CaSn(OH)6的结构及其在锌镍电池中的应用
杨占红,王升威,廖建平,胡俊,易师
(中南大学 化学化工学院,湖南 长沙,410083)
摘 要:为了改善锌负极的循环性能,采用共沉淀法合成CaSn(OH)6,并将其作为锌镍电池的负极添加剂,研究其对锌负极电化学性能的影响。研究结果表明:通过共沉淀方法合成的CaSn(OH)6粒度分布均匀,平均粒径在1 μm左右,并且具有较高的纯度;添加CaSn(OH)6的锌负极在循环过程中游离出了Ca(OH)2和金属锡等有益物质,并进一步与ZnO反应生成锌酸钙,降低了锌负极活性物质在电解液中的溶解性,从而显著提高了锌电极的循环性能;以CaSn(OH)6与ZnO的混合物为负极活性物质的模拟锌镍电池具有较高的放电平台和优良的循环性能,经过50次循环后,电池放电容量保持为起始容量的72.6%。
关键词:添加剂;锌酸钙;锌负极;循环伏安法;共沉淀
中图分类号:TM 912.2 文献标志码:A 文章编号:1672-7207(2010)01-0073-04
Structure of CaSn(OH)6 and its application in Ni/Zn battery
YANG Zhan-hong, WANG Sheng-wei, LIAO Jian-ping, HU Jun, YI Shi
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: In order to improve the cycling performance of zinc electrode, CaSn(OH)6 was prepared by coprecipitation method and added in zinc anode. The influences of CaSn(OH)6 on electrochemical properties of Zn/Ni batteries were studied. The results show that the sample is well dispersed particles with high purity and an average size of about 1 μm. Favorable substances of Ca(OH)2 and Sn appear during the cycling of zinc electrode containing CaSn(OH)6, Ca(OH)2 has continues to react with ZnO to produce calcium zincate, which can highly reduce the dissolvability of anode active materials and greatly improve the cycling performance of zinc electrode. The simulated Ni/Zn battery using CaSn(OH)6 and ZnO as the active material of zinc electrode has a high discharge plateau and an excellent cycling ability, and it can retain about 72.6% of initial discharge capacity after 50 charge-discharge cycles.
Key words: additive; calcium zincate; zinc electrode; cyclic voltammetry; coprecipitation
碱性可充锌基电池因其具有高能量密度、无环境污染等特点,而引起人们的广泛关注,但电极材料的形变、枝晶等问题严重制约了锌电极的开发利用[1]。为了解决这些问题,人们尝试了许多解决方法,如:向电解液中加入KF,K3BO3,K3PO4等物质以降低放电产物的溶解度[2-3];往电极添加PbO,In2O3,In (OH)3,Bi2O3或TiO2等物质,通过改变电流的分配来减缓枝晶和形变[4-6];选用优良的隔膜阻止枝晶的穿透和锌酸盐的传递[7]等等。其中,以向电极中添加Ca(OH)2效果较显著[8-9]。其依据是:在充放电过程中,Ca(OH)2与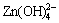 反应形成溶解度更小的锌酸钙(Ca(OH)2?2Zn(OH)2?2H2O),抑制了
反应形成溶解度更小的锌酸钙(Ca(OH)2?2Zn(OH)2?2H2O),抑制了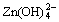 的迁移,从而增强了电极的稳定性,并提高电极的循环寿命。预先合成具有电化学活性的Ca(OH)2?2Zn(OH)2?2H2O化合物比直接由ZnO和Ca(OH)2进行相互混合形成的混合物具有更好的性能[10-11]。但是,由于锌酸钙具有比容量低、导电性差、合成工艺较复杂等缺点,锌酸钙并不适用于大规模的锌电极工业化生产。目前,在锌电极中直接添加Ca(OH)2成为工业化研究的热点。然而,由于Ca(OH)2具有较强的碱性,会对黏合剂的使用造成一定的影响,造成锌负极浆料流动性差,不方便拉浆等。CaSn(OH)6是一种pH值为中性的化学物质,主要作为制备CaSnO3的前驱体,人们对CaSn(OH)6的制备与表征研究较少[12-13],对于CaSn(OH)6在锌镍电池方面的应用研究还未见文献报道。为此,本文作者采用共沉淀法合成CaSn(OH)6,并对其在锌镍电池中的应用进行研究。
的迁移,从而增强了电极的稳定性,并提高电极的循环寿命。预先合成具有电化学活性的Ca(OH)2?2Zn(OH)2?2H2O化合物比直接由ZnO和Ca(OH)2进行相互混合形成的混合物具有更好的性能[10-11]。但是,由于锌酸钙具有比容量低、导电性差、合成工艺较复杂等缺点,锌酸钙并不适用于大规模的锌电极工业化生产。目前,在锌电极中直接添加Ca(OH)2成为工业化研究的热点。然而,由于Ca(OH)2具有较强的碱性,会对黏合剂的使用造成一定的影响,造成锌负极浆料流动性差,不方便拉浆等。CaSn(OH)6是一种pH值为中性的化学物质,主要作为制备CaSnO3的前驱体,人们对CaSn(OH)6的制备与表征研究较少[12-13],对于CaSn(OH)6在锌镍电池方面的应用研究还未见文献报道。为此,本文作者采用共沉淀法合成CaSn(OH)6,并对其在锌镍电池中的应用进行研究。
1 实验
1.1 样品的制备
参照文献[14],将物质的量比为1?1的CaCl2溶液和SnCl4溶液充分搅拌均匀,在连续搅拌的条件下,将过量的NaOH溶液缓慢滴加到混合溶液中。继续搅拌5 h,静置15 h,减压抽滤,所得沉淀物经蒸馏水洗涤至中性后于80 ℃真空干燥器中干燥6 h,得白色粉末。化学反应方程式为:
Ca2++Sn4++6OH-→CaSn(OH)6 (1)
1.2 样品的X线衍射和SEM分析
X线衍射分析采用日本Digaku D/max 2550VB+ 18 kW转靶X线衍射仪,选用Cu靶Kα辐射,加速电压为40 kV,电流为30 mA,扫描速度为5(?)/min。采用日本电子公司生产的JSM-6360LV型扫描电子显微镜对CaSn(OH)6样品的表面形态进行观察。
1.3 锌负极片的制作
将质量分数为15%的CaSn(OH)6与70% ZnO、3%石墨以及10%锌粉混合均匀,再加入粘合剂(0.5% HPMC干粉和1.5% PTFE乳液)和适量水调制成浆,涂覆在铜网集流体上,经烘干、压片后制成锌电极。
为了对比实验结果,采用85% ZnO、3% 石墨、10% 锌粉、0.5% HPMC干粉、1.5% PTFE乳液的配方按上述方法制备不添加CaSn(OH)6的锌电极。
1.4 循环伏安测试
循环伏安实验采用Princeton Applied Research公司生产的PARSTAT 2273电化学测试系统进行测试。工作电极是分别以纯氧化锌和氧化锌与CaSn(OH)6的混合物为活性物质的粉末微电极,具体做法参考文献[15],以Hg/HgO电极为参比电极,大面积镍片为辅助电极。隔膜采用聚乙烯接枝膜,电解液为6 mol/L KOH溶液。扫描范围为-1.5~ -1.2 V,扫描速率为50 mV/s。
1.5 锌镍模拟电池恒电流充放电实验
将制得的锌负极片用聚乙烯接枝膜包裹2层,并放置于2片正极之间组成模拟电池极组,放入1个自制有机玻璃盒中,加入一定量的6 mol/L KOH电解液,组装成锌镍模拟电池。正极为大面积烧结式镍正极,其容量远大于锌负极容量。采用BS-9300R二次电池性能检测装置进行模拟电池的恒电流充放电测试。先以0.1C(C为倍氧,指电池在规定时间内放出额定容量时的电流)的电流对电池进行化成,然后进行充放电循环,充放电循环制度为:以0.3C的电流充电3.5 h,然后以0.3C放电,充电限制电压为2.5 V,放电终止电压为1.4 V,测试环境温度为25 ℃。为保证负极充满,充电倍率电流按负极活性物质的理论容量计算。
2 结果与讨论
2.1 CaSn(OH)6材料循环前后的X线衍射检测
新制得的CaSn(OH)6的X线衍射分析结果如图1(a)所示。从图1(a)可以看出:通过共沉淀法制备出的样品与JCPDS卡片上73-2383号非常相符,这说明该方法所制备的CaSn(OH)6样品具有非常高的纯度。图中衍射峰的宽化说明样品的颗粒粒径较小,结晶度不高。
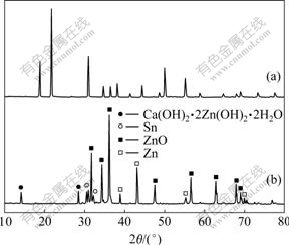
(a) CaSn(OH)6样品;(b)循环后的负极活性物质
图1 CaSn(OH)6样品和循环后的负极活性物质的XRD谱
Fig.1 XRD patterns of CaSn(OH)6 sample and active material after cycling
图1(b)所示为充放电循环后的负极材料(添加CaSn(OH)6的电极)的X线衍射结果。由图1(b)可见,循环后负极材料的化学组分发生了改变,CaSn(OH)6的衍射峰消失,而出现了一些其他物质的衍射峰,经分析,是锌酸钙(Ca(OH)2?2Zn(OH)2?2H2O)和金属锡的特征峰。
2.2 CaSn(OH)6材料的表观形貌
图2所示为共沉淀法制备的CaSn(OH)6样品的SEM照片。从图2可以看出:该样品的颗粒粒径较小,粒度分布较均匀,平均粒径约为1 μm左右。较小的粒径有利于在电极材料中的分散,所以,采用该方法制备的CaSn(OH)6样品比较适合作为电极添加剂。

图2 CaSn(OH)6的SEM照片
Fig.2 SEM image of CaSn(OH)6
2.3 CaSn(OH)6材料的循环伏安特性
图3所示为氧化锌与CaSn(OH)6混合物和纯氧化锌的粉末微电极的第6次循环伏安曲线。从图3可以看出:曲线1只在-1.50 V处有1个还原峰,此为氧化锌的特征还原峰,而曲线2在-1.50 V和-1.39 V处各有1个还原峰。结合对循环后负极材料的XRD检测结果可知,该电极中的锌酸钙(Ca(OH)2?2Zn(OH)2? 2H2O)是在充放电循环过程中产生的。其原因是CaSn(OH)6中含有的Sn元素在充电过程中被还原成金属单质,而Ca元素则被游离出来生成Ca(OH)2,Ca(OH)2接着在强碱性条件下与电极中的氧化锌反应,生成了锌酸钙。其化学反应方程式如下:
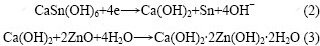
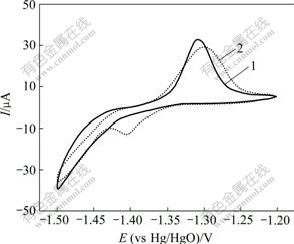
1—纯氧化锌粉末;2—添加CaSn(OH)6的粉末
图3 2种粉末微电极的循环伏安曲线
Fig.3 Cyclic voltammograms of two powder microelectrodes
2.4 锌镍模拟电池的恒电流放电曲线
图4所示为含2种锌电极的锌镍电池恒电流循环的第8次放电曲线。从图4可以看出:未添加CaSn(OH)6的电极能放出较多的电量,其原因是氧化锌在负极材料中含量较高,具有较高的理论比容量。但是,添加CaSn(OH)6的电极具有较高的放电平台,这是由于CaSn(OH)6在循环过程中生成了具有良好导电性能的金属锡,金属锡在充放电过程中将不再被氧化,而一直以单质金属形式存在,能在锌电极中形成导电网络,从而大大减弱了锌电极在放电过程中的极化现象。
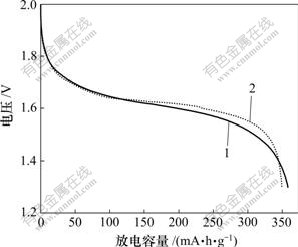
1—纯氧化锌负极;2—添加CaSn(OH)6的锌负极
图4 锌镍电池的第8次恒电流放电曲线
Fig.4 Discharge curves of simulated Ni/Zn batteries at 8th cycle
2.5 锌镍模拟电池恒电流充放电循环性能
图5所示为锌镍模拟电池的恒电流放电容量与循环次数的关系。从图5可以看出:纯氧化锌电极的容量衰减很快,在30次充放电循环后,放电容量已衰减到起始容量的45.3%。这是由于纯氧化锌在强碱性溶液中具有较大的溶解性,而氧化锌在电解液中溶解后,会不可逆地扩散到电解液中,造成锌电极活性物质的损失和锌电极放电容量的衰减。而添加CaSn(OH)6的电极的放电容量衰减较为缓慢。其原因是CaSn(OH)6在循环过程中能够产生Ca(OH)2、锌酸钙和金属锡等有益物质,在Ca(OH)2和锌酸钙的作用下,锌电极放电产物在电解液中的溶解度会大大减小,在一定程度上减少了锌电极活性物质的损失,缓解了负极的形变、枝晶等问题,而锌镍电池的循环性能也得到提高。经过50次循环,电池放电容量仍保持为起始容量的72.6%。而化成过程中生成的金属锡在充电过程中将不再会被氧化,从而在锌电极中形成导电网络,由于金属锡具有较大的析氢过电位,能有效抑制锌电极的析氢反应,所以,金属锡对锌镍电池容量的保持也具有一定的促进作用。
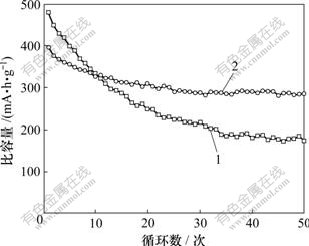
1—纯氧化锌负极;2—添加CaSn(OH)6的锌负极
图5 放电容量与循环次数的关系
Fig.5 Relationship between discharge capacity and cycle number
3 结论
(1) 采用共沉淀法合成出的CaSn(OH)6样品粒度分布均匀,粒径较小,并且纯度较高,比较适合作为电极添加剂。
(2) CaSn(OH)6在充电过程中能游离出金属锡和Ca(OH)2,并能在充放电循环过程中进一步生成锌酸钙。
(3) 添加CaSn(OH)6样品的锌镍电池具有较高的放电平台和更好的循环性能。锌酸钙的生成可以降低锌电极活性物质在电解液中的溶解度,从而在一定程度上减少了锌负极的形变和枝晶等,提高了锌电极的循环性能。
参考文献:
[1] 金达莱, 岳林海, 徐铸德. 锌负极材料锌酸钙的晶体形貌和物化性质研究[J]. 无机化学学报, 2005, 21(2): 265-269.
JIN Da-lai, YUE Lin-hai, XU Zhu-de. Morphology and physical properties of calcium zincate[J]. Chinese Journal of Inorganic Chemistry, 2005, 21(2): 265-269.
[2] Zhu J L, Zhou Y H, Gao C Q. Influence of surfactants on electrochemical behavior of zinc electrodes in alkaline solution[J]. Journal of Power Sources, 1998, 72(2): 231-235.
[3] Chen J S, Mclarnon E R, Cairns E J. Investigations of low-zinc-solubility electrodes and electrolytes in zinc/silver oxide cells[J]. Journal of Power Sources, 1992, 39(3): 333-348.
[4] McBreen J, Gannon E. The electrochemistry of metal oxide additions in pasted zinc electrode[J]. Electrochimica Acta, 1981, 26(10): 1439-1446.
[5] Shivkumar R, Kalaignan G P, Vasudevan T. Studies with porous zinc electrodes with additives for secondary alkaline batteries[J]. Journal of Power Sources, 1998, 75(1): 90-100.
[6] Lan C J, Lee C Y, Chin T S. Tetra-alkyl ammonium hydroxides as inhibitors of Zn dendrite in Zn-based secondary batteries[J]. Electrochimica Acta, 2007, 52(17): 5407-5416.
[7] Zhu J L, Zhou Y H. Effects of ionomer films on secondary alkaline zinc electrodes[J]. Journal of Power Sources, 1998, 72(2): 266-270.
[8] Mclarnon F R, Cairns E J. The secondary alkaline zinc electrode[J]. Journal of the Electrochemical Society, 1991, 138(22): 645-664.
[9] ZHU X M, YANG H X, AI X P, et al. Structural and electrochemical characterization of mechanochemically synthesized calcium zincate as rechargeable anodic materials[J]. Journal of Applied Electrochemistry, 2003, 33(7): 607-612.
[10] 张春, 王建明, 张昭, 等. 钙添加剂对可充锌电极性能的影响[J]. 中国有色金属学报, 2001, 11(5): 780-784.
ZHANG Chun, WANG Jian-ming, ZHANG Zhao, et al. Effects of calcium additive on performance of pasted zinc electrode[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(5): 780-784.
[11] 杨占红, 王升威, 曾利辉, 等. 化学合成法制备锌镍电池负极材料锌酸钙[J]. 中南大学学报: 自然科学版, 2008, 39(5): 1-5.
YANG Zhan-hong, WANG Sheng-wei, ZENG Li-hui, et al. Chemosynthesis of calcium zincate as negative electrode material for Ni/Zn battery[J]. Journal of Central South University: Science and Technology, 2008, 39(5): 1-5.
[12] Mandal K D, Sastry M S, Parkash O. Preparation and characterization of calcium stannate[J]. Journal of Materials Science Letters, 1995, 14(17): 1412-1413.
[13] Pfaff G. Chemical synthesis of calcium stannates from peroxo precursors[J]. Materials Science and Engineering B, 1995, 33(2/3): 156-161.
[14] HE Ze-qiang, LI Xin-hai, LIU En-hui, et al. Preparation of calcium stannate by modified wet chemical method[J]. Journal of Central South University of Technology, 2003, 10(3): 195-197.
[15] Cha C S, Li C M, Yang H Y, et al. Powder microelectrodes[J]. Journal of Electroanalytical Chemistry, 1994, 368(1/2): 47-54.
收稿日期:2009-03-03;修回日期:2009-08-10
基金项目:国家科技支撑计划项目(2006BAE03B03)
通信作者:杨占红(1969-),男,河南新乡人,教授,博士生导师,从事电化学研究;电话:0731-88879616;E-mail: zhanhongyang@126.com
(编辑 赵俊)