Cu-Ni-Pb-Zn-Cl-H2O体系的热力学分析
赵中伟,石玉臣,陈爱良,霍广生,李洪桂
(中南大学 冶金科学与工程学院 稀有金属冶金与材料制备湖南省重点实验室,湖南 长沙,410083)
摘 要:结合已有的热力学数据,对镍阳极液的Cu-Ni-Pb-Zn-Cl-H2O体系进行热力学平衡计算,绘制在298.15 K时Cu-Ni-Pb-Zn-Cl-H2O体系中不同金属离子和各级配离子的浓度与Cl-浓度和pH的关系图。研究结果表明:当pH为4.5时,镍阳极液中镍、铜主要以游离态离子和配合阳离子形式存在,铅、锌主要以配合阴离子形式存在,因此,可以采用阴离子树脂交换除去其中的铅、锌;提高溶液中氯离子的浓度有利于金属配合离子的形成,使铅锌的配合阴离子含量增多,有利于采用阴离子树脂交换除铅、锌;镍阳极液经过D201树脂交换除锌和D363树脂交换除铅后,铅质量浓度降至0.32 mg/L以下,锌质量浓度降至0.30 mg/L以下,达到了镍电解精炼的要求。
关键词:Cu-Ni-Pb-Zn-Cl-H2O系;镍阳极液;热力学
中图分类号:TQ 021.2 文献标志码:A 文章编号:1672-7207(2010)05-1680-06
Thermodynamic analysis of Cu-Ni-Pb-Zn-Cl-H2O system
ZHAO Zhong-wei, SHI Yu-chen, CHEN Ai-liang, HUO Guang-sheng, LI Hong-gui
(Key Laboratory of Hunan Province for Metallurgy and Material Processing of Rare Metals,
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The thermodynamic equilibrium of Cu-Ni-Pb-Zn-Cl-H2O system for nickel anode electrolyte was calculated according to the known thermodynamic data, and the c-c[Cl]T and c-pH diagrams for Cu-Ni-Pb-Zn-Cl-H2O system were drawn at 298.15 K. The results show that, in nickle electrolysis anode solution, the occurrence states of copper and nickel are free ions and complex cations at pH 4.5, while the lead and zinc exist as complex anions that can be removed by anion exchange resin. In addition, the amount of complex anions of lead and zinc are increased with the improvement of Cl- concentration, which is beneficial to the removing process of lead and zinc. The results of verification experiments for thermodynamic analysis demonstrate that the concentration of lead and zinc can be decreased to 0.32 mg/L and 0.30 mg/L by ion exchange technic, respectively.
Key words: Cu-Ni-Pb-Zn-Cl-H2O system; nickel anode electrolyte; thermodynamics
镍电解阳极液是复杂的Cu-Ni-Pb-Zn-Cl-H2O体系,电镍产品对杂质特别是铅、锌的要求极为严格。为防止铅、锌在电解过程中与镍一起在阴极析出,必须在阳极液净化过程中除去微量的杂质铅、锌,以保证电镍产品质量。许多学者进行了大量的研究工作,提出了很多阳极液除杂方法[1-3]。但由于目前缺乏针对该复杂体系的理论研究,很难从理论上完整地解释各种离子的行为。在此,本文作者引入活度对该体系进行热力学计算,充分考虑各级配位反应,计算平衡时各离子的形态与平衡浓度,获取溶液中各离子平衡浓度与氯离子浓度和pH的关系,并结合文献采用离子交换法对计算结果进行实验验证。采用离子交换法将阳极液中铅、锌除去,使溶液达到镍电解精炼的要求。
1 Cu-Ni-Pb-Zn-Cl-H2O系热力学计算
镍电解阳极液成分如表1所示。由于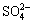 和H3BO3难于与铜、镍等离子形成配合物,不考虑其与金属离子的配位反应。为简化计算,只考虑溶液中镍、铜、铅、锌离子与水溶液中的OH-和Cl-形成配位化合物的情况。
和H3BO3难于与铜、镍等离子形成配合物,不考虑其与金属离子的配位反应。为简化计算,只考虑溶液中镍、铜、铅、锌离子与水溶液中的OH-和Cl-形成配位化合物的情况。
表1 镍电解阳极液浓度
Table 1 Components of nickel electrolysis anolyte mol/L

1.1 阳极液中配合离子浓度计算
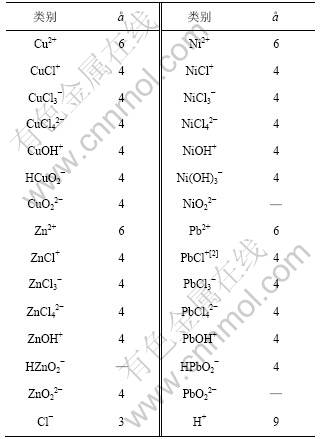
溶液中配合离子的形成使自由金属离子的活度降低。在一定条件下,自由金属离子的活度以及形成的各级配合离子的浓度可以应用配合离子的各级稳定常数进行近似的计算。在同时含有Ni,Cu,Zn和Pb的镍电解阳极液中,其Ni,Cu,Zn和Pb离子均有可能与Cl-形成各种配合物。现查得各反应生成相应配合物的累积平衡常数β(βMei代表Me离子i级配合物累积平衡常数,其中,Me表示金属离子)如表2所示[4-7]。
对于配位反应Mei++iL-=MeLi(式中i=1,2,3,4),其累积稳定常数为:
 (1)
(1)
设[Cu]T,[Ni]T,[Pb]T,[Zn]T和[Cl]T分别为铜、镍、铅、锌和氯的总浓度,根据质量守恒定律和同时平衡原理,Cu,Ni,Pb,Zn和Cl自由离子及其配合物的浓度总和为:
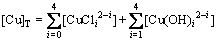 (2)
(2)
 (3)
(3)
表2 镍、铜、铅、锌各级配合物累积平衡常数[5-7]
Table 2 Coordination constants of nickel-ligand, copper-ligand, lead-ligand, zinc-ligand at 298.15 K

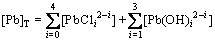 (4)
(4)
 (5)
(5)
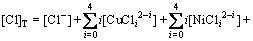
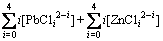 (6)
(6)
根据溶液中正负离子电荷平衡原理,有:
[H+]+[Na+]+[CuCl+]+[CuOH+]+[NiCl+]+[NiOH+]+[PbCl+]+[PbOH+]+[ZnOH+]+[ZnCl+]+2([Cu2+]+[Ni2+]+
[Pb2+]+[Zn2+])=[Cl]+[OH]+[HCuO2]+[CuCl3]+
[Ni(OH)3]+[NiCl3]+[PbCl3]+[HPbO2]+[ZnCl3-]+[HZnO2-]+2([SO42-]+[CuO22-]+[PbO22-]+[ZnO22-]) (7)
根据公式计算溶液的离子强度为:
 (8)
(8)
式中:zi为离子i的价数;mi为离子i的质量摩尔浓度。
根据德拜-休克公式,溶液中各离子的活度系数为:
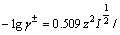 (1+B
(1+B )
)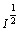 (9)
(9)
式中:z为溶液中各离子所带电荷数; 为溶液中各离子半径。溶液中各离子的半径
为溶液中各离子半径。溶液中各离子的半径 见表3。
见表3。
表3 溶液中各离子半径 [8]
[8]
Table 3 Ionic radius in solusion 10-9 m
随着溶液pH升高,金属离子会生成难溶电解质而沉淀,此时,溶液中金属自由离子的浓度应该由溶液中难溶电解质的容度积常数Ksp来确定。各难溶电解质的Ksp如表4所示。
表4 难溶电解质的溶度积常数Ksp
Table 4 Ksp of indiscerptible electrolyte

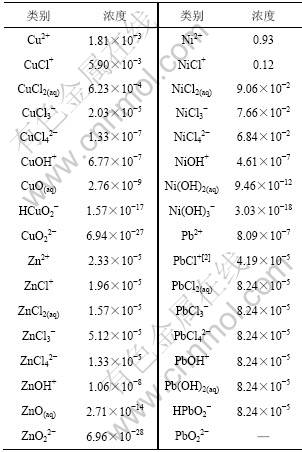
显然,在计算过程中有一个矛盾,即在不知道溶液中水溶物种分布的情况下,便无法得知溶液的离子强度,从而无法正确选择数据,无法进行正确的计算。本文参考Wang等[8-10]提出的热力学分析方法,进一步优化赵中伟等[11-14]提出的平衡浓度计算法来克服这一困难,即先求出溶液的表观离子强度I,以此为起点,算出各物种的平衡浓度,然后,据此计算溶液的离子强度,再选用此离子强度下的数据计算各物种的平衡浓度。经过如此多次运算,逐步逼近,直到前后2次运算的结果误差足够小(≤0.1‰)为止。镍电解在pH为4.5左右进行。pH为4.5的镍电解阳极液中各离子的平衡浓度如表5所示。
表5 pH=4.5时镍电解阳极液中各离子的平衡浓度
Table 5 Balance concentration of ions in nickel anodic electrolyte at pH=4.5 mol/L
1.2 镍电解液中各离子浓度与[Cl-]T关系
在温度为298.15 K,pH为4.5时,据表1,按上述方法计算得到各离子浓度c与氯离子浓度cClT的关系如图1所示(其中,部分配离子浓度过低,所以在图中被省略)。
1.3 镍电解液中各离子浓度与pH关系
在298.15 K时,据表1,按上述方法计算得到的阳极液中各离子浓度c与pH的关系如图2所示(其中,部分配离子浓度过低,所以在图中被省略)。
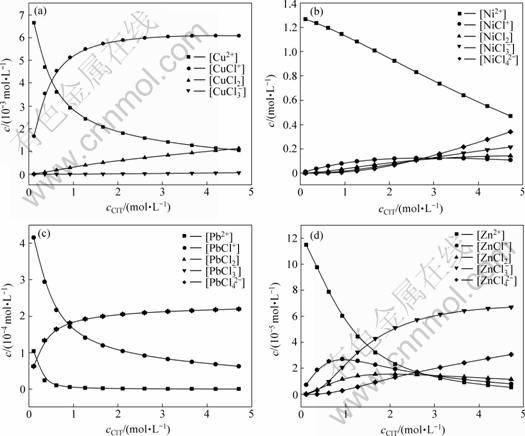
图1 Cl-浓度cClT对各配合离子浓度c的影响
Fig.1 Effects of Cl- concentration on complex anions
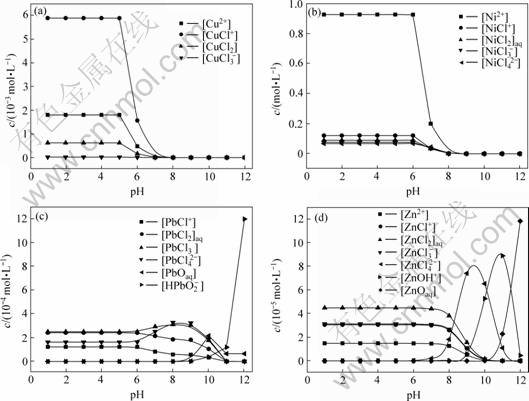
图2 镍电解阳极液中铜、镍、铅、锌各级配合离子浓度c与pH的关系
Fig.2 Relationship between concentration of complex anions and pH value in nickel anodic electrolyte system
2 分析和验证
从图1可以看出:随着cClT的增大,溶液中金属自由离子Cu2+的浓度呈明显的下降趋势。这是因为cClT增大,溶液中配合金属离子总体能力增强,在金属总含量不变的情况下,与氯的配合离子浓度增加,金属自由离子减少。
从图2可以看出:当pH超过7时,各[Me2+]和[MeCli2-i]迅速降低。这主要是pH增大,OH-对金属自由离子的配合能力增强,[Me(OH) i2-i]增加。此外,该体系中铅具有不同于铜、锌、镍的特性,即其主要配合物浓度随pH的变化不大。
从表5可以看出:在温度为298.15 K,pH为4.5时,镍电解液中,镍和铜主要以配合阳离子的形式存在,而铅、锌主要以配合阴离子的形式存在,因此,可以采用阴离子交换树脂选择性地除去镍电解液中的铅、锌。赵中伟等[12]使用阴离子交换树脂从镍电解液中除铅锌以验证以上分析。原料为金川公司提供的除铜后液,图3所示为反应温度T=333.15 K,ρ0=4.20 mg/L,pH=3.48,接触时间为25 min时D201树脂吸附锌的流出曲线,其中,ρzinc为流出液中锌的质量浓度,V为流出液体积,VR为树脂体积,测得交后液中锌的质量浓度小于0.30 mg/L;图4所示为反应温度T=298.15 K,ρ0=2.80 mg/L,pH=2.67,接触时间为30 min时D363树脂吸附铅的流出曲线(其中,ρlead为流出液中铅的质量浓度)。测得交后液中铅的质量浓度小
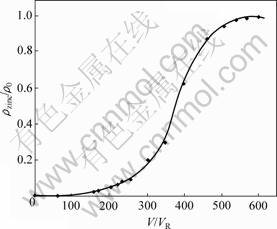
图3 D201交换吸附锌的流出曲线
Fig.3 Effuse curve of zinc in ion exchange process of D201 resin
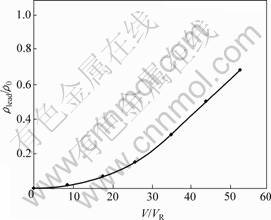
图4 D363交换吸附铅的流出曲线
Fig.4 Effuse curves of lead in ion exchange process of D363 resin
于0.32 mg/L。经过吸附,溶液中铅锌浓度完全达到镍电解精炼的要求。从图3和图4可知:铅锌被阴离子交换树脂吸附,因此,镍电解液中金属铅、锌主要是以配阴离子存在。
3 结论
(1) 引入活度概念对镍阳极液的Cu-Ni-Pb-Zn- Cl-H2O体系进行了热力学平衡计算,计算出298.15 K时不同离子浓度cClT和不同pH条件下的镍电解阳极液的物相分布,并根据计算结果绘制了298.15 K时Cu-Ni-Pb-Zn-Cl-H2O体系中不同金属自由离子和各级配离子的浓度与Cl-浓度和pH的关系图。
(b) 提高溶液中氯离子的浓度有利于铅、锌配合离子的形成,有利于采用阴离子树脂交换除铅、锌;在酸性体系下改变溶液pH对离子交换除杂工艺影 响小。
(3) 经过D201树脂交换除锌和D363交换除铅后,镍阳极液中铅质量浓度降至0.32 mg/L以下,锌质量浓度降至0.30 mg/L以下,达到了镍电解精炼的要求,证明了该热力学分析的准确性。
参考文献:
[1] Huang T C, Lin Y K, Chen C Y. Selective separation of nickel and copper from a complexing solution by a cation-exchange membrane[J]. Journal of Membrane Science, 1988, 37(2): 131-144.
[2] Lilga M A, Orth R J, Sukamto J P H, et al. Metal ion separations using electrically switched ion exchange[J]. Separation and Purification Technology, 1997, 11(3): 147-158.
[3] Caiteux J L H, Kampunzu A B, Lerouge C, et al. Genesis of sediment-hosted stratiform copper-cobalt deposits, central African Copperbelt[J]. Journal of African Earth Sciences, 2005, 42(1): 134-158.
[4] Hazra S, Majumder S, Fleck M, et al. Syntheses, structures, absorption and emission properties of a tetraiminodiphenol macrocyclic ligand and its dinuclear Zn(Ⅱ) and Pb(Ⅱ) complexes[J]. Polyhedron, 2009, 28: 2871-2878.
[5] Kong D, Martell A E, Motekaitis R J, et al. Two novel homodinuclear Ni(Ⅱ) and Cu(Ⅱ) complexes with a 24-membered octadentate hexaazamacrocyclic ligand: Stability and X-ray crystal structures[J]. Inorganica Chimica Acta, 2001, 317(1/2): 243-251.
[6] Gobi K V, Ohsaka T. Electrochemical and spectral properties of novel dinickel(Ⅱ) and dicopper(Ⅱ) complexes with N,N-linked bis(pentaazacyclotetradecane)[J]. Electrochimica Acta, 1998, 44(2/3): 269-278.
[7] Dean J A. 兰氏化学手册[M]. 尚久方, 译. 北京: 科学出版社, 1991: 1467-1532.
Dean J A. Langeps handbook of chemistry[M]. SHANG Jiu-fang, translation. Beijing: Science Press, 1991: 1467-1532.
[8] WANG Ming-sheng, ZHANG Yu, Muhammed M. Critical evaluation of thermodynamics of complex formation of metal ions in aqueous solutions I.A description of evaluation methods[J]. Hydrometallurgy, 1997, 45(1): 21-36.
[9] ZHANG Yu, Muhammed M. Critical evaluation of thermodynamics of complex formation of metal ions in aqueous solutions VI. Hydrolysis and hydroxo-complexes of Zn2+ at 298.15 K[J]. Hydrometallurgy, 2001, 60(3): 215-236.
[10] 马玉天, 龚竹青, 陈文汨, 等. 化学法制备高纯碲过程中杂质元素脱除的热力学分析[J]. 中南大学学报: 自然科学版, 2006, 37(2): 257-262.
MA Yu-tian, GONG Zhu-qing, CHEN Wen-mi, et al. Thermodynamic analysis of impurities removal in purification of tellurium by chemical method[J]. Journal of Central South University:Science and Technology, 2006, 37(2): 257-262.
[11] 赵中伟, 刘旭恒. Li-Fe-P-H2O系热力学分析[J]. 中国有色金属学报, 2006, 16(7): 1264-1269.
ZHAO Zhong-wei, LIU Xu-heng. Thermodynamic analysis of Li-Fe-P-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(7): 1264-1269.
[12] 赵中伟, 曹才放, 李洪桂. 碳酸钠分解白钨矿的热力学分析[J]. 中国有色金属学报, 2008, 18(2): 356-360.
ZHAO Zhong-wei, CAO Cai-fang, LI Hong-gui. Thermodynamics on soda decomposition of scheelite[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(2): 356-360.
[13] 张刚, 赵中伟, 李江涛, 等. 氢氧化钠分解钼酸铅矿的热力学分析[J]. 中南大学学报: 自然科学版, 2008, 39(5): 902-906.
ZHANG Gang, ZHAO Zhong-wei, LI Jiang-tao, et al. Thermodynamics analysis on sodium hydroxide decomposition of wulfenite[J]. Journal of Central South University: Science and Technology, 2008, 39(5): 902-906.
[14] 张贵清. 连续离子交换除镍电解液中微量铅锌的研究[D]. 长沙: 中南大学冶金科学与工程学院, 2003: 33-35.
ZHANG Gui-qing. Study on removing trace lead and zinc from nickel electrolyte by continuous ion exchange process[D]. Changsha: Central South University. School of Metallurgy Science and Engineering, 2003: 33-35.
(编辑 刘华森)
收稿日期:2009-11-23;修回日期:2010-03-15
基金项目:国家重点基础研究发展计划(“973”计划)项目(2007CB613603);新世纪优秀人才支持计划资助项目(NCET-05-0692)
通信作者:赵中伟(1966-),男,河北永年人,博士,教授,从事冶金、功能材料研究;电话:0731-88830476;E-mail: Zhaozw@mail.csu.edu.cn