DOI:10.19476/j.ysxb.1004.0609.2018.11.20
铝灰中活性物相的反应行为
刘桂华1,黄文强1,熊德芬2,王洪阳1,李小斌1,周秋生1,彭志宏1,齐天贵1
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 云南文山铝业有限公司,文山 663099)
摘 要:采用XRD和SEM-EDS (微区分析)研究了铝灰的工艺矿物学以及其活性物相Al和AlN在水和氢氧化钠溶液中的反应行为。结果表明:铝灰中活性物相Al、AlN等含量较多,且AlN含量多于Al的。铝灰中Al多以球形颗粒单独存在,也常与Si嵌布形成合金;AlN以大颗粒块状或针状颗粒单独存在,也可与α-Al2O3共存;刚玉(α-Al2O3)存在的形态不一;NaCl大多单独聚集存在,少部分被包裹和分散。活性含铝物相Al和AlN在与水反应时,反应缓慢,反应率小;AlN比Al更易反应;Al与水反应生成的氢氧化铝包裹了Al颗粒,阻碍了反应进行。而与浓氢氧化钠溶液反应时,铝灰中的Al和AlN迅速发生化学反应,常温下0.5 h反应率均达到80%以上,并且Al比AlN更易反应。Si和SiO2也会发生反应。同时,温度升高有利于Al和AlN与水或碱反应。
关键词:铝灰;铝;氮化铝;工艺矿物学;水;氢氧化钠
文章编号:1004-0609(2018)-11-2341-10 中图分类号:TF09 文献标志码:A
铝电解和铝熔炼过程中,均会大量产生铝灰[1-2]。铝灰中主要存在α-Al2O3、AlN、SiO2、MgO、Al和少量的Si、Al4C3物质,也可能含有较多的CaF2、NaCl、KCl和MgAl2O4等[3-5]。尽管铝灰是一种含铝资源,但铝灰是一种危废。铝灰与溶液接触,其Al、AlN、Si等活性物相反应生成易爆H2和刺鼻难闻NH3,而氯化盐和氟化盐可进入溶液中,从而污染空气和水体[1, 6]。因此,铝灰的安全经济利用一直是研究的热点,也是世界性难题[1, 7]。
传统火法转盐炉(RSF)可回收金属铝;火法处理铝灰还可制备混凝土砖、耐火材料等非金属产品和低品质氧化铝等。上述工艺流程简单,但存在铝回收率低(或产品品质低)、成本偏高、含氯和氟气体难处理、设备腐蚀严重等缺点,同时工艺中实现完全隔离铝灰与水(溶液)接触也存在较多困难[8-11]。而以铝灰为原料,湿法合成聚合氯化铝(PAC)、AlPO4-5/CrAlPO-5、η(α)-Al2O3、X–Al型水滑石(X=Mg、Ca和Zn)、(Mg,Si)Al2O4尖晶石、Al-Si合金和棕刚玉[7, 12-14]等时成本低,但需要安全处理氢气、氨气和进入溶液中的氯化盐和氟化盐等。因此,铝灰的安全利用都离不开活性物相(Al、AlN、Si)和氯化盐、氟化盐的处理。SVEDBERG等[15]研究了不同pH值下85 ℃时AlN与水的反应规律,发现在水中可生成具有不同比例的AlOOH和Al(OH)3相。KRNEL等[16]发现,AlN水解反应之前存在诱导期,该诱导期随着温度的升高而缩短,随着溶液pH值的降低而延长。FUKUMOTO等[17]研究了在室温到100℃条件下,粉末和用该粉末烧结的AlN陶瓷块体的水解行为,也认为AlN在发生水解反应之前存在诱导期,诱导期随着温度的升高而缩短;烧结后的AlN陶瓷块体在室温下不发生水解,在373 K下有轻微的水解,水解后的pH值仅达到8。上述纯AlN与水的反应机制并未充分阐明。姜澜等[14]、张勇等[18]不考虑铝灰中其他物相的反应,研究AlN的水解行为,明确了温度、液固比、搅拌等因素的影响规律,但铝灰中AlN是和Al、Al2O3、Si等物相共存,难以忽略它们对AlN反应行为的影响。本文作者在实验中也发现,铝灰中Al、AlN、Si反应行为复杂,在水中比在碱液中氢气和氨气释放时间长。主要原因可能铝灰中各物相工艺矿物学复杂,在反应过程中各物相反应行为相互影响显著。而这一方面的研究尚无明确结论,也不利于铝灰的清洁安全综合利用。
旨在湿法安全处理铝灰,本文作者着眼于铝灰中活性含铝物相(铝、氮化铝)反应行为,研究了铝灰中各物相的工艺矿物学,分析了其在碱性和水体系下的反应行为,探讨了各因素对铝灰活性含铝物相反应的影响规律。
1 实验
1.1 实验原料
铝灰从云南某铝厂堆场中取出,是铝电解和铝熔铸过程中产生的铝灰。铝灰的粒度在40~200 μm之间,d50为82.358 μm(见图1)。

图1 铝灰的粒度分布
Fig. 1 Particle size distribution of aluminum dross
铝灰的X荧光光谱(XRF)分析结果(见表1)表明。铝灰中的主要元素为Al、O、Cl、Na、F、Mg、Si、K、Ca,其他含量约1.5%的元素包括Ti、Fe、Sr、S、V、Mn、In、Zn、P等。
表1 铝灰中主要元素含量
Table 1 Content of elements in aluminum dross (mass fraction, %)

1.2 实验过程
称取3 g铝灰加入到250 mL盛有溶液的单口圆底烧瓶中,恒温水浴锅控制反应温度,磁力搅拌,三级稀硫酸吸收氨,采用排水法收集氢气。实验连接如图2所示。
反应结束后,反应后浆液经真空抽滤、开水洗涤,滤渣在60 ℃下烘干24 h,然后对滤渣再进行形貌和物相分析。

图2 实验装置连接示意图
Fig. 2 Schematic diagram of experimental apparatus
1.3 测试方法
使用JSM-6360LV型高低真空扫描电子显微镜(日本电子公司)观察样品微观形貌。同时,为分析各物相粒子嵌布规律,取一定量的铝灰经煮胶,用无水乙醇代替水进行磨片和抛光,然后再进行电镜面扫描分析。
使用PW2424型X荧光光谱仪分析铝灰中各元素的含量。使用LS-POP型激光粒度分布仪分析铝灰的中位径(d50)。物相组成采用日本理学3014Z型X射线衍射分析仪(XRD)进行分析,其衍射条件为:Cu靶Kα辐射源(λ=1.5406 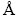 ),管电压为40 kV,扫描速度10 (°)/min,步长0.02 (°)/步,扫描角度5°~75°。
),管电压为40 kV,扫描速度10 (°)/min,步长0.02 (°)/步,扫描角度5°~75°。
硫酸收集氨后,采用水杨酸分光光度法测定氮含量[19]。采用排水法测定氢气体积。
XRD物相半定量分析方程[20-21]:
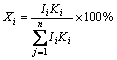 (1)
(1)
式中:Xi为i相的质量分数;Ii、Ij分别为i、j相最强衍射峰的积分强度;Ki、Kj分别为 和
和 的比值(α-Al2O3为参比相);n为铝灰中物相数。
的比值(α-Al2O3为参比相);n为铝灰中物相数。
此方法在没有内标的情况下也可进行分析,考虑到分析误差和物相结晶度不同,半定量分析误差范围低于5%。
铝灰中活性物相Al和AlN的反应率η:
 (2)
(2)
式中:V1为反应释放出的H2或NH3体积;V0为铝灰中Al或AlN完全反应可释放出H2或NH3体积。
2 结果与讨论
2.1 铝灰中主要物相和分布
铝灰XRD图谱见图3。结果显示,铝灰中的主要物相为α-Al2O3、AlN、Al、NaCl、Si、CaF2、MgAl2O4、KAl11O17和SiO2,其中Si和SiO2的衍射峰最为微弱,含量少。由于α-Al2O3在常温常压下稳定不易与溶液反应,因而本研究中定义其为非活性物相,而AlN、Al和Si等定义为活性物相。单质Si在铝灰中含量很少,且与Al反应行为类似,故不重点考察。

图3 铝灰的XRD谱
Fig. 3 XRD pattern of aluminum dross
进一步对铝灰中44.69%铝中含铝物相进行分析(见表2)。结果显示,总铝中68.58%为活性含铝物相,表明该铝灰中活性含铝物相量多,氧化铝物相相对较少。也预示着该铝灰危害性较大。
表2 铝灰含铝物相中铝的化学组成
Table 2 Al content in aluminum bearing substances of aluminum dross
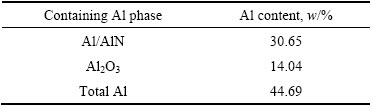
基于XRD谱,进一步半定量分析活性含铝物相分布规律,结果显示活性含铝物相中AlN占65.4%,每3 g铝灰中理论可释放185 mL NH3,单质Al占34.6%,每3 g铝灰中理论可释放223 mL H2。
铝灰的SEM像如图4所示。由图4可知,铝灰的粒度分布从几十到200 μm不等,与粒度分布图1一致。铝灰中各物相颗形貌各异,各物相可呈聚集状态,颗粒形貌有呈球状、棱片状、颗粒状、长柱状和丝网状等;不同亮度物相之间分布比较散,但有少量的连生颗粒以及包裹颗粒。

图4 铝灰的SEM像
Fig. 4 SEM image of aluminum dross
对不同类型颗粒区域进一步研究Al、Si、O、Na和Cl元素的分布,元素面扫描结果如图5所示。
图5(a)所示为大块颗粒,内含有致密和疏松多空不同布局。致密处主要是Na和Cl元素,且分布均匀,构成NaCl物相;同时还含有Al和O元素,为Al2O3物相;也含有少量的Si元素。区域Ⅱ是球形颗粒和形状不均的连生颗粒组成,球形颗粒主要含有Al和Si元素,以Al元素为主,构成Al-Si互相嵌布,且外围未观测到O元素的包裹,以铝硅合金为主。连生疏松颗粒主要由Al和O元素组成,以α-Al2O3形式结合;连生密实部位主要是可溶性NaCl相。由上分析可知,铝灰中Al和O结合形成α-Al2O3,形貌无规则,有呈丝网状或颗粒块状等;NaCl聚集存在,可以独立物相颗粒存在,也可与Al2O3物相互相嵌布;还有单质Al和Si呈球形,Al-Si互相嵌布形成稳定合金,表面无氧化膜。
由于铝灰中主要物相既有单独存在的物相,也有相互嵌布、包裹的物相,因而活性物相与水、碱反应行为应该是复杂的。
2.2 铝灰与水反应行为

图5 铝灰的SEM像和微区元素面分布
Fig. 5 SEM images of aluminum dross and section- distribution of elements (Al, Si, O, Na and Cl)
铝灰中活性物相Al和AlN在水体系下可能发生的化学反应方程式如下:
 (1)
(1)
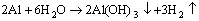 (2)
(2)
在本文实验条件中,铝灰中Al和AlN的反应率随时间的变化规律如图6所示。从图6中可以看出,25 ℃时,8 h内Al与水几乎不反应,8 h开始缓慢反应,24 h反应率约为9%;相对而言,AlN易与水反应,且随反应时间的延长,反应率逐渐升高,24 h反应率约12%,不存在诱导期,这与纯AlN反应行为不同[16-17]。提高反应温度为60 ℃,Al和AlN与水反应规律类似,但反应率均明显增大,这与姜澜等[14]的结果一致。总之,在水中,Al和AlN的反应行为不一致,反应率不高,Al反应率后期变化很小;AlN更易反应,且温度对反应的影响大于铝灰中Al。为此,针对反应渣的物相和物相分布来分析铝灰中Al和Al与水的反应行为。
2.3 铝灰与水反应渣分析
铝灰与水反应渣的XRD谱如图7所示。对比图3,铝灰与水反应1 h后,图7(a)中除NaCl的衍射峰消失外,渣中的α-Al2O3、KAl11O17、MgAl2O4、CaF2、Si、SiO2、Al和AlN等的衍射峰明显。反应24 h后,图7(b)中出现了新相Al(OH)3的衍射强峰,也仍可检测到α-Al2O3、KAl11O17、MgAl2O4、CaF2和AlN的衍射峰,NaCl、SiO2、Si和Al衍射峰消失。因此,60 ℃下1 h内,铝灰中NaCl可溶解进入溶液中,Al和AlN几乎不反应,与图6结果相符。

图6 时间对铝和氮化铝与水反应率的影响
Fig. 6 Effect of time on reaction rate of Al and AlN in water at different temperatures (Reaction time: t=0-24 h, aluminum dross: 30 g/L)

图7 铝灰与水反应后残渣的XRD谱
Fig. 7 XRD patterns of residue after aluminum dross reacting with water (θ=60 ℃)
反应后渣的SEM和元素面扫描结果如图8所示。从图8中可知,反应渣中存在Al、AlN和Al2O3等物相,同时可观测到局部Si-O的存在,氧化铝没有发生反应。密实球形颗粒为单质Al(或含少量的铝硅合金),且在Al颗粒的周边观察到丰富的O元素(见图8(b)和8(c))。结合图5和7结果可以认为,铝灰与水反应生成了氢氧化铝,即可以认为Al被氢氧化铝包覆。正是这一包裹层,阻碍铝与水的反应,导致Al的反应率维持在一定值(见图6(a))。同时,嵌布在Al中少量的NaCl没有被充分溶解,说明铝灰中相互嵌布的物相影响了可溶性NaCl的溶解;Si无明显反应。
2.4 铝灰与氢氧化钠溶液反应行为
铝灰中Al、AlN在氢氧化钠溶液体系下可能发生的化学反应方程式如下:
 (3)
(3)
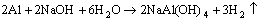 (4)
(4)
铝灰中Al和AlN与氢氧化钠溶液反应率随时间变化如图9所示。从图9中可以看出,铝灰与300 g/L氢氧化钠在25 ℃和60 ℃下均剧烈反应,接近反应完全,且都在0.5 h内反应率达80%以上。在25 ℃下,3 h内单质Al和AlN与氢氧化钠反应率达97%以上;而在60 ℃时,Al的反应率1 h之后趋于稳定,达98.6%,AlN的反应率2 h之后趋于恒定,达97.3%。由此可见,在300 g/L氢氧化钠溶液下,铝灰中的Al和AlN极易与其反应释放出H2和NH3;且温度越高,反应速率越大。相对而言,Al与氢氧化钠的反应率高于AlN与氢氧化钠的反应率,这与在水中反应行为不同。Al和AlN在碱液中的快速反应,为湿法碱性体系中回收铝带来一定的困难。
2.5 铝灰与氢氧化钠溶液反应渣分析
铝灰与氢氧化钠溶液反应渣的XRD谱见图10。从图10(a)中可见,反应渣中的物相主要有α-Al2O3、KAl11O17、All2MgO4、CaF2等;AlN的衍射峰减弱,Al的衍射峰较弱,说明Al和AlN已明显反应;NaCl特征峰消失,NaCl溶于溶液中。而图10(b)中AlN的衍射峰微弱,Al的衍射峰消失。从铝灰与氢氧化钠溶液反应渣的XRD谱也可知,铝灰中的Al和AlN很容易与浓氢氧化钠溶液反应,且可以看出Al的反应更快。结合图7中结果还可知,氟化钙是惰性能物质,由此可使预处理-火法综合利用复杂化。
从图10(a)和(b)中还能发现,Si和SiO2的衍射峰都消失,这说明在浓碱中,还发生了如下反应,并生成了含硅铝酸钠溶液。
 (5)
(5)
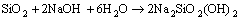 (6)
(6)
反应渣的SEM和元素面扫描结果见图11。由图11可知,与浓碱反应0.5 h后,反应渣中大量存在的元素仍是Al和O,有Al和O面扫图像均能重叠区域,也有单质Al-Si共存区域(与图5中区域II的不同),还有Al单独存在区域。这一现象与图8有所不同,在碱体系与Al反应生成铝酸钠溶液不会形成氢氧化铝膜。
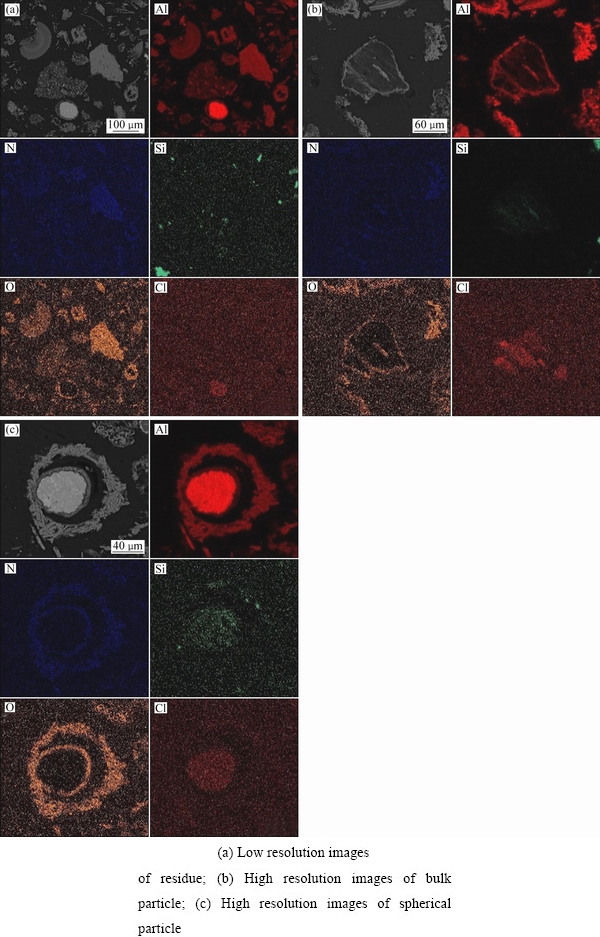
图8 铝灰与水在60 ℃下反应1 h后残渣微区元素面分布
Fig. 8 Section-distribution of elements (Al, N, Si, O and Cl) for residue after aluminum dross reacting with water at 60 ℃ for 1 h

图9 时间对铝和氮化铝与氢氧化钠反应率的影响
Fig. 9 Effect of time on reaction rate of Al and AlN in NaOH solution at different temperatures (φNaOH=300 g/L, aluminum dross: 30 g/L)
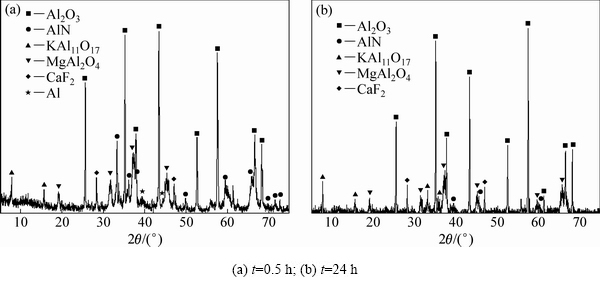
图10 铝灰与氢氧化钠反应渣的XRD谱
Fig. 10 XRD patterns of residue after aluminum dross reacting with NaOH solution (θ=25 ℃)
还可观察到Al、N和O元素面扫图像重叠区域,AlN以方形块状呈现,颗粒较大;部分Si还可以颗粒状或与其他物相嵌布形式存在。上述结果表明,Al、AlN和Si相均发生了反应,这与XRD分析结果一致。
3 结论
1) 铝灰中主要物相为α-Al2O3、AlN、Al、NaCl、Si、CaF2、MgAl2O4、KAl11O17和SiO2。铝灰中Al多以球形颗粒存在,常与Si嵌布,生成铝硅合金;AlN多以大颗粒块状物形式存在;刚玉(α-Al2O3)存在的形态不一;NaCl多聚集存在,也可嵌布于α-Al2O3中。铝灰无论是在水还是在浓碱中,其中活性物相可反应,氯化盐可进入溶液,而氟化盐仍留在残渣中。
2) 常温下铝灰中Al和AlN与水反应率低,反应缓慢,AlN比Al更易反应;温度升高有利于Al和AlN与水反应,Al与水反应生成氢氧化铝包裹了Al颗粒,阻碍了反应进行;包裹在铝或氧化铝中的氯化盐溶解速率也较慢;Si相似没有反应。
3) 浓氢氧化钠溶液中,铝灰中的Al和AlN迅速发生化学反应,常温下0.5 h反应率均达到80%以上,2~3 h后,反应率达97%以上,反应放出H2和NH3;Si和SiO2也会发生反应生成硅酸钠进入溶液。同时,在浓碱条件下,Al的反应活性强于AlN的,不会出现氢氧化铝包裹层。
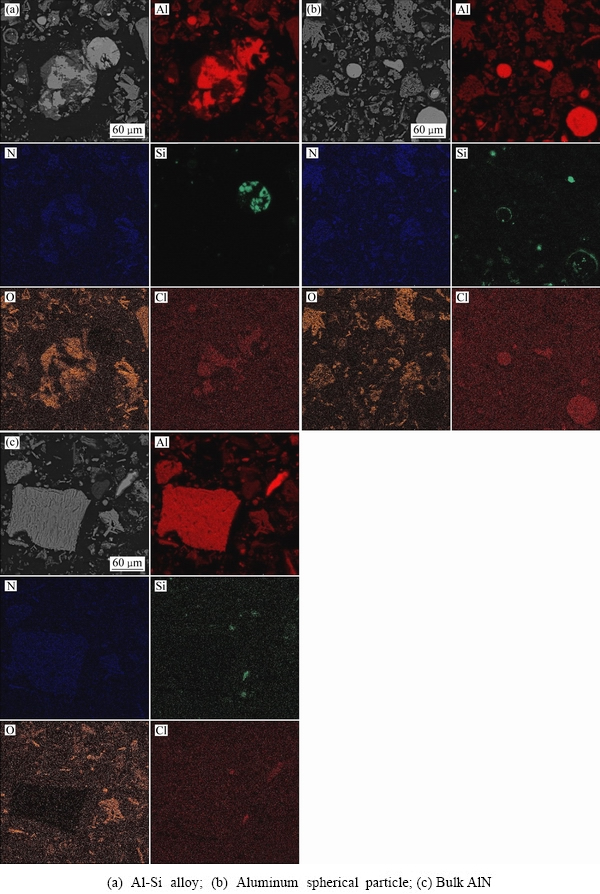
图11 铝灰与氢氧化钠溶液在25 ℃下反应0.5 h后残渣的SEM像和微区元素面分布
Fig. 11 SEM images of residue and section-distribution of elements (Al, N, Si, O and Cl) for residue after aluminum dross reacting with NaOH solution at 25 ℃ for 0.5 h
REFERENCES
[1] HONG J P, WANG J, CHEN H Y, SUN B D, LI J J, CHEN C. Process of aluminum dross recycling and life cycle assessment for Al-Si alloys and brown fused alumina[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(11): 2155-2161.
[2] 郭学益, 李 菲, 田庆华, 计 坤. 二次铝灰低温碱性熔炼研究[J]. 中南大学学报(自然科学版), 2012(3): 809-814.
GUO Xue-yi, LI Fei, TIAN Qing-hua, JI Kun. Recovery of aluminium from secondary aluminum dross using low-temperature alkaline smelting[J]. Journal of Central South University(Science and Technology), 2012(3): 809-814.
[3] MANFREDI O, WUTH W, BOHLINGER I. Characterizing the physical and chemical properties of aluminum dross[J]. JOM, 1997, 49(11): 48-51.
[4] FUKUMOTO S, HOOKABE T, TSUBAKINO H. Reaction between aluminum and water[J]. Journal of Japan Institute of Light Metals, 1998, 48(4): 199-203.
[5] 张庆建, 丁仕兵, 冯丽丽, 宋 飞, 管 嵩, 郭 兵. X射线技术在铝灰分析中的应用[J]. 分析测试学报, 2012, 31(s1): 219-221.
ZHANG Qing-jian, DING Shi-bing, FENG Li-li, SONG Fei, GUAN Song, GUO Bing. Application of X-ray techinque in analysis of aluminium dross[J]. Journal of Instrumental Analysis, 2012, 31(s1): 219-221.
[6] 吴 龙, 胡天麒, 郝以党. 铝灰综合利用工艺技术进展[J]. 有色金属工程, 2016, 6(6): 45-49.
WU Long, HU Tian-lin, HAO Yi-dang. Progress of utilization technology for aluminum ash[J]. Nonferrous Metals Engineering, 2016, 6(6): 45-49.
[7] 柴登鹏, 周云峰, 李昌林, 汪艳芳, 刘 卓. 铝灰综合回收利用的国内外技术现状及趋势[J]. 轻金属, 2015(6): 1-4.
CHAI Deng-peng, ZHOU Yun-feng, LI Chang-lin, WANG Yan-fang, LIU Zhuo. The technology status and trends of comprehensive recovery and utilization of aluminum dross both at home and abroad[J]. Light Metals, 2015(6): 1-4.
[8] üNL N, DROUET M G. Comparison of salt-free aluminum dross treatment processes[J]. Resources Conservation & Recycling, 2002, 36(1): 61-72.
[9] YOSHIMURA H N, ABREU A P, MOLISANI A L, DE CAMARGO A C, PORTELA J C S, NARITA N E. Evaluation of aluminum dross waste as raw material for refractories[J]. Ceramics International, 2008, 34(3): 581-591.
[10] EWAIS E M M, KHALIL N M, AMIN M S, AHMED Y M Z, BARAKAT M A. Utilization of aluminum sludge and aluminum slag (dross) for the manufacture of calcium aluminate cement[J]. Ceramics International, 2009, 35(8): 3381-3388.
[11] LUCHEVA B, TSONEV T, PETKOV R. Non-waste aluminum dross recycling[J]. Journal of the University of Chemical Technology and Metallurgy, 2005, 40(4): 335-338.
[12] 张宁燕, 宁 平, 谢天鉴, 段 刚. 铝灰有价组分回收及综合利用研究进展[J]. 硅酸盐通报, 2017, 36(6): 1951-1956.
ZHANG Ning-yan, NING Ping, XIE Tian-jian, DUAN Gang. Research progress on recycling and comprehensive utilization of aluminum dross[J]. Bulletin of the Chinese Ceramic Society, 2017, 36(6): 1951-1956.
[13] 李 菲, 郭学益, 田庆华. 二次铝灰制备α-Al2O3工艺[J]. 北京科技大学学报, 2012, 34(4): 383-389.
LI Fei, GUO Xue-yi, TIAN Qing-hua. Preparation process of α-Al2O3 from secondary aluminium dross[J]. Journal of University of Science and Technology Beijing, 2012, 34(4): 383-389.
[14] 姜 澜, 邱明放, 丁友东, 苏 楠, 姚 泉. 铝灰中AlN的水解行为[J]. 中国有色金属学报, 2012, 22(12): 3555-3561.
JIANG Lan, QIU Ming-fang, DING You-dong, SU Nan, YAO Quan. Hydrolysis behavior of AlN in aluminum dross[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(12): 3555-3561.
[15] SVEDBERG L M, ARNDT K C, CIMA M J. Corrosion of aluminum nitride (AlN) in aqueous cleaning solutions[J]. Journal of the American Ceramic Society, 2010, 83(1): 41-46.
[16] KRNEL K, KOSMAC T. Protection of AlN powder against hydrolysis using aluminum dihydrogen phosphate[J]. Journal of the European Ceramic Society, 2001, 21(10): 2075-2079.
[17] FUKUMOTO S, HOOKABE T, TSUBAKINO H. Hydrolysis behavior of aluminum nitride in various solutions[J]. Journal of Materials Science, 2000, 35(11): 2743-2748.
[18] 张 勇, 郭朝晖, 王 硕, 肖细元. 响应曲面法对铝灰中AlN的水解行为[J]. 中国有色金属学报, 2016, 26(4): 919-927.
ZHANG Yong, GUO Zhao-hui, WANG Shuo, XIAO Xi-yuan. Hydrolysis behavior of AlN in aluminum dross with response surface methodology[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 919-927.
[19] HJ 536—2009. 水质 氨氮的测定 水杨酸分光光度法[S].
HJ 536—2009. Water quality—Determination of ammonia nitrogen —Salicylic acid spectrophotometry[S].
[20] LI X, XU X, ZHOU Q, QI T, LIU G, PENG Z, CUI Y, LI J. Ca3WO6 prepared by roasting tungsten-containing materials and its leaching performance[J]. International Journal of Refractory Metals & Hard Materials, 2015, 52: 151-158.
[21] AL-JAROUDI S S, UL-HAMID A, MOHAMMED A R I, SALIH S. Use of X-ray powder diffraction for quantitative analysis of carbonate rock reservoir samples[J]. Powder Technology, 2007, 175(3): 115-121.
Reaction behavior of active phases in aluminum dross
LIU Gui-hua1, HUANG Wen-qiang1, XIONG De-fen2, WANG Hong-yang1, LI Xiao-bin1, ZHOU Qiu-sheng1, PENG Zhi-hong1, QI Tian-gui1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Yunan Wenshan Aluminium Co., Ltd., Wenshan 663099, China)
Abstract: The mineralogy of aluminum dross and the reaction behavior of the active phase (Al and AlN) in water and sodium hydroxide solution were investigated by XRD and SEM-EDS (microanalysis). The results indicate that more Al and AlN occur in aluminum dross, and AlN content is more than Al content. Al exists alone as spherical particles or appears in Al-Si alloy. AlN can exist alone or co-exist with α-Al2O3 in the shape of large particles or needle-like particles, respectively, corundum (α-Al2O3) appearance is irregular. NaCl occurs alone or appears together with other phases, Al or AlN reacts with water slowly. AlN is more active compared with Al in water. Moreover, the formation of aluminum hydroxide on Al particle limits the reaction of Al in water. However, Al or AlN quickly reacts with the concentrated NaOH solution with reaction rate of above 80% at 25 ℃ for 0.5 h, Al reacts more quickly as comparison with AlN in NaOH solution. Meanwhile, Si and SiO2 also react with the concentrated NaOH solution. In addition, elevating temperature promotes the reaction of AlN and Al in water or NaOH solution.
Key words: aluminum dross; aluminum; aluminum nitride; mineralogy; water; sodium hydroxide
Foundation item: Project(51604309) supported by the National Natural Science Foundation of China
Received date: 2017-08-24; Accepted date: 2018-01-05
Corresponding author: LIU Gui-hua; Tel: +86-731-88830453; E-mail: liugh303@163.com
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51604309)
收稿日期:2017-08-24;修订日期:2018-01-05
通信作者:刘桂华,教授;电话:0731-88830453;E-mail:liugh303@163.com