Trans. Nonferrous Met. Soc. China 23(2013) 456-461
Affinity and fluorescent detection of surfactants/ssDNA and single-walled carbon nanotube
Jiao ZHOU1, Juan-ping LI1, Yu-hong NIE1, Ji-shan LI1, Jin-feng YANG1,2
1. State Key Laboratory for Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China;
2. Department of Anesthesiology, Tumor Hospital, Xiangya School of Medicine, Central South University, Changsha 410013, China
Received 16 November 2012; accepted 6 January 2013
Abstract: A new biosensor platform was explored for detection of surfactant based on fluorescence changes from single strand DNA (ssDNA) and single-walled carbon nanotubes (SWNTs). Thermodynamics assay was performed to value the stability of probe. The affinities of SWNT to five common surfactants (SDS, DBS, Triton X-100, Tween-20 and Tween-80) were investigated by real-time fluorescence method. The effects of Mg2+ and pH on the fluorescence intensity of self-assembled quenched sensor were performed. The fluorescent emission spectra were used to measure the responses of self-assembled quenched fluorescent of ssDNA /SWNTs to different concentration surfactant(Triton X-100). The FAM-DNA wrapped SWNTs probe was stable in a wide temperature range (5 °C to 80 °C). The binding strength of surfactants and single-stranded DNA (ssDNA) on SWNTs surfaces was shown as follows: Triton X-100>DBS>Tween-20>Tween-80>ssDNA>SDS, and the optimized reaction conditions included pH 7.4 and 10 mmol/L Mg2+. The fluorescence of FAM-ssDNA wrapped SWNTs was proportionally recovered as a result of adding different concentrations of Triton X-100, which realizes the quantitative detection of Triton X-100.
Key words: single-stranded DNA; single-walled carbon nanotubes; surfactant; fluorescent sensor; affinity
1 Introduction
Surfactants are usually organic compounds that are amphiphilic, meaning that they contain both hydrophobic groups and hydrophilic groups and can lower the surface tension of a liquid. So, surfactants are usually designed to have cleaning or solubilization properties, which are widely applied in industrial and medical fields, and then routinely deposited in numerous ways on land and into water systems [1-3]. However, some of them are known to be toxic to animals, ecosystems, and humans, and can increase the diffusion of other environmental contaminants [4,5]. Thus, it is very important to detect surfactants in water samples. Although there are many well-established analytical methods for the determination of surfactants [6,7], they still show some limitations in their applicability, such as tedious procedures, large amount of toxic solvents, irreproducibility and signal instability. Therefore, it is especially attractive to develop new methods for detection of surfactants in water.
A unique ability for DNA adsorbing [8] as well as its super quenching capacity with a wide energy transfer range has shown single-walled carbon nanotubes (SWNTs) to be a robust artificial nanomaterial in bionanotechnology, with applications in DNA analysis [9], protein assays [10], cellular imaging [11], etc. Our group have also reported several effective, novel self-assembled SWNTs complex with oligonucleotides and demonstrated their feasibility in recognizing and detecting specific DNA sequences and proteins in a homogeneous solution [12-15]. Recently, CHEN et al [16] have used surfactant sodium dodecyl sulfate (SDS) to disperse SWNTs before they were modified by DNA probe. This process will avoid the DNA sequences damage resulting from acutely sonication. However, the SWNTs/DNA probe-based sensors for detection of surfactants, and even an integrated research about the affinity between SWNTs and surfactants, have not been reported yet. Therefore, as the continuation of our studies on SWNTs-based fluorescent biosensor designs, we report herein our initial attempt for the exploration of affinity between SWNTs and surfactants, and then fabricate a simple fluorescent ssDNA/SWNTs-based sensing platform for the detection of surfactants in homogeneous aqueous solution.
The detection mechanism of this method is shown in Fig. 1. The FAM-ssDNA, which is a random linear stranded DNA sequence labeled with a carboxy- fluorescein dye (FAM) at its 5' terminus, is first wrapped on the SWNTs surface through p-p stacking interaction [8] to form a stable fluorescence-quenched FAM-ssDNA/ SWNT complex. In the presence of surfactant, the affinity between the surfactant and SWNT is stronger than that between ssDNA and SWNT, so the FAM-ssDNA will be liberated from the SWNTs surface due to the competitive adsorption of surfactant, resulting in the fluorescence recovery of the probe system. Based on the above principle, the affinity of SWNTs to oligonucleotide and five common surfactants (sodium dodecyl sulfate, sodium dodecylbenzene sulfonate, Triton X-100, Tween-20, Tween-80) was investigated, and a novel simple fluorescent biosensor was developed for the detection of Triton X-100.
2 Experimental
2.1 Materials and apparatus
Single-walled carbon nanotubes (SWNTs) were purchased from Carbon Nanotechnologies Inc., Houston (TX, USA). Sodium dodecyl sulfate (SDS), sodium dodecyl benzene sulfonate (DBS), Triton X-100, Tween-20 and Tween-80 were all purchased from Changsha Tianheng Scientific Instrument & Equipment Co., Ltd., China Fluorescent oligonucleotide (FAM- ssDNA, 5'-FAM-TGTGGTAGTTGGAGCTGA- 3') was synthesized by Takara Biotechnology Co., Ltd. (Dalian, China). The stock solution was obtained in highly pure water (sterile Minipore water, 18.3 MΩ) and the concentration was estimated by a Hitachi U-4100 UV-vis spectrophotometer (Kyoto, Japan) using sequence-dependent absorption coefficients [17]. All work solutions were prepared with 20 mmol/L tris-HCl buffer (pH 7.4).
All steady-state fluorescence measurements were performed on a Hitachi F-7000 fluorescence spectro-fluorometer (Kyoto, Japan). Under the excitation wavelength of 480 nm, the fluorescence spectra were recorded from 490 nm to 650 nm. Both the excitation and emission slits were set as 5 nm and voltage of light was 700 V. Fluorescence emission spectra were collected using a 0.2×1 cm2 quartz cuvettes containing 500 mL solution. The pH was measured by a model 868 pH meter (Orion). Temperature was controlled by PolyScience 9112 refrigerating/heating circulator.
2.2 Preparation of FAM-ssDNA/SWNTs conjugates
Enough SWNTs were introduced into 100 nmol/L FAM-ssDNA solution (10 mmol/L Mg2+, 20 mmol/L tris-HCl, pH 7.4), followed by 10 min sonication twice (WD-9415B, Beijing, China) at a power level of 100 W, and then incubated for 40 min at room temperature. The mixture was centrifuged at 1500 r/min for 5 min to get rid of the aggregated SWNTs without DNA coating. The obtained supernatant containing FAM-ssDNA-wrapped SWNTs was stored in a refrigerator at 4 °C before usage.
2.3 Measurement procedures
Surfactants were added into 500 μL solution containing FAM-ssDNA/SWNTs in 20 mmol/L tris-HCl buffer (10 mmol/L Mg2+, pH 7.4). After the mixture was incubated for 30 min, the fluorescence was detected by the Hitachi F-7000 spectrofluorometer. Thermodynamics assay was performed to explore the stability of FAM- ssDNA/SWNTs conjugates. The temperature was slowly increased at the rate of 5 °C /min from 5 °C to 80 °C.
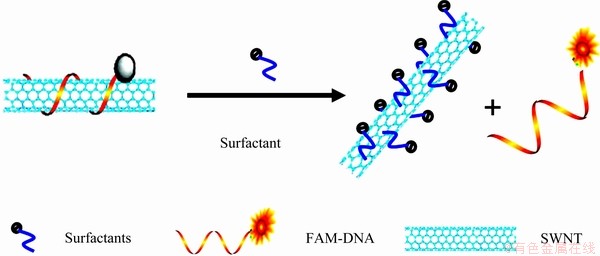
Fig. 1 Schematic principle of FAM-ssDNA/SWNT-based biosensing platform for detection of surfactant
The effects of Mg2+ concentration and pH value on the fluorescence intensity of self-assembled quenched sensor were performed. The affinity of SWNT to oligo- nucleotide and the five common surfactants (SDS, DBS, Triton X-100, Tween-20 and Tween-80) was investigated by real-time fluorescence method.
3 Results and discussion
3.1 Thermodynamics stability of FAM-ssDNA/SWNTs conjugates
Excellent thermodynamics stability is one of the most important parameters for a suitable fluorescent sensing platform. So, before the FAM-ssDNA/SWNTs- based sensing platform was used to explore the affinity between surfactants and SWNTs, the thermodynamics stability of the FAM-ssDNA/SWNTs conjugates should be explored carefully. As a control, the effect of temperature on the FAM-ssDNA was first investigated. One can see from Fig. 2 that the fluorescence intensity (F) of FAM-ssDNA has no significant difference in the temperature range of 5-80 °C (curve b), that is to say, the temperature has no effect on the FAM-ssDNA in the range of 5-80 °C. Then the effect of temperature on the stability of FAM-ssDNA/SWNTs conjugates was also investigated. It can be observed from Fig. 2 that for the conjugates of FAM-ssDNA/SWNTs, the fluorescence was kept quenched-state and was quite stable in a wide temperature range of 5-80 °C (curve a), indicating that the self-assembled quenched sensor will not be affected by temperature and it can be used to detect surfactant at a wide temperature range.
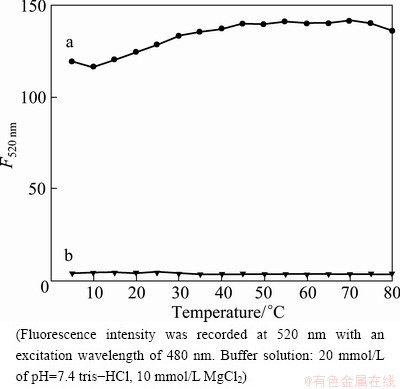
Fig. 2 Fluorescence intensity responds of FAM-ssDNA (curve a) and FAM-ssDNA/SWNTs (curve b) as function of temperature
3.2 Affinity between surfactants and SWNTs
In the present approach, SWNT has been proved an excellent quencher to fluorophore via the energy-transfer and electron-transfer processes, and the ssDNA can be adsorbed well on its surface through p-p stacking interaction [8]. So, the FAM-ssDNA-wrapped SWNT as the probe platform was chosen as the standard to explore the affinity of surfactants with SWNT. One can find from Fig. 3(a) that the fluorescence intensities at 520 nm of FAM-ssDNA/SWNTs-contained solutions are all increased after additions of DBS, Triton X-100, Tween-20 and Tween-80 except SDS, indicating that the affinity of DBS, Triton X-100, Tween-20 or Tween-80 with SWNT is stronger than that of ssDNA with SWNT, resulting in the liberation of FAM-ssDNA from the surface of SWNTs due to the competitive adsorption of these surfactants, while SDS has a weaker affinity to SWNT compared with ssDNA and the FAM-ssDNA can not be released from the SWNT’s surface. In order to compare the affinity strength of these surfactants with SWNT, real-time fluorescence responses of the FAM-ssDNA/SWNTs-contained solutions were recorded after additions of these surfactants (Fig. 3(b)). From the recovery rate and the recovery efficiency of the fluorescence intensity in Fig. 3(b), one can conclude that the binding strength of surfactants and ssDNA on SWNT surface is as follows: Triton X-100>DBS>Tween-20> Tween-80>ssDNA>SDS. Therefore, we could use this kind of novel, simple and convenient FAM-ssDNA/ SWNTs-based sensing platform for detection of most surfactants.
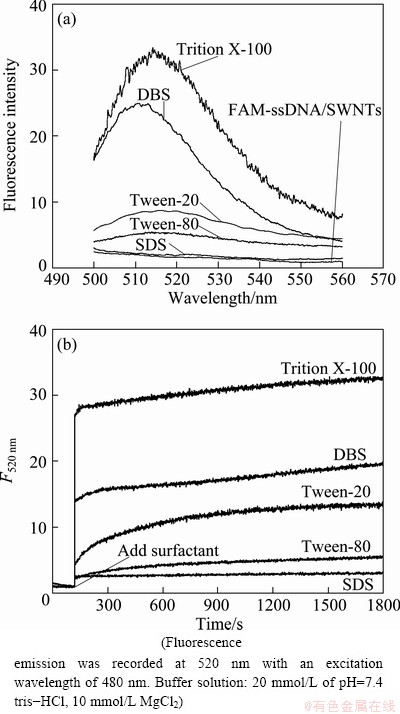
Fig. 3 Fluorescence emission spectra of FAM-ssDNA/SWNTs- contained solutions after addition of SDS, Tween-80, Tween-20, DBS or Triton X-100 with concentration of 0.5 mg/mL and incubated for 30 min at room temperature (a), real-time fluorescence response of FAM-ssDNA/SWNTs upon addition of five surfactants (SDS, Tween-80, Tween-20, DBS and Triton X-100) with concentration of 0.5 mg/mL (b)
3.3 Effects of pH and Mg2+ concentration on FAM- DNA/SWNTs-based sensing platform
Divalent metal ions such as Mg2+ have effect on the dispersion of carboxylic SWNTs, and the wrap of DNA sequence on the SWNT’s surface will also be affected by the divalent metal ions, then affecting the performance of the FAM-ssDNA/SWNTs-based sensing platform. As shown in Fig. 4(a), the fluorescence intensity of the FAM-ssDNA/SWNTs conjugates has no significant difference in a wide Mg2+ concentration range from 0 to 20 mmol/L, while the fluorescence response is the largest at 10 mmol/L Mg2+ for addition of surfactant (for example, Triton X-100). This phenomenon might be resulted from the reason that high Mg2+ concentration will increase the hybridization force of DNA sequence, making the liberation of FAM-ssDNA from SWNTs easier due to the formation of rigid DNA secondary structures. However, the high concentration of Mg2+ will also change the electrostatic balance among the dispersed nanotubes, resulting in the aggregation of SWNTs.
The pH is another important factor for the proposed fluorescence method. It is well known that both the fluorescence property of fluorescein (FAM) and the bone structure of DNA sequence can be affected by the pH value, thus affecting the performance of the FAM- ssDNA/SWNTs-based sensing platform. As shown in Fig. 4(b), the fluorescence intensity of the detection system in the presence of surfactant of Triton X-100 is increased with the increase of pH value, while the background fluorescence also will be increased when the pH value is more than 7.5, indicating that both the fluorescence property of FAM-ssDNA and the interactions between the FAM-ssDNA and SWNTs will be affected by the pH value. Considering requirement for a good signal/background, so pH 7.4 was selected in the following experiments.

Fig. 4 Effects of Mg2+ concentration (a) and pH value (b) on performance of FAM-ssDNA/SWNTs-based sensing platform
3.4 Detection of surfactant Triton X-100
The fluorescence emission spectra of FAM- ssDNA/SWNTs conjugates are sensitive to the presence of surfactant of Triton X-100 and the fluorescence recovery at 520 nm can be utilized to estimate the concentration of Triton X-100. Figure 5(a) shows fluorescence emission spectra of the self-assembled quenched sensors, FAM-ssDNA/SWNTs conjugates, with varying concentrations of target surfactant of Triton X-100 under the optimum conditions (pH 7.4, 10 mmol/L MgCl2). The fluorescence emission almost cannot be observed when the target surfactant of Triton X-100 was not present, indicating a high quenching efficiency by SWNTs. While, the fluorescence emission will be increased with increasing in the Triton X-100 concentration, and a quantitative determination range of 0.1-2.5 mg/mL was obtained with the detection limit of 0.05 mg/mL (Fig. 5(b)). The experimental data show that the proposed approach may work well for probing surfactants.
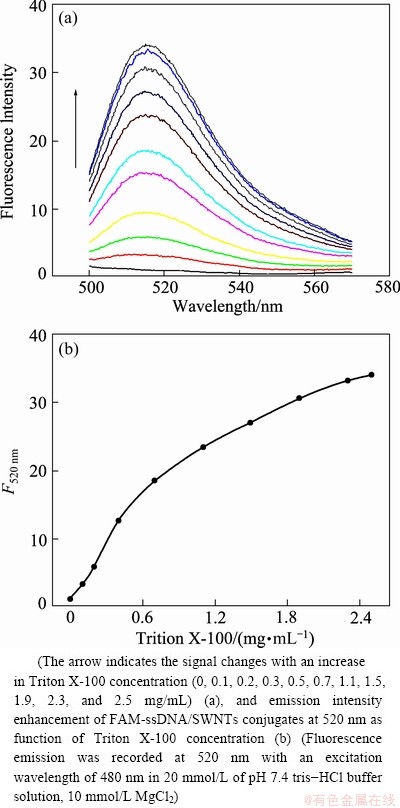
Fig. 5 Fluorescence emission spectra of FAM-ssDNA/SWNTs conjugates in the presence of different concentrations of Triton X-100
4 Conclusions
1) A advanced fluorescence probe for detection of surfactant based on the functional oligonucleotides and SWNTs is successfully developed, and the probe is stable in a wide temperature range (5-80 °C).
2) The binding strength of five common surfactants and single-stranded DNA (ssDNA) on SWNTs surfaces is shown as follows: Triton X-100>DBS>Tween-20> Tween-80>ssDNA>SDS, and the optimized reaction conditions are included pH 7.4 and 10 mmol/L Mg2+.
3) The advanced fluorescence probe can be used to detect surfactant such as Triton X-100.
References
[1] JIAN Jin, WAKAYAMA Y, PENG Xin-sheng, ICHINOSE I. Surfactant-assisted fabrication of free-standing inorganic sheets covering an array of micrometre-sized holes [J]. Nature Materials, 2007, 6(9): 686-691.
[2] JI Ying, LIU Chen, PEI Yuan-ying. Artificial pulmonary surfactant as a carrier for intratracheally instilled insulin [J]. Acta Pharmacol Sin, 2007, 28(5): 744-750.
[3] LI Wen-wen, YOON J A, MATYJASZEWSKI K. Dual-reactive surfactant used for synthesis of functional nanocapsules in miniemulsion [J]. J Am Chem Soc, 2010, 132(23): 7823-7825.
[4] KLAMMER A A, MACCOSS M J. Effects of modified digestion schemes on the identification of proteins from complex mixtures [J]. Proteome Res, 2006, 5(3): 695-700.
[5] METCALFE T L, DILLON, P J,METCALFE C D. Detecting the transport of toxic pesticides from golf courses into watersheds in the precambrian shield region of Ontario, Canada [J]. Environmental Toxicology and Chemistry, 2008, 27(4): 811-818.
[6] COLL C, MARTINEZ-MANEZ R, DOLORES M, SANCENON F, SOTO J. A simple approach for the selective and sensitive colorimetric detection of anionic surfactants in water [J]. Angew ChemInt Ed Engl, 2007, 46(10): 1675-1678.
[7] HEINING K, VOGT C. Determination of surfactants by capillary electrophoresis [J]. Electrophpresis, 1999, 20(15-16): 3311-3328.
[8] ZHENG Ming, JAGOTA A, STRANO M S, SANTOS A P, BARONE P, CHOU S G, DINER B A, DRESSELHAUS M S, MCLEAN R S, ONOA G B, SAMSONIDZE G G, SEMKE E D, USREY M, WALLS D J. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly [J]. Science, 2003, 302(5650): 1545-1548.
[9] YANG Rong-hua, JIN Jian-yu, CHEN Yan, SHAO Na, KANG Huai-zhi, XIAO Ze-yu, TANG Zhi-wen, WU Yan-rong, ZHU Zhi, TAN Wei-hong. Carbon nanotube-quenched fluorescent oligonucleotides: Probes that fluoresce upon hybridization [J]. J Am Chem Soc, 2008, 130(26): 8351-8358.
[10] ZHU Zhi, TANG Zhi-wen, PHILLIPS J A, YANG Rong-hua, WANG Hui, TAN Wei-hong. Regulation of singlet oxygen generation using single-walled carbon nanotubes [J]. J Am Chem Soc, 2008, 130(33): 10856-10857.
[11] WELSHER K, LIU Zhuang, DARANCIANG D, DAI Hong-jie. Selective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent molecules [J]. Nano Lett, 2008, 8(2): 586-590.
[12] WU Yan-rong, PHILLIPS J A, LIU Hai-peng, YANG Rong-hua, TAN Wei-hong. Carbon nanotubes protect DNA strands during cellular delivery [J]. ACS Nano, 2008, 2(10): 2023-2028.
[13] YANG Rong-hua, TANG Zhi-wen, YAN Ji-lin, KANG Huai-zhi, KIM Y M, ZHU Zhi, TAN Wei-hong. Noncovalent assembly of carbon nanotubes and single-stranded DNA: An effective sensing platform for probing biomolecular interactions [J]. Anal Chem, 2008, 80(19): 7408-7413.
[14] LIU Yue, WANG Yong-xiang, JIN Jian-yu, WANG Hao, YANG Rong-hua, TAN Wei-hong. Fluorescent assay of DNA hybridization with label-free molecular switch: reducing background-signal and improving specificity by using carbon nanotubes [J]. Chem Commun (Camb), 2009, 14(6): 665-667.
[15] OUYANG Xiang-yuan, YU Ru-qin, JIN Jian-yu, LI Ji-shan, YANG Rong-hua, TAN Wei-hong, YUAN Jing-li. New strategy for label-free and time-resolved luminescent assay of protein: Conjugate Eu3+ complex and aptamer-wrapped carbon nanotubes [J]. Anal Chem, 2011, 83(3): 782-789.
[16] CHEN Yi, LIU Hai-peng, YE Tao, KIM J, MAO Cheng-de. DNA-directed assembly of single-wall carbon nanotubes [J]. J Am Chem Soc, 2007, 129(28): 8696-8697.
[17] SZEJTLI  Introduction and general overview of cyclodextrin chemistry [J]. Chem Rev, 1998, 98(5): 1743-1753.
Introduction and general overview of cyclodextrin chemistry [J]. Chem Rev, 1998, 98(5): 1743-1753.
单链DNA/表面活性剂与单壁碳纳米管的亲合力及荧光检测性能
周 姣1,李娟萍1,聂钰洪1,李继山1,杨金凤1,2
1. 湖南大学 化学化工学院 化学生物传感与计量学国家重点实验室,长沙 410082;
2. 中南大学 湘雅医学院 附属肿瘤医院 麻醉科,长沙 410013
摘 要:研制一种基于单链DNA与单壁碳纳米管荧光变化的新型荧光探针用于表面活性剂物质的检测方法。考察了温度对探针稳定性的影响,通过观察实时荧光光谱变化来评估单壁碳纳米管与5种常见表面活性剂物质的亲和性,观察镁离子及pH对探针检测表面活性物质的影响,通过观察荧光发射光谱变化来考察加入不同浓度表面活性物质曲拉通X-100后荧光探针的荧光恢复情况。结果表明,在5~80 °C的温度范围内,探针十分稳定;单壁碳纳米管与5种常见表面活性物质的亲和力由强至弱依次为曲拉通X-100、十二烷基苯磺酸钠、吐温-20、吐温-80、十二烷硫酸钠;荧光探针检测的最佳Mg2+浓度为10 mmol/L,pH 为7.4;当加入不同浓度的表面活性剂物质曲拉通X-100时,探针体系的荧光逐渐得到恢复。
关键词:单链DNA;单壁碳纳米管;表面活性剂;荧光传感器;亲和力
(Edited by Hua YANG)
Foundation item: Projects (21075032, 21005026, 21135001) supported by the National Natural Science Foundation of China; Project (llJJ5012) supported by Hunan Provincial Natural Science Foundation, China
Corresponding author: Jin-feng YANG; Tel: +86-13875985950; E-mail: xiaofengjie2000@yahoo.com.cn
DOI: 10.1016/S1003-6326(13)62485-1