
Hydro-chemical conversion of galena in FeCl3-KCl solution
LONG Huai-zhong(龙怀中)1, CHAI Li-yuan(柴立元)1, LIU Hui(刘 辉)2, QIN Wen-qing(覃文庆)2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 20 May 2009; accepted 10 August 2009
Abstract: The behaviours of complexation and dissolution of PbCl2 on the surface of galena were investigated to explore the process of hydro-chemical conversion of galena (PbS) in chloride media. By means of solution chemistry calculation, the production and dissolution of the products PbCl2 were studied. And the passivation of the galena was studied by Tafel curve. The results show that PbCl42- is the main form of PbCl2 presented in the saturated potassium chloride (KCl) solution. The PbCl2 crystal is easy to precipitate when the total concentration of chloride ion ([Cl-]T) is equal to 0.92 mol/L, and it is inclined to dissolve when [Cl-]T is more than 0.92 mol/L. The chloride complexing reaction rate strongly depends on the Fe3+ ion concentration when it is less than 6×10-4 mol/L, while passivation occurs on the surface of the electrode when Fe3+ concentration is larger than 6×10-4 mol/L. The reaction rate increases obviously when KCl is added, since the activity of Cl- increases; thus accelerates the dissolution of PbCl2.
Key words: FeCl3-KCl solution; galena; complexation; solution chemistry
1 Introduction
At present, pyro-metallurgy is still a main method in plumbery. However, traditional sintering technology as melting in the blast furnace, has many disadvantages, such as high energy consumption and serious environment pollution[1]. Recently, the basic study on a direct extract of Pb and preparation of material from original resource has been paid more attention to, with continuously increasing need of Pb and environment protection problem[2]. Chemistry extraction of Pb has attracted much attention because of its low energy consumption and friendly environment. Many methods have been reported in hydrometalurgy ways using FeCl3 [3], Fe2(SO4)3[4-5], silicofluoric acid[6], HNO3[7] and EDTA[8]. The study on extraction technique using FeCl3, especially the thermodynamics and dynamics in the transformation process[9-18] was mostly performed because of its high transformation speed, efficiency and easy manipulation. However, there was less report about the complexation and dissolution behavior of reaction production on the surface of galena (PbS). In this study, the complexation and dissolution of chloride on the surface of galena (PbS) in FeCl3-KCl media was investigated by means of solution chemistry calculation and corrosion electrochemistry.
2 Experimental
The galena used in this study consists of 82.20% Fe, 16.20% S and 1.6% SiO2, and was taken from Fankou Mine of Guangdong Province in China. Section cut out from the highly mineralized galena was fashioned into the form of electrodes for electrochemical measurement. The cut section of the mineral was mounted on the tip of a perspex tubule of d 7 mm using epoxy resin and the exposed outer surface was well polished. The exposed surface area of the electrode was about 1 cm2. FeCl3·6H2O and KCl were analytical reagents. A conventional three-electrode system was used, including a Ag/AgCl reference electrode, a platinum plate as the counter electrode and galena (PbS) as working electrode. An EG&GPARC Model 273 bi-potentiostat and 686 rotating disk electrode were used for electrochemistry experiments. The Tafel experiment was performed at room temperature (298 K) and normal atmosphere with a rotating speed of 500 r/min. The potentials throughout this study were referenced to standard hydrogen electrode(SCE).
3 Results and discussion
3.1 Solution chemistry on surface of galena in chloride medium
In FeCl3-KCl system, the surface of galena contacts with Fe3+ by the following reaction:
PbS(s)+2Fe3+(aq)+6Cl-(aq)→
PbCl2(s)+2Fe2+(aq)+4Cl-(aq)+S(s) (1)
The strong oxidation capability of PbCl+ and Fe3+ facilitates the fracture of Pb—S bond on the surface of galena. S2- is oxidized to S solid, and Pb(Ⅱ) combines with Cl- to form PbCl2 crystal adhering on the surface of galena. The following complexing equilibriums of PbCl2 crystal exist on the surface of galena in chloride medium:
PbCl2 Pb2++2Cl-,
Pb2++2Cl-,
 (2)
(2)
Pb2++Cl- PbCl+,
PbCl+,
 (3)
(3)
Pb2++2Cl- PbCl2(aq),
PbCl2(aq),
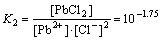 (4)
(4)
Pb2++3Cl-

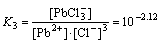 (5)
(5)
Pb2++4Cl-
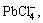
 (6)
(6)
Associating with Eqs.(2)-(6), the concentration of each component can be calculated by the following equations:
 (7)
(7)
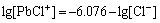 (8)
(8)
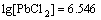 (9)
(9)
 (10)
(10)
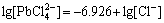 (11)
(11)
According to Eqs.(7)-(11), the logarithmic solubility curve of PbCl2 is drawn as shown in Fig.1. It can be seen that the decrease of Cl- concentration facilitates the dissolution of PbCl2. As a result, the concentration of Pb2+ increases, but the probability of Pb2+ complexation with Cl- to form high-coordination anion 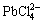 decreases because of the low concentration of Cl-. Until the concentrations of Pb2+ and
decreases because of the low concentration of Cl-. Until the concentrations of Pb2+ and 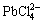 are equal, the system reaches the balance. On the contrary, if the concentration of Cl- increases, so that of Pb2+ decreases, and the complexation reaction takes place easily, resulting in high concentration of
are equal, the system reaches the balance. On the contrary, if the concentration of Cl- increases, so that of Pb2+ decreases, and the complexation reaction takes place easily, resulting in high concentration of 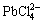 . As a result,
. As a result, 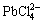 is in a dominant position when the concentration of Cl- is high in the solution. So, in the FeCl3-KCl system with saturated KCl medium, PbCl2 exists as a form of complex
is in a dominant position when the concentration of Cl- is high in the solution. So, in the FeCl3-KCl system with saturated KCl medium, PbCl2 exists as a form of complex 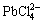 .
.
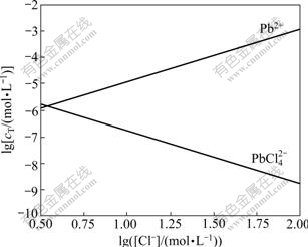
Fig.1 Logarithmic solubility diagram of lead chloride at 25 ℃
In order to study the formation and dissolution of PbCl2 in KCl medium, the total concentrations of Pb2+ ([Pb2+]T) and Cl- ([Cl-]T) were calculated by the following equations that depended on -lg[Cl-].
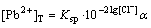 ,
,
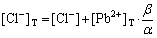 (12)
(12)
where
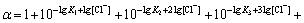
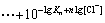
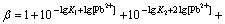
 (13)
(13)
If n=4, the total concentration of Pb2+ can be calculated from the following equation:
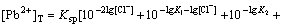
 (14)
(14)
The best point for producing PbCl2 is estimated by
differentiating Eq.(14). Assuming  we
we
have
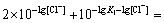

Approximately, it can be written as
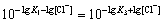
As a result, 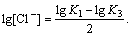
The solubility of PbCl2 at different values of lg[Cl-] could be calculated by Eqs.(12) and (13), and the results are listed in Table 1.
Table 1 Calculation results of solubility of PbCl2
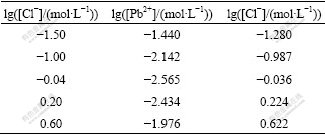
As a result, the relationship of lg[Pb2+] and lg[Cl-] with lg[Cl-] is shown in Fig.2.
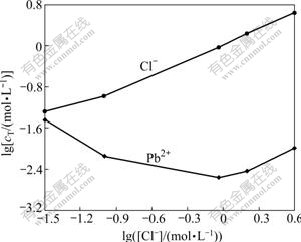
Fig.2 Relationship of lg[Pb2+]T and lg[Cl-]T with lg[Cl-] at 25 ℃
As can be seen in Fig.2, the total concentration of Cl- increases with increasing lg[Cl-], while that of Pb2+ decreases. When the value of lg([Cl-]/(mol?L-1)) is -0.04 and the total concentration of Cl- is 0.92 mol/L, the concentration of Pb2+ reaches the minimal value of 0.002 8 mol/L, then the equilibrium point of production and dissolution occurs. When the value of lg([Cl-]/(mol?L-1)) is lower than -0.04, the dissolution rate of PbCl2 is less than that of production, so the production reaction is dominant. When the value of lg([Cl-]/(mol?L-1)) is larger than -0.04, the dissolution rate of PbCl2 is more than that of production, indicating that at a high concentration of Cl-, the complexation reaction of Pb2+ with Cl- is dominant, and the dissolution rate of PbCl2 becomes rapid. As a result, the surface of galena can contact with Fe3+ and dissolve continuously.
3.2 Corrosion electrochemistry on surface of galena in chloride medium
Galena has the character of semiconductor. Actually in chloride medium, galena (PbS) reacts with Fe3+ as a corrosion primary cell. In this study, the corrosion electrochemistry technique, Tafel curve was used to investigate the corrosion and dissolution behavior of galena in chloride medium.
On the surface of electrode in FeCl3-KCl solution system, the following reactions occur:
Anode
PbS(s)+2Cl-→PbCl2(s)+S0(s)+2e (15)
Cathode
2Fe3+(aq)→2Fe2+(aq)-2e (16)
The Tafel curve test was carried out with the M352 software using 686 rotating electrode. The rotating speed of the electrode was 400 r/min, the scanning rate was 1.5 mV/s, and the step was 0.20 s. The pH value was adjusted to 3.0 at each time and pure N2 was pumped into the solution to remove the oxygen before the measurement. The electrokinetic potential polarization curves of anode dissolution are shown in Fig.3 and Fig.4, from which the corrosion potential(φcorr) and corrosion current density(Jcorr) were calculated and the results are listed in Table 2.

Fig.3 Tafel curves of lead sulfide electrode under different ferric chloride concentrations without KCl: (a) [FeCl3]=0; (b) [FeCl3]=2×10-4 mol/L; (c) [FeCl3]=5×10-4 mol/L; (d) [FeCl3]=8×10-4 mol/L; (e) [FeCl3]=1.0×10-3 mol/L
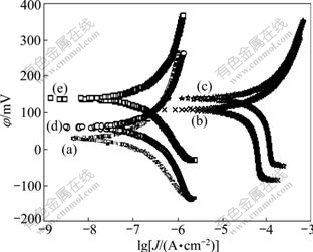
Fig.4 Tafel curves of lead sulfide electrode in saturated KCl solution under different ferric chloride concentrations: (a) [FeCl3]=0; (b) [FeCl3]=2×10-4 mol/L; (c) [FeCl3]=5×10-4 mol/L; (d) [FeCl3]=8×10-4 mol/L; (e) [FeCl3]=1×10-3 mol/L
Table 2 Tafel parameters of lead sulfide electrode in saturated KCl solution and without KCl under different ferric chloride oncentrations
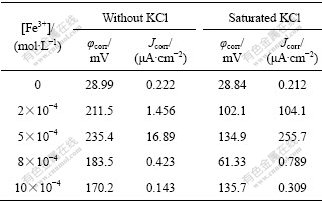
It can be seen from Fig.3 and Table 2 that, when the concentration of Fe3+ ([Fe3+]) is 0-5×10-4 mol/L, φcorr and Jcorr increase with the increase of [Fe3+]. While when [Fe3+] is more than 5×10-4 mol/L, the corrosion current density decreases with the increase of [Fe3+]. It is illuminated that there is passivation reaction on the surface of galena. The electrochemistry reaction production PbCl2 crystal agglomerates on the surface of electrode, resulting in the increase of resistance and decrease of current. At the open circuit voltage ([Fe3+]=0), φcorr and Jcorr do not change any more with and without KCl addition, indicating that KCl has little effect on the rest potential. When [Fe3+] is 5×10-4 mol/L, Jcorr reaches the maximum value.
From Fig.4 and Table 2, φcorr and Jcorr in saturated KCl system have the same trend of change as the in the system without KCl. That is, with the increase of [Fe3+], φcorr and Jcorr increase firstly. When [Fe3+] is more than 5×10-4 mol/L, φcorr and Jcorr decrease. The difference is when [Fe3+] is equal to 5×10-4 mol/L, the current density increase abruptly. The reason is that with the addition of KCl, the concentration of Cl- increases. The complexation reaction of Pb2+ with Cl- accelerates, which facilitates the dissolution of PbCl2 crystal on the surface of electrode. As a result, the electrode surface resistance decreases, and the current density increases.
In FeCl3-KCl system, when [Fe3+] is less than 5×10-4 mol/L, the corrosion rate depends on the existence of Fe3+, and increases rapidly with increasing Fe3+ concentration. When [Fe3+] is more than 5×10-4 mol/L, passivation reaction takes place on the surface of galena. With the addition of KCl, the complexation reaction of Pb2+ and Cl- facilitates the dissolution of PbCl2, so the electrode surface resistance decreases, and the corrosion current density increases.
4 Conclusions
1) In FeCl3-KCl system, the strong oxidation of Fe3+ facilitates the transformation of galena to PbCl2 crystal, which is dissolved by complexation with Cl-. This equilibrium state is verified by liquid chemistry calculation. PbCl2 exists as complex compound PbCl42- in saturated KCl solution.
2) When [Cl-]T is equal to 0.92 mol/L, PbCl2 precipitates in solid state. When [Cl-]T is more than 0.92 mol/L, PbCl2 dissolves because of complex reaction.
3) With the addition of KCl, the activity of Cl- increases, and the complex reaction becomes stronger. Therefore, the electrode surface resistance decreases, and corrosion current density increases.
References
[1] KHOLMOGOROV A, KONONOVAB O N. Recovery of lead from sulfide concentrate after mechanochemical activation using nitric acid [J]. Chinese J Chem Eng, 2005, 13(1): 91-95.
[2] KHOLMOGOROV A, PASHKOV G, MIKHLINA E, MIKHLIN Y.  Electrochemical aspects of the nitric acid leaching of lead sulfide concentrates: On the way to a highly effective method for Pb recovery [J]. ECS Transactions, Electrochemistry in Mineral and Metal Processing VII, 2006, 2(3): 221-230.
Electrochemical aspects of the nitric acid leaching of lead sulfide concentrates: On the way to a highly effective method for Pb recovery [J]. ECS Transactions, Electrochemistry in Mineral and Metal Processing VII, 2006, 2(3): 221-230.
[3] WANG S, FANG Z, WANG Y, CHEN Y. Electrogenerative leaching of galena with ferric chloride [J]. Minerals Engineering, 2003, 16(9): 869-872.
[4] DUTRIZAC J E. The leaching of sulphide minerals in chloride media [J]. Hydrometallurgy, 1992, 29(1/3): 1-45.
[5] GABRIEL D S. Kinetics and mechanism of the bacterial and ferric sulphate oxidation of galena [J]. Hydrometallurgy, 2004, 75(1/4): 99-110.
[6] ALAN A. The ferric fluosilicate leaching of lead concentrates [J]. Metallurgy Transactions B, 1994, 25: 473-478.
[7] WRIGHT K, HILLIER I H, VAUGHAN D J, VINCENT M A. Cluster models of the dissociation of water on the surface of galena (PbS) [J]. Chemical Physics Letters, 1999, 299(6): 527-531.
[8] ROGER S, GREET C. Diagnostic leaching of galena and its oxidation products with EDTA [J]. Minerals Engineering, 2002, 15(7): 515-522.
[9] ZHANG Sheng, LI Jian-ping, WANG Yu-rong, HU Guang-qian. Dissolution kinetics of galena in acid NaCl solutions at 25-75 ℃ [J]. Applied Geochemistry, 2004, 19: 835-841.
[10] AYDO?ANA S, ERDEMO?LUB M, U?ARA G, ARASA A. Kinetics of galena dissolution in nitric acid solutions with hydrogen peroxide [J]. Hydrometallurgy, 2007, 88(1/4): 52-57.
[11] DAI Zhong-xu, WANG Di-hua, ZOU Jin-yun. Studies on the behaviors of PbSO4/Pb and PbO2/PbSO4 electrodes prepared from lead carbonate by powder microelectrode technique [J]. Wuhan University Journal of Natural Science, 2000, 5(4): 474-478. (in Chinese)
[12] BALAZ P. Influence of solid state properties on ferric chloride leaching of mechanically activated galena [J]. Hydrometallurgy, 1996, 40(3): 359-368.
[13] da SILVA G. Kinetics and mechanism of the bacterial and ferric sulphate oxidation of galena [J]. Hydrometallurgy, 2004, 75(1/4): 99-110.
[14] PRITZKER M. Model for the ferric chloride leaching of galena [J]. Metallurgical Trans B, 1998, 29: 953-959.
[15] GONG Qian, GONG Ya-jun. Oxidation of galena in ammonium carbonate solution and applied reactor [J]. Trans Nonferrous Met Soc China, 1995, 5(3): 41-44.
[16] KOBAYASHI M, et al. A critical review of the ferric chloride leaching of galena [J]. Canadian Metallurgical Quarterly, 1990, 29(3): 201-211.
[17] WANG Dian-zuo, HU Yue-hua. Flotation solution chemistry [M]. Changsha: Hunan Science and Technology Press, 1988.
[18] LI Shu-tang. Elements of X-ray crystallography [M]. Beijing: Metallurgical Industry Press, 1990. (in Chinese)
Foundation item: Project(50774094) supported by the National Natural Science Foundation of China
Corresponding author: LONG Huai-zhong; Tel/Fax: +86-731-88876765; E-mail: ysxblong@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60445-8
(Edited by YUAN Sai-qian)