol, which may be attributed to the high similar chemical structure of catechol and guaiacol. However, the relative responses obtained from most of these interferents are found to be negligible (<1.19%). Therefore, the proposed laccase biosensor exhibits the ability to reduce the influences of possible interferences and can be used to selectively determine catechol without interference.
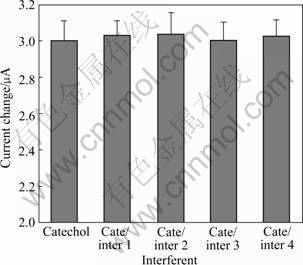
Fig.6 Effect of different interferents on response of laccase biosensor for catechol
4 Conclusions
1) The MMCNT combining CS hybrid membrane is successfully applied to immobilize laccase on magnetic carbon paste electrode, and the multilayer structure of MMCNT/laccase/CS hybrid membrane provides a microenvironment to maintain the bioactivity of laccase, ensuring the long life and the stability of biosensor.
2) The biosensor shows satisfied performances for detection of catechol, under optimal conditions of pH 5.5 and working potential of -0.052 V, and it exhibits a wide detection range from 10-7 to 0.165×10-3 mol/L with the detection limit of 3.34×10-8 mol/L. In terms of reproducibility, the RSD≤3.01%, and among the interferents of phenol, guaiacol, vanillin and N,N-dimethylaniline, the interference is less than 1.2%. Therefore, it might be feasible for applications in relation to the detection of catechol.
3) The proposed strategy for constructing laccase biosensor is simple, rapid and has low cost. It only takes 50 s to reach response equilibrium and the results are reliable, when being used for detection of catechol. Therefore, the methodology employed in this work might be applied for fabrication other biosensors based on oxidases, such as biosensor for cholesterol, lactate, alcohol and glucose.
References
[1] VARSAMIS D G, Touloupakis E, Morlacchi P, Ghanotakis D F, Giardi M T, Cullen D C. Development of a photosystem II-based optical microfluidic sensor for herbicide detection [J]. Talanta, 2008, 77(1): 42-47.
[2] TANG Lin, ZENG Guang-ming, SHEN Guo-li, LI Yuan-ping, LIU Can, LI Zhen. Sensitive detection of lip genes by electrochemical DNA sensor and its application in polymerase chain reaction amplicons from phanerochaete chrysosporium [J]. Biosensor and Bioelectronics, 2009, 24(5): 1474-1479.
[3] WANG S G, QING Zhan-ga, WANG Rui-li, YOON S F. A novel multi-walled carbon nanotube-based biosensor for glucose detection [J]. Biochemical and Biophysical Research Communications, 2003, 311(3): 572-576.
[4] TANG Lin, ZENG Guang-ming, SHEN Guo-li, LI Yuan-ping, ZHANG Yi, HUANG Dan-lian. Rapid detection of picloram in agricultural field samples using a disposable immunomembrane- based electrochemical sensor [J]. Environmental Science and Technology, 2008, 42(4): 1207-1212.
[5] TKAC J, NAVRATIL M, STURDIK E, GEMEINER P. Monitoring of dihydroxyacetone production during oxidation of glycerol by immobilized Gluconobacter oxydans cells with an enzyme biosensor [J]. Enzyme and Microbial Technology, 2001, 28(4/5): 383-388.
[6] CRESPILHO F N, GHICA M E, CARIDADE C G, OLIVEIRA O N J, BRETT C M A. Enzyme immobilization on electroactive nanostructured membranes (ENM): Optimised architectures for biosensing [J]. Talanta, 2008, 76(4): 922-928.
[7] Freire R S, Duran N, Kubota L T. Effects of fungal laccase immobilization procedures for the development of a biosensor for phenol compounds [J]. Talanta, 2001, 54(4): 681-686.
[8] FU Guang-lei, YUE Xiu-li, DAI Zhi-fei. Glucose biosensor based on covalent immobilization of enzyme in sol-gel composite film combined with Prussian blue/carbon nanotubes hybrid [J]. Biosensors and Bioelectronics, 2011, 26(9): 3973-3976.
[9] Tiwari A, Aryal S, Pilla S, Gong S Q. An amperometric urea biosensor based on covalently immobilized urease on an electrode made of hyperbranched polyester functionalized gold nanoparticles [J]. Talanta, 2009, 78(4/5): 1401-1407.
[10] Guerrieri A, Benedetto G E, Palmisano F, Zambonin P G. Electrosynthesized non-conducting polymers as permselective membranes in amperometric enzyme electrodes: A glucose biosensor based on a co-crosslinked glucose oxidase/overoxidized polypyrrole bilayer [J]. Biosensor and Bioelectronics, 1998, 13(1): 103-112.
[11] Barbadillo M, Casero E, Petit D M D, Vazquez L, Pariente F, Lorenzo E. Gold nanoparticles-induced enhancement of the analytical response of an electrochemical biosensor based on an organic-inorganic hybrid composite material [J]. Talanta, 2009, 80(1): 797-802.
[12] LI Yang, LIU Xiao-yan, YUAN Hong-yan, XIAO Dan. Glucose biosensor based on the room-temperature phosphorescence of TiO2/SiO2 nanocomposite [J]. Biosensor and Bioeletronics, 2009, 24(12): 3706-3710.
[13] Rossi A M, Wang L L, Rabbi V, Murphy T E. Porous silicon biosensor for detection of viruses [J]. Biosensor and Bioelectronics, 2007, 23(5): 741-745.
[14] Luais E, Thobie G C, Tailleur A, Djouadi M A, Granier A, Tessier P Y, Debarnot D, Poncin E F, Boujtita M. Preparation and modification of carbon nanotubes electrodes by cold plasmas processes toward the preparation of amperometric biosensors [J]. Electrochimica Acta, 2010, 55(26): 7916-7922.
[15] DENG Chun-yan, CHEN Jin-hua, NIE Zhou, SI Shi-hui. A sensitive and stable biosensor based on the direct electrochemistry of glucose oxidase assembled layer-by-layer at the multiwall carbon nanotube-modified electrode [J]. Biosensors and Bioelectronics, 2010, 26(1): 213-219.
[16] Wisitsoraat A, Sritongkham P, Karuwan C, Phokharatkul D, Maturos T, Tuantranont A. Fast cholesterol detection using flow injection microfluidic device with functionalized carbon nanotubes based electrochemical sensor [J]. Biosensors and Bioelectronics, 2010, 26(4): 1514-1520.
[17] Zhang Yi, Zeng Guang-ming, Tang Lin, Huang Dan-lian, Jiang Xiao-yun, Niu Chen-gang. A hydroquinone biosensor using modified core-shell magnetic nanoparticles supported on carbon paste electrode [J]. Biosensor and Bioelectronics, 2007, 22(9/10): 2121-2126.
[18] Ho K C, Tsai P Y, Lin Y S, Chen Y C. Using biofunctionalized nanoparticles to probe pathogenic bacteria [J]. Analytical Chemistry, 2004, 76(24): 7162-7168.
[19] Kouassi G K, Irudayaraj J. Magnetic and gold-coated magnetic nanoparticles as a DNA sensor [J]. Analytical Chemistry, 2006, 78(10): 3234-3241.
[20] Liang Yuan-yuan, Zhang Li-ming. Bioconjugation of papain on superparamagnetic nanoparticles decorated with carboxymethylated chitosan [J]. Biomacromolecules, 2007, 8(5): 1480-1486.
[21] LIU Ying, LEI Jian-ping, JU Huang-xian. Amperometric sensor for hydrogen peroxide based on electric wire composed of horseradish peroxidase and toluidine blue-multiwalled carbon nanotubes nanocomposite [J]. Talanta, 2008, 74(4): 965-970.
[22] QU Song, WANG J, KONG Jie-lie, YANG Peng-yuan, CHEN Guang. Magnetic loading of carbon nanotube/nano-Fe3O4 composite for electrochemical sensing [J]. Talanta, 2007, 71(3): 1096-1102.
[23] YAN Xu-xu, PANG Dai-wei, LU Zhe-xue, LU Jian-quan, TONG Hua. Electrochemical behavior of L-dopa at single-wall carbon nanotube-modified glassy carbon electrodes [J]. Journal of Electroanalytical Chemistry, 2004, 569(1): 47-52.
[24] Santhosh P, Manesh K M, Gopalan A, Lee K P. Fabrication of a new polyaniline grafted multi-wall carbon nanotube modified electrode and its application for electrochemical detection of hydrogen peroxide [J]. Analytica Chimica Acta, 2006, 575(1): 32-38.
[25] WU Fang-hui, ZHAO Guang-chao, WEI Xian-wen. Electrocatalytic oxidation of nitric oxide at multi-walled carbon nanotubes modified electrode [J]. Electrochemistry Communications, 2002, 4(9): 690- 694.
[26] QU Sheng-chun, YANG Hai-bin, REN Da-wei, KAN Shi-hai, ZOU Guang-tian. Magnetite nanoparticles prepared by precipitation from partially reduced ferric chloride aqueous solutions [J]. Journal of Colloid and Interface Science, 1999, 215(1): 190-192.
[27] ZHANG Mao-gen, Gorski W. Electrochemical sensing based on redox mediation at carbon nanotubes [J]. Analytical Chemistry, 2005, 77(13): 3960-3965.
[28] YANG Da-peng, JI Hong-fang, TANG Guang-yan, REN Wei, ZHANG Hong-yu. How many drugs are catecholics [J]. Molecules, 2007, 12(4): 878-884.
[29] Sies H. Oxidative stress: Oxidants and antioxidants [J]. Experimental physiology, 1997, 82(7): 291-295.
[30] YANNG Shao-ming, LI Yang-mei, JIANG Xiu-ming, CHEN Zhi-chun, LIN Xian-fu. Horseradish peroxidase biosensor based on layer-by-layer technique for the determination of phenolic compounds [J]. Sensors and Actuators B: Chemical, 2006, 114(2): 774-780.
[31] Kochana J, Nowak P, Wilkolazka A J, Bieroń M. Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds [J]. Microchemical Journal, 2008, 89(2): 171-174.
[32] Tembe S, Inamdar S, Haram S, Karve M, Souz S F. Electrochemical biosensor for catechol using agarose–guar gum entrapped tyrosinase [J]. Journal of Biotechnology, 2007, 128(1): 80-85.
[33] WANG Sheng-fu, TAN Yu-mei, ZHAO Dong-ming, LIU Guo-dong. Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles-chitosan nanocomposite [J]. Biosensor and Bioelectronics, 2008, 23(12): 1781-1787.
[34] Banks C E, Compton RG. Exploring the electrocatalytic sites of carbon nanotubes for NADH detection: An edge plane pyrolytic graphite electrode study [J]. Analyst, 2005, 130(9): 1232-1239.
[35] JIA Jian-bo, WANG Bing-quan, WU Ai-guo, CHENG Guang-jin, LI Zhuang, DONG Shao-jun. A method to construct a third-generation horseradish peroxidase biosensor: Self-assembling gold nanoparticles to three-dimensional sol-gel network [J]. Analytical Chemistry, 2002, 74(9): 2217-2223.
[36] LEI Cun-xi, HU Shun-qin, SHEN Guo-li, YU Ru-qin. Immobilization of horseradish peroxidase to a nano-Au monolayer modified chitosan-entrapped carbon paste electrode for the detection of hydrogen peroxide [J]. Talanta, 2003, 59(5): 981-988.
(Edited by HE Yun-bin)
Foundation item: Project(IRT0719) supported by the Program for Changjiang Scholars and Innovative Research Team in University, China; Projects (50978088, 51039001) supported by the National Natural Science Foundation of China; Project(2009FJ1010) supported by the Hunan Key Scientific Research Program, China; Project(10JJ7005) supported by the Natural Science Foundation of Hunan Province, China; Projects(CX2009B080, CX2010B157) supported by the Hunan Provincial Innovation Foundation For Postgraduate; Project supported by the Fundamental Research Funds for the Central Universities, Hunan University, China
Received date: 2010-12-22; Accepted date: 2011-04-28
Corresponding author: ZENG Guang-ming, Professor, PhD; Tel: +86-731-88822754; E-mail: zgming@hnu.cn

