DOI: 10.11817/j.issn.1672-7207.2015.02.006
酸法制羟基氧化铁催化降解甲基橙研究
张丽清,刘志国,周华锋,吴昊,姜文文
(沈阳化工大学 应用化学学院 辽宁省稀土化学与应用重点实验室,辽宁 沈阳,110142)
摘要:采用酸法制备羟基氧化铁纳米粒子,以其为催化剂,以过氧化氢为氧化剂,进行甲基橙的催化降解反应并推断反应机理。研究催化剂加入量、氧化剂浓度、反应温度和反应pH等对甲基橙降解率的影响,并对催化剂的溶铁量进行测量。研究结果表明:当H2O2浓度为0.23 mmol/L,催化剂质量浓度为0.28 g/L,pH为2.54时,在60 ℃下反应30 min,质量浓度为9.41 mg/L的甲基橙的降解率为97.3%。在催化反应过程中发挥主要作用的OH·由均相催化反应和非均相的表面催化反应提供。由催化剂溶铁而进行的均相催化反应在甲基橙降解过程中可能发挥了重要作用。
关键词:FeOOH;甲基橙;降解;反应机理
中图分类号:O643.13 文献标志码:A 文章编号:1672-7207(2015)02-0416-05
Catalytic oxidation of methyl orange using FeOOH catalyst prepared by acid method
ZHANG Liqing, LIU Zhiguo, ZHOU Huafeng, WU Hao, JIANG Wenwen
(Key Laboratory of Rare-earth Chemistry and Applying of Liaoning Province, School of Applied Chemistry,
Shenyang University of Chemical Engineering, Shenyang 110142, China)
Abstract: FeOOH nanoparticles were prepared by acid method. The degradation of methyl orange in water was investigated using FeOOH as catalyst and H2O2 as oxidant. The effects of the amount of the catalyst, concentration of H2O2, temperature, and pH on the degradation of methyl orange were investigated. The amount of ferrous ions dissolved from FeOOH was measured. The results show that when the mass concentration of FeOOH is 0.28 g/L, the concentration of H2O2 is 0.23 mmol/L, the pH is 2.54, the removal of methyl orange with mass concentration of 9.41 mg/L reaches 97.3% after reacting for 30 min at 60 ℃.In the process of the reaction, the most important oxidant OH· is produced by the homogeneous reaction and heterogeneous reaction. The amount of ferrous ions dissolved from FeOOH plays an important role in catalyzing degradation of methyl orange.
Key words: FeOOH; methyl orange; degradation; reaction mechanism
铁氧化物主要包括铁的氢氧化物和铁的氧化物,广泛存在于自然环境中[1]。其中,α-FeOOH,γ-FeOOH等由于价廉易得,且具有较稳定的物理化学性质,受到研究者的广泛关注。以α-FeOOH,γ-FeOOH等为非均相Fenton反应催化剂、光催化剂氧化降解废水中的有害有机物,取得了较好的效果[2-3]。马军等[4]以α-FeOOH为催化剂,研究了在水相中O3氧化痕量硝基苯,研究表明:在α-FeOOH催化下,O3的一级分解速率常数提高了108%。雷静等[5]以γ-FeOOH为光催化剂,进行了光催化脱色橙黄Ⅰ的研究,并对反应机理做了探讨,认为催化反应过程中产生了具有强氧化性的OH· 。催化剂的制备方法和表面形貌决定催化剂的性能,FeOOH的主要制备方法有酸法、碱法、水热合成法以及电化学法等[6-8],目前采用较多的为碱法与水热合成法[9]。从工业应用角度考虑,酸法反应条件温和,碱的利用率高,废液量较小。本文作者采用酸法制备了α-FeOOH和γ- FeOOH的共存的纳米粒子,以其为催化剂氧化降解甲基橙,并对甲基橙降解机理进行探讨,为处理废水中难降解有毒有机物提供参考。
1 实验
1.1 试剂及仪器
实验试剂为七水合硫酸亚铁(分析纯,天津市大茂化学试剂厂生产)、氢氧化钠(分析纯,天津市大茂化学试剂厂生产)、甲基橙(国药集团化学试剂有限公司生产)和过氧化氢(国药集团化学试剂有限公司生产)。所用水均为去离子水。
主要仪器为DF-101S集热式恒温水浴锅(郑州长城科工贸有限公司生产)、PHS-25数显pH计(上海精密科学仪器有限公司生产)、D8 Advance X线衍射仪(德国布鲁克公司生产)、扫描电镜(SEM)和 722N型可见分光光度计。
1.2 实验方法
催化剂的制备:取一定量的七水合硫酸亚铁溶于蒸馏水中,磁力搅拌下,逐滴加入1 mol/L氢氧化钠溶液,在15 ℃反应13 h,过滤,室温干燥48 h,制得FeOOH催化剂。
甲基橙降解:取一定量的甲基橙配成20 mg/L的储备液备用,实验用H2O2质量分数为30%,以0.01 mol/L高锰酸钾溶液标定。
称取一定量羟基氧化铁置于烧杯中,加入一定量的甲基橙溶液和H2O2,调节溶液pH,密封,在一定温度下定时反应,定时取样,于甲基橙最大吸收波长463 nm处测其吸光度,并计算甲基橙的降解率。
加入OH·捕捉剂的甲基橙降解:在实验确定的最佳条件下,加入甲醇溶液,使反应体系的甲醇浓度为20 mg/L,测定甲基橙的降解率。
溶铁量测量:以本实验选取的最佳条件下,进行溶铁实验。以邻菲罗啉分光光度法测量反应后溶液中二价铁离子质量浓度和总铁质量浓度。
均相催化对比研究:以溶铁实验测得的铁离子浓度为参照,在本实验确定的最佳条件下,进行均相催化甲基橙降解实验。
2 结果与讨论
2.1 催化剂的表征
催化剂的XRD谱图如图1所示。
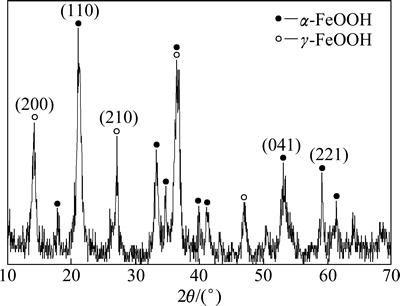
图1 催化剂XRD谱图
Fig. 1 XRD patterns of catalyst
从图1可以看出:2θ在21.04°,33.39°,34.70°,36.75°,39.88°,41.13°,53.14°等处有明显的吸收峰,与标准卡片(PDF:29-0713)及其相关文献[10]比较,此为α-FeOOH的特征吸收峰;2θ在14.22°,27.08°,36.41°,47.05°等处也有较强的吸收峰,与标准卡片及相关文献[11]比较发现,此为γ-FeOOH的特征吸收峰。由此可见,催化剂主要为α-FeOOH和γ-FeOOH的混合物相,且结晶性较好。催化剂的SEM照片如图2所示。从图2可以看出:产物为近纺锤状,直径为30~50 nm,长度为400~500 nm,粒度较均一。
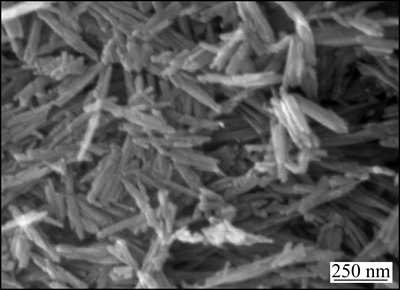
图2 催化剂的SEM照片
Fig. 2 SEM image of catalyst
2.2 甲基橙降解
本实验固定甲基橙溶液质量浓度为9.41 mg/L,考察催化氧化降解甲基橙反应温度、pH、H2O2浓度和催化剂投量对甲基橙降解的影响。实验结果如图3~6 所示。
由图3可知:温度对甲基橙的降解有较大影响,随着反应温度的升高,甲基橙的降解速率逐渐增大,降解率逐渐升高,当温度为60 ℃时,反应30 min后甲基橙的降解率大于97%。这主要是因为随着温度的升高,分子的平均动能增大,会加速生成OH·;另外,温度升高导致反应物分子的运动速度加快,提高反应物之间的碰撞频率和不同相间的传质速率,从而提高了甲基橙的降解反应速率。
反应体系的pH在一定程度上对甲基橙的降解起决定性作用。从图4可知:当pH大于4.1时,反应 90 min内,甲基橙降解率低于10%,当pH小于3.0时,甲基橙才能迅速被降解。
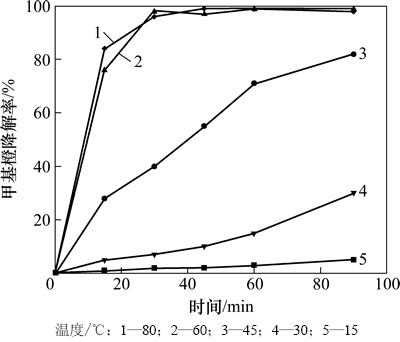
图3 温度对甲基橙降解的影响
Fig. 3 Effect of temperature on methyl orange degradation
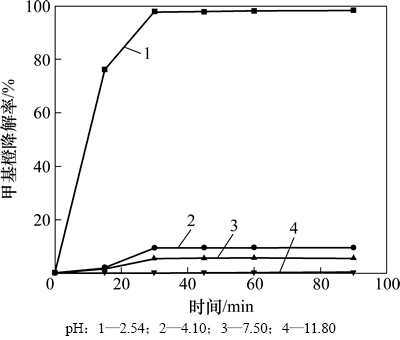
图4 pH对甲基橙降解的影响
Fig. 4 Effect of pH on methyl orange degradation
由图5可知:随着H2O2浓度的增大,甲基橙的降解速率也逐渐增大,当H2O2浓度大于0.23 mmol/L时,甲基橙在30 min内降解率大于97%。反应过程中,催化剂催化H2O2分解,生成具有强氧化性的的OH·,溶液中H2O2含量越高,生成的OH·越多,甲基橙的降解速率越大。
催化剂的用量对甲基橙的降解有一定程度的影响。从图6可知:催化剂的投量增大,甲基橙的降解速率增大,但当催化剂的投量增加到0.28 g/L时,再增加催化剂投量,甲基橙的降解速率变化缓慢。
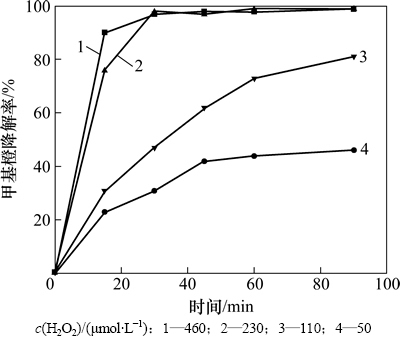
图5 H2O2浓度对甲基橙降解的影响
Fig. 5 Effect of H2O2 concentration on methyl orange degradation
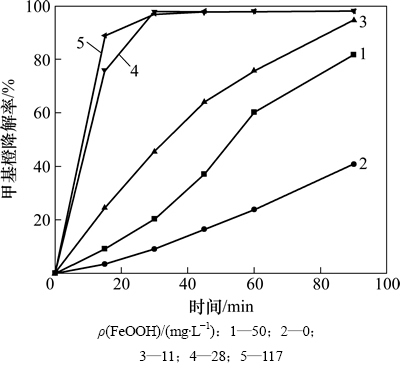
图6 催化剂投量对甲基橙降解的影响
Fig. 6 Effect of catalyst concentration on methyl orange degradation
由以上实验可以确定羟基氧化铁催化过氧化氢氧化降解甲基橙的最佳反应条件为:H2O2浓度0.23 mmol/L,催化剂投量为0.28 g/L,pH 2.54,反应温度 60℃,反应时间30 min,在此条件下,质量浓度为9.41 mg/L的甲基橙的降解率为97.3%。
2.3 反应机理探讨
在最佳反应条件下,反应后溶液中二价铁离子浓度及总铁质量浓度见表1。以此铁离子质量浓度为参照进行均相催化反应,实验结果如图7所示。
表1 催化反应的溶铁量
Table 1 Fe(Ⅱ/III) concentrations in catalyst experiment ρ/(mg·L-1)

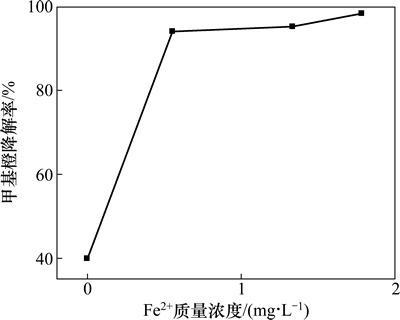
图7 最佳条件下均相催化反应结果
Fig. 7 Results of homogeneous catalysis under optimum conditions
不同的制备方法所制得的催化剂具有不同的微观形貌,对于羟基氧化铁催化剂而言,不同的制备方法获得的催化剂的微观形貌差异巨大,甚至晶相结构也不相同。而催化剂的晶相结构和微观形貌不仅影响催化剂的催化效率,而且会改变催化反应机理。
在本实验所确定的最佳条件下,加入OH·的淬灭剂甲醇后,甲基橙的降解率明显降低,仅为47.9%。对比图6和图7可见:羟基氧化铁的加入明显加快了甲基橙的降解速率,改变了反应历程,产生了具有强氧化性的OH· [12]。
据研究,OH·的来源主要为均相催化反应和非均相表面催化反应,反应(1)为均相催化反应[13]。反应(2)为非均相表面催化反应[14]。
Fe2++H2O2→Fe3++OH·+OH_ (1)
≡FeOH+H2O2→OH·+OH-+≡FeOH+ (2)
在催化降解甲基橙过程中,值得注意的是,反应温度与反应体系的pH对甲基橙的降解率影响很大,特别是当pH大于4.1时,甲基橙在90 min的降解率小于10%。而且,随着pH的增大,甲基橙的降解率逐渐降低,当pH大于11时,甲基橙基本无降解。推测原因可能是:1)在催化降解反应过程中,H+发挥了重要作用,不仅提供酸性环境,而且参与了表面催化反应;2)与催化剂的溶铁作用有关,pH越高,铁离子的溶出量降低,均相Fenton反应减弱,导致甲基橙降解率下降。也可能与两者均有关,是两者共同作用的结果。为此,在实验确定的最佳条件下,对催化剂的溶铁量(反应(3))进行测定,包括二价铁离子质量浓度和总铁质量浓度。
FeOOH+3H+→Fe3++2H2O (3)
最佳反应条件下,反应结束后溶液中的平均总铁质量浓度为1.79 mg/L,二价铁量占总铁量的70%左右;与此相比,在未加目标物甲基橙的反应体系中,总铁量与催化降解反应的总铁量基本相当,但二价铁离子量仅占总铁量的不足5%。由此可见,在加入目标物甲基橙后,整个反应体系中基本没有或仅有微量的二价铁(羟基氧化铁表面吸附的微量二价铁)出现,且其质量浓度达到了1.26 mg/L。Lu等[15]认为有3种机制可以产生二价铁离子。
FeOOH+3H++e-→Fe2++2H2O (4)
Fe3++1/2H2O2→Fe2++1/2O2+H+ (5)
Fe3++还原的有机物→还原的有机物-Fe3+配合物→Fe2++氧化的有机物 (6)
在催化降解甲基橙反应过程中,未加入任何还原剂,反应(4)很难发生,据表1可知反应(5)影响可忽略。Fe2+最有可能的生成机制是反应(6),即Fe3+与目标物生成了配合物,通过配合物从而生成了Fe2+。
以Fe2+为均相催化剂,进行均相催化反应。由图7可知:在本实验所确定的最佳条件下进行均相催化反应,含0.55 mg/L Fe2+的溶液的甲基橙的降解率达到了94%,含1.33 mg/L Fe2+的溶液的甲基橙的降解率已经大于95%。这与文献[16]报道的羟基氧化铁催化苯酚降解的过程有显著不同,两者相比,催化苯酚降解过程中,总铁质量浓度为0.8~1.0 mg/L,此条件下,由溶铁而产生的均相催化反应对苯酚的降解影响很小。而催化甲基橙降解过程中铁离子溶出量较大,这主要与目标物及催化剂的制备方法有关。由此推测,在酸法制备羟基氧化铁催化降解甲基橙的反应过程中,由溶铁作用而进行的均相Fenton反应对甲基橙的降解也起了重要作用。
3 结论
1) 采用酸法制备了FeOOH,并以此为催化剂,催化降解甲基橙,在反应过程中溶液的pH和反应温度对甲基橙的降解起决定性作用,在最佳条件下甲基橙的降解率大于97%。
2) FeOOH催化降解反应过程中,强氧化性的基团OH·主要由均相催化反应产生。
3) 催化降解反应过程中,H+发挥了重要作用,提供酸性环境,使催化剂的溶铁量增加,由催化剂溶铁作用而进行的均相Fenton反应对甲基橙的降解起了重要作用。
参考文献:
[1] 巩志坚, 田原宗, 李文华, 等. 国内铁氧化物研究现状[J]. 材料导报, 2006, 7(7): 19-21.
GONG Zhijian, TIAN Yuanyu, LI Wenhua, et al. The domestic preparation of iron oxides[J]. Materials Review, 2006, 7(7): 19-21.
[2] 姜利荣, 赵超, 黄应平. 可见光照射下α-FeOOH光催化降解有机污染物研究[J]. 环境化学, 2007, 26(4): 434-438.
JIANG Lirong, ZHAO Chao, HUANG Yingping, et al. Photocatalytic degradation of organic pollutants by α-FeOOH under visible light[J]. Environmental Chemistry, 2007, 26(4): 434-438.
[3] 杜卫平, 李臻, 冷文华, 等. 氧化铁和羟基氧化铁光催化还原银离子[J]. 物理化学学报, 2009, 25(8): 1530-1534.
DU Weiping, LI Zhen, LENG Wenhua, et al. Photocatalytic reduction of silver ions on ferric oxides and ferric hydroxides[J]. Acta Physico-Chimica Sinica, 2009, 25(8): 1530-1534.
[4] 马军, 张涛, 陈忠林, 等. 水中羟基氧化铁催化臭氧分解和氧化痕量硝基苯的机理探讨[J]. 环境科学, 2005, 26(3): 299-303.
MA Jun, ZHANG Tao, CHEN Zhonglin, et al. Pathway of aqueous ferric hydroxide catalyzed ozone decomposition and ozonation of trace nitrobenzen[J]. Environmental Chemistry, 2005, 26(3): 299-303.
[5] 雷静, 李芳柏, 刘承帅, 等. γ-FeOOH-草酸系统中橙黄Ⅰ的光化学脱色[J]. 环境科学学报, 2005, 25(10): 1385-1390.
LEI Jing, LI Fangbai, LIU Chengshuai, et al. The photochemical bleaching for orange I in the lepidocrocite-oxalate complex system[J]. Acta Scientiae Circumstantia, 2005, 25(10): 1385-1390.
[6] 张丽清, 刘新锋, 王雅静, 等. 利用工业废铁泥制备α-FeOOH[J]. 化工环保, 2011, 31(3): 244-245.
ZHANG Liqing, LIU Xinfeng, WANG Yajing, et al. Preparation of α-FeOOH from industrial iron sludge[J]. Environmental Protection of Chemical Industry, 2011, 31(3): 244-245.
[7] 朱建育, 施利毅, 张仲燕, 等. 纳米α-FeOOH颗粒的形态和结构[J]. 功能材料, 2002, 33(2): 225-227.
ZHU Jianyu, SHI Liyi, ZHANG Zhongyan, et al. Morphology and structure of nanometer α-FeOOH particles[J]. Journal of Functional Materials, 2002, 33(2): 225-227.
[8] 王国田, 李久义, 田秉晖, 等. 纳米氧化铁絮凝剂的制备及应用研究[J]. 环境工程, 2009, 27(增刊): 606-610.
WANG Guotian, LI Jiyi, TIAN Binghui et al. Preparation of nano-sized iron oxide flocculant and the applied research[J]. Environmental Engineering, 2009, 27(Suppl): 606-610.
[9] 张辉, 邬建波, 杨德仁. 氧化铁和羟基氧化铁纳米结构的水热法制备及其表征[J]. 无机材料学报, 2007, 22(2): 213-218.
ZHANG Hui, WU Jianbo, YANG Deren. Synthesis and characterization of Fe2O3 and FeOOH nanostructures prepared by ethylene glycol assisted hydrothermal process[J]. Journal of Inorganic Materials, 2007, 22(2): 213-218.
[10] 董玉明, 蒋平平, 张爱民. 介孔结构的α-FeOOH对苯酚的催化臭氧化降解[J]. 无机化学学报, 2009, 25(9): 1595-1600.
DONG Yuming, JIANG Pingping, ZHANG Aimin. Catalytic ozonation degradation of phenol in water by mesoporous α-FeOOH[J]. Chinese Journal of Inorganic Chemistry, 2009, 25(9): 1595-1600.
[11] 车阿小, 李春忠, 朱以华, 等. EDTA对下γ-FeOOH制备过程的影响[J]. 化学物理学报, 1999, 12(2): 209-213.
CHE Axiao, LI Chunzhong, ZHU Yihua, et al. Effect of EDTA on preparation of γ-FeOOH[J]. Chinese Journal of Chemical Physics, 1999, 12(2): 209-213.
[12] 高真, 雷国元, 姜成春. Si-FeOOH 非均相Fenton 降解活性艳红MX-5B的效能研究[J]. 环境科学学报, 2011, 31(4): 765-769.
GAO Zhen, LEI Guoyuan, JIANG Chengchun. Degradation of reactive red MX-5B by heterogeneous Fenton reaction over Si-FeOOH[J]. Acta Scientiae Circu-Mstantia, 2011, 31(4): 765-769.
[13] 林志荣, 赵玲, 董元华, 等. 针铁矿催化过氧化氢降解PCB28[J]. 环境科学学报, 2011, 31(11): 2403-2407.
LIN Zhirong, ZHAO Ling, DONG Yuanhua, et al. Degradation of PCB28 by goethite-catalyzed hydrogen peroxide[J]. Acta Scientiae Circumstantia, 2011, 31(11): 2403-2407.
[14] Christina R K, David L S. Factors affecting the yield of oxidants from the reaction of nanoparticulate zero-valent iron and oxygen[J]. Environ Sci Technol, 2008, 42(4): 1262-1267.
[15] LU Mingchun, CHEN Jongnan, HUANG Hushui. Role of goethite dissolution in the oxidation of 2-chlorphenol with hydrogen peroxide[J]. Chemosphere, 2002, 46(1): 131-136.
[16] 吴大清, 刁桂仪, 袁鹏. 针铁矿纤铁矿催化降解苯酚动力学速率及其反应产物研究[J]. 生态环境, 2006, 15(4): 714-719.
WU Daqing, DIAO Guiyi, YUAN Peng. Catalytic decomposition products and kinetic rates of phenol by hydrogen peroxide with goethite and lepidocrocite[J]. Ecology and Environment, 2006, 15(4): 714-719.
(编辑 赵俊)
收稿日期:2014-01-13;修回日期:2014-04-20
基金项目(Foundation item):辽宁省高校重点实验室基金资助项目(LS2010122);辽宁省自然科学基金资助项目(2014020139)(Project (LS2010122) supported by the Key Laboratory Funds of Universities of Liaoning Province; Project (2014020139) supported by the Natural Science Foundation of Liaoning Province of China)
通信作者:张丽清,博士,教授,从事矿物分离及材料制备研究;E-mail:zlqsyuct@163.com