
Preparation of calcium phosphate coatings on Mg-1.0Ca alloy
ZHANG Chun-yan(张春艳)1, 3, ZENG Rong-chang(曾荣昌)1, CHEN Rong-shi(陈荣石)2,
LIU Cheng-long(刘成龙)1, GAO Jia-cheng(高家诚)3
1. School of Materials Science and Engineering, Chongqing University of Technology, Chongqing 400050, China;
2. Environmental Corrosion Center, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China;
3. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
Received 23 September 2009; accepted 30 January 2010
Abstract: The calcium phosphate coatings were prepared by virtue of electrochemical deposition in order to improve the corrosion resistance of Mg-1.0Ca alloys in simulated body fluids. The chemical compositions, structures and morphologies of the coatings were investigated by energy dispersive spectroscopy (EDS), X-ray diffractometry (XRD) and scanning electron microscopy (SEM), respectively. The potentiodynamic electrochemical technique was employed to investigate the bio-degradation behavior of Mg-1.0Ca alloys with Ca-P coatings in Hank’s solutions. The experimental results show that the deposited coatings predominately consist of flake-shape brushite (DCPD, CaHPO4·2H2O) crystallites. The corrosion resistance of the substrates with coatings is improved in Hank’s solutions significantly.
Key words: coating; magnesium alloy; corrosion; biomaterials; simulated body fluids
1 Introduction
The magnesium alloys exhibit an attractive combination of biocompatibility, biodegradability and mechanical properties analogous to natural bones[1-2]. These properties make them become ideal candidates for biodegradable implant applications. In recent years, there is an increasing research on magnesium alloys used as biodegradable metal implants in potential orthopedic surgery. Currently, the studies are mainly focused on commercial alloys, such as Mg-Al and Mg-RE alloys, due to their relatively high strength and good corrosion resistance[3-7]. However, it has been reported that Al element can cause nerve toxicity and restraining growth to human body[8]. And the rare earth elements, such as cerium, praseodymium and yttrium, may lead to a severe hepatotoxicity[9]. The alloying elements Ca, Zn, Mn can be tolerated in human body and can also retard the biodegradation of magnesium alloys[10]. Calcium is one of the most attractive elements due to the fact that it is a major component of human bones and essential in chemical signaling with cells. In addition, Mg-Ca alloys have a similar density to bones and magnesium is necessary for the incorporation of calcium into bones[11]. Nevertheless, these alloys have issues identical to other magnesium alloys, and this shows that they have a low corrosion resistance[11].
One of the effective approaches to reduce their degradation rates is to coat the base materials. The calcium phosphates, including hydroxyapatite (HA), octacalcium phosphate(OCP) and brushite (DCPD), have been widely utilized as the coatings of bone implant materials because of their excellent biocompatibility, non-toxicity, bioactivity and bone inductivity[12-13]. Up to now, some scientists attempt to prepare calcium phosphate coatings on magnesium alloys to manipulate the degradation rates[14-17]. The results indicate the calcium phosphate coatings can improve the corrosion properties of Mg-Al alloys. No report dealing with the study on the degradation rate of Mg-Ca alloys with calcium phosphate coatings can be found in the literatures[1-2]. Thus, this study aims to prepare calcium phosphate coatings on Mg-1.0Ca alloy by electrochemical deposition and investigates their bio-degradation behavior by means of potentiodynamic electrochemical technique in Hank’s solutions.
2 Experimental
The substrate material used was Mg-1.0Ca magnesium alloy (nominal chemical composition: 1.0% Ca (mass fraction), balance Mg) with size of 20 mm×20 mm×2 mm. The sample surfaces were ground by 200- 1 200 grit SiC papers, and then cleaned ultrasonically in acetone for 15 min and finally dried with hot air. Samples 1, 2, 3 and 4 were electro-deposited on Mg-1.0Ca alloys in a bath, containing the solution of 42 mmol/L Ca(NO3)2·4H2O and 25 mmol/L NH4H2PO4 with pH value of 5.0 for 20, 60, 120 and 240 min, respectively. The Mg-1.0Ca alloy substrate was used as the cathode for electro-deposition with an applied cathode potential of -2.5 V versus saturated calomel electrode (SCE). The coating structure was analyzed by means of X-ray diffractometry (XRD). The morphology was observed by virtue of a JSM-6460LV type scanning electron microscopy (SEM) and its affiliated energy dispersive spectroscopy (EDS) was used to probe the chemical compositions.
The electrochemical tests were performed on an EG&G 273 type potentiostat in a classical three-electrode cell with platinum as counter electrode, saturated calomel electrode (SCE) as reference electrode and the uncoated or coated samples as working electrode with an exposed surface area of 2.84 cm2. The potential was scanned from -300 mV to 300 mV versus free corrosion potential (φcorr) at rate of 0.5 mV/s. Hank’s solutions consisted of 8 g/L NaCl, 0.4 g/L KCl, 0.14 g/L CaCl2, 0.35 g/L NaHCO3, 1.0 g/L C6H6O6, 0.1 g/L MgCl2·6H2O, 0.06 g/L MgSO4·7H2O, 0.06 g/L KH2PO4 and 0.06 g/L Na2HPO4·12H2O with pH of 7.4. All potentials correspond to SCE.
3 Results and discussion
3.1 Morphologies of coatings
The morphologies of the coatings formed on the Mg-1.0Ca substrates with deposition time of 20, 60, 120 and 240 min are illustrated in Fig.1. It is obvious that the deposition time has a significant influence on the morphologies of the coatings. After 20 min deposition, a few dot-like and lamellar crystallites have already been formed on the surfaces of the substrates. The magnitude of area B in Fig.1(a) shows a fissure surface with white floccules observed under the crystallites. After 60 min deposition, the crystallites partly cover the surface of the substrate. The coating consists of lamellar crystallites distributed irregularly (Fig.1(b)). Just as exhibited by the
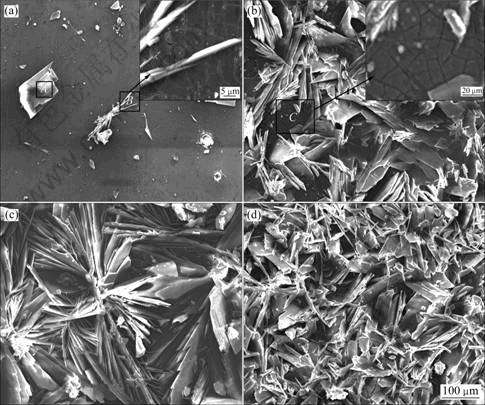
Fig.1 SEM images of samples 1(a), 2(b), 3(c) and 4(d)
magnitude of area B in Fig.1(a), a fissure surface can also be seen of area C in Fig.1(b). After being deposited for 2 h, the lamellar crystallites grow larger. The crystallites of the coating appear regular flower-like structure diverging from centre towards periphery (see Fig.1(c)). Fig.1(d) displays that the surface of sample 4 is fully covered with dense lamellar crystallites which are finer and fairly uniform distributed compare with those of the other samples. And the lamellar crystallites of the coating interlace together and form micropores. The microporous structures may be helpful for the bone tissues to infiltrate into the implants then to accelerate the healing of the damaged bones[12]. The thickness of the Ca-P coating (shown in Fig.2) is 20-25 μm on sample 4.
EDS results show that the lamellar crystallite (area A) in Fig.1(a) consists of oxygen, phosphorus and calcium, and n(Ca)/n(P)=1.01 which is approximate the n(Ca)/n(P) value of DCPD(Fig.3(a)). The area C in Fig.1(b) is rich in magnesium, oxygen and a small amount of phosphorus and calcium (Fig.3(b)), indicating Mg(OH)2 film containing phosphorus and calcium forms on the surface of substrate. The fissure surface may be caused by the dehydration of the layer after drying.
3.2 XRD analysis of coatings
Fig.4 shows the XRD patterns of the coatings formed on Mg-1.0Ca substrates after electro-deposition for different times. These patterns represent crystallographic structures. Besides the peaks of α-Mg and Mg2Ca phases in the substrates, some special peaks correspond to brushite (DCPD, CaHPO4 2H2O) of the (020), (021), (040) and (041) planes. No other calcium phosphates, e.g. OCP and HA, are observed, suggesting that the deposition time has no influence on the transformation of DCPD to other calcium phosphate phases. But the intensity of the diffraction peaks of
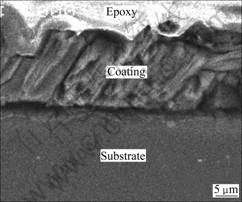
Fig.2 SEM image of sample 4 on cross-sectional view
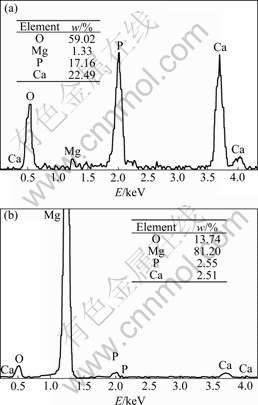
Fig.3 EDS spectra of areas A(a) and B(b) in Fig.1(a)
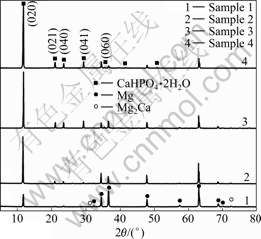
Fig.4 XRD patterns of coatings formed on substrates
DCPD increases from 20 min to 120 min, while that of α-Mg decreases. Moreover, the narrow line widths and high intensities of the diffraction peaks reveal that the crystallinity of DCPD is relatively high. It was reported that a highly crystalline structure yields less dissolution of the coating[18]. Therefore, the high degree of crystallinity may be beneficial to decreasing the biodegradation rate of Ca-P coating.
The formation of DCPD among calcium phosphate phases is closely dependent upon the super-saturation and the pH values of the solutions[19]. At lower pH, DCPD may be involved as a precursor phase. It is reported that magnesium ion markedly inhibits HA crystal growth in a solution supersaturated only with respect to this phase, has a modest influence on the kinetics of OCP growth, and has practically no effect on DCPD crystallization[19]. Therefore, in the case of pH=5.0 of the solutions, the deposited coatings on Mg-1.0Ca predominately consist of DCPD (CaHPO4·2H2O) crystallites.
3.3 Corrosion behaviour
The polarization curves for the substrate and Ca-P coated samples in Hank’s solutions are shown in Fig.5. Tafel fits are employed to analyze the polarization curves. On one hand, as can be seen in Fig.5, the Mg-1.0Ca alloy substrate exhibits a lowest φcorr of -1.65 V. The φcorr values of the coated samples 1, 2, 3 and 4 are approximately -1.60, -1.54, -1.51 and -1.46 V, respectively, which are fairly higher than that of the substrates. The coated sample 4 has a highest φcorr, followed by sample 3, 2 and 1, which implies that the thickness and the coverage of coating influence the φcorr. On the other hand, the corrosion current density of coated samples is lower than that of substrates. The coated sample 4 with best coverage and dense crystallites, has the lowest corrosion current density of 7.47×10-6 A/cm2, nearly two orders of the magnitude lower than that (5.9×10-4 A/cm2) of the substrates.
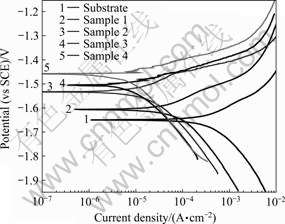
Fig.5 Polarization curves of Mg1.0-Ca alloy and its coatings
After a three-day immersion in Hank’s solution, the corrosion pits occur on the substrate, while merely slight corrosion appears on the coated samples. But the lamellar crystallites of the coatings are partly dissolved. The mass loss of the coated sample 4 is just one fourth that of its substrate, further revealing the degradation rates of the substrates with the coatings decrease significantly.
4 Conclusions
1) A calcium phosphate coating was successfully prepared on Mg-1.0Ca alloys by means of electrochemical deposition. The deposited coatings consist of flake-shape brushite (DCPD, CaHPO4·2H2O) crystallites. The deposition time has obvious influence on the morphologies of the coatings, but has no impact on the transformation of DCPD.
2) The free corrosion potential of the coated samples is fairly higher than that of substrate, and the corrosion current density of coated samples is two orders of the magnitude lower than that of the Mg-1.0Ca substrate, which indicates that the Ca-P coating can improve the corrosion resistance of Mg-1.0Ca alloy in Hank’s solutions.
3) The thickness and morphology of the coating have significant impact on the corrosion behavior of Mg-1.0Ca alloy.
Acknowledgements
The material used was provided by Dr. CHEN Rong-shi in Institute of Metals Research, Chinese Academy of Sciences.
References
[1] MARK P S, ALEXIS M P, JERAWALA H, GEORGE D. Magnesium and its alloys as orthopedic biomaterials: A review [J]. Biomaterials, 2006, 27(9): 1728-1734.
[2] ZENG Rong-chang, DIETZEL W, WITTE F, HORT N, BLAWERT C. Progress and challenge for magnesium alloys as biomaterials [J]. Adv Eng Mater, 2008, 10: B03-B14.
[3] WITTE F, KAESE V, SWITZER H, MEYER L A, WIRTH C J, WINDUAG H. In vivo corrosion of four magnesium alloys and the associated bone response [J]. Biomaterials, 2005, 26(17): 3557-3563.
[4] LI Long-chuan, GAO Jia-cheng, WANG Yong. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid [J]. Surf Coating Technol, 2004, 185(7): 92-98.
[5] WITTEA F, FISCHER J, NELLESEN J, CROSTACK H, CROSTAC H A. In vitro and in vivo corrosion measurements of magnesium alloys [J]. Biomaterials, 2006, 27(7): 1013-1018.
[6] ZARTNER P, CESENJEVAR R, SINGER H, WEYAND M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby [J]. Catheter Cardiovasc Interv, 2005, 66(4): 590-594.
[7] HONG Yan-song, YANG Ke, ZHANG Guang-dao, HUANG Jing-jing, HAO Yu-quan, AI Hong-jun. The role of bone induction of a biodegradable magnesium alloy [J]. Acta Metallurgica Sinica, 2008, 44(9): 1035-1041. (in Chinese)
[8] ZHANG Er-lin, LEI Yang. Microstructure, mechanical properties and bio-corrosion properties of Mg-Zn-Mn-Ca alloy for biomedical application [J]. Mater Sci Eng A, 2008, 497(12): 111-118.
[9] YUMIK O N, YUKARI T, YASUHIDE T, TADASHI S, YOSHHIO I. Differences in behavior among the chlorides of seven rare earth elements administered intravenously to rats [J]. Fundam Appl Toxicol, 1997, 37(2): 106-116.
[10] SONG Guang-ling. Control of biodegradation of biocompatable magnesium alloys [J]. Corros Sci, 2007, 49(4): 1696-1701.
[11] LI Zi-jian, GU Xu-nan, LOU Si-quan, ZHENG Yu-feng. The development of binary Mg-Ca alloys for use as biodegradable materials within bone [J]. Biomaterials, 2008, 29 (10): 1329-1344.
[12] SUHANEC W, YOSHIMURA M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants [J]. J Mater Res, 1998, 13(1): 94-117.
[13] ZHANG Chun-yan, GAO Jia-cheng, LI Long-chuan, WANG Yong. Calcium phosphate bioceramics coating deposited on Ti6Al4V by wet chemical reaction [J]. The Chinese Journal of Nonferrous Metals, 2002, 11(s12): 117-121. (in Chinese)
[14] SONG Y W, SHAN D Y, HAN E H. Electrodeposition of hydroxyapatite coating on AZ31D magnesium alloy for biomaterial application [J]. Mater Lett, 2008, 62(6): 3276-3279.
[15] CUI Fu-zai, YANG Jing-xin, JIAO Yan-ping, YIN Qing-shui, ZHANG Yin, LEE I S. Calcium phosphate coating on magnesium alloy for modification of degradation behavior [J]. Front Mater Sci China, 2008, 2(2): 143-148.
[16] ZHANG Chun-yang, ZENG Rong-cang, CHEN Jun, YANG Hui, TAN Zong-qing. A study on chemical deposition of calcium phosphate bioceramics coating on magnesium alloy AZ31 [J]. Rare Met Mater Eng, 2009, 38(8): 1363-1367. (in Chinese)
[17] HIROMOTO S, YAMAMOTO A. High corrosion resistance of magnesium coated with hydroxyapatite directly synthesized in an aqueous solution [J]. Electrochimica Acta, 2009, 54(27): 7085-7093.
[18] ELIA N, SRIDHAR T M, KAMACHI M U, RAJ B. Electrochemical and electrophoretic deposition of hydroxyapatite for orthopaedic applications [J]. Surf Eng, 2005, 21(3): 238-242.
[19] MATS S, JOHNSON A, GEORGE H. The role of brushite and octacalcium phosphate in apatite formation [J]. Critical Reviews in Oral Biology and Medicine, 1992, 3(1/2): 61-82.
(Edited by LI Yan-hong)
Foundation item: Projects(CSTC2009AB4008) supported by Key Technologies R&D Program and Natural Science Foundation of Chongqing Science and Technology commission, China; Project(KJ100808, KJ08065) supported by Science and Technology Research Fund of Chongqing Municipal Education commission, China
Corresponding author: ZENG Rong-chang; Tel: +86-23-68665616; E-mail: rczeng2001@yahoo.com.cn