DOI:10.19476/j.ysxb.1004.0609.2019.04.22
真空下CO2/CO对镁蒸气冷凝的影响
熊 能1, 2, 3, 4,田 阳1, 2, 3, 4,杨 斌1, 2, 3, 4,刘大春1, 2, 3, 4,徐宝强1, 2, 3, 4,曲 涛1, 2, 3, 4,戴永年1, 2, 3, 4,刘 海1, 2, 3
(1. 昆明理工大学 省部共建复杂有色金属资源清洁利用国家重点实验室,昆明 650093;
2. 昆明理工大学 真空冶金国家工程实验室,昆明 650093;
3. 云南省有色金属真空冶金重点实验室,昆明 650093;
4. 昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:针对真空碳热还原法炼镁过程镁蒸气发生逆反应这一关键问题,研究了真空条件下CO2/CO分别对镁蒸气冷凝的影响,以期为该过程中镁蒸气的安全高效冷凝并收集提供理论依据。结果表明:30 Pa下镁蒸气的冷凝是直接由气态变为固态的凝华过程;在系统压强30 Pa、加热温度700~800 ℃保温30 min的条件下,CO2会使冷凝物表面结晶形貌不平整,但不会与镁蒸气发生化学反应;在相同实验条件下,CO会与镁发生化学反应,所得到的冷凝物含镁、氧、碳元素,这阻碍镁蒸气冷凝成形貌规则的晶体。
关键词:镁蒸气;CO;CO2;真空;冷凝
文章编号:1004-0609(2019)-04-0851-07 中图分类号:TF131;TF818 文献标志码:A
金属镁是仅次于钢铁和铝的第三大金属结构材料,也是迄今工程应用最轻的金属结构材料。镁基材料被称为绿色环保工程材料,因其具有比强度和比刚度高、减振性、磁屏蔽性、耐腐蚀性好以及易加工等优点,广泛应用于航空、汽车、计算机、通讯、军事等领域[1-3]。随着金属镁的扩大化应用,原镁需求量逐年攀升,在诸多制镁的方法中,因真空碳热法具有还原剂廉价、能耗低、废气和废渣排放量少等优点备受关注[4]。但是,经过多年研究[5-7],该方法未能实现产业化,主要瓶颈是金属镁蒸气冷凝时发生逆反应:Mg+CO=MgO+C,使镁蒸气难以安全有效地冷凝并收集。
针对碳热还原法制镁过程中镁蒸气的冷凝问题,各国学者展开了大量研究,例如澳大利亚CSIRO研究院利用拉法尔喷嘴急速冷却镁蒸气,可获得逆反应率较低的镁粉[8-10],但是收集结晶较好的块状金属镁以及提高金属镁直收率等问题仍没有解决。对此,昆明理工大学真空冶金国家工程实验室通过研究分析及自行设计冷凝器,获得了直收率高的金属镁块,并在此基础上研究了冷凝温度等因素对镁蒸气冷凝的影响[11-14]。本文借助扫描电镜(SEM)、能谱仪(EDS)、背散射电子成像(BSE)等实验手段,针对真空高温下CO2和CO分别对镁蒸气的冷凝影响进行了实验研究,以期为实现真空碳热还原法制备金属镁过程中镁蒸气的安全高效冷凝并收集冷凝物提供理论依据。
1 实验
1.1 实验原料和装置
实验所用原料金属镁为山西某企业采用皮江法生产的含镁96.54%的镁锭,其主要化学成分如表1所列。CO2由昆明广瑞达工贸有限责任公司提供,纯度达99.9%以上。CO由广东华特气体股份有限公司提供,纯度达99.9%以上。镁锭经处理后制成的细镁条在压片机中压制成如图1(a)所示直径20 mm、高度6 mm的圆柱体样品。实验反应装置如图1(b)所示,将压制好的样品放入右侧石墨加热器中,左侧冷凝器通过石墨管与加热器相连,整套反应装置置于真空管式炉内。
1.2 实验方法
实验时首先将准备好的样品准确称量后放入反应装置的加热器中,将反应装置置于石英管式炉内,确保加热器的位置处于电炉的加热区,冷凝器的上方插入一根K型热电偶用于测量冷凝温度。待管式炉密封后,开真空泵抽真空,当炉内压强降至30 Pa以下时通电升温,控制升温速率为7~8 ℃/min。
表1 原料镁的化学成分
Table 1 Chemical composition of magnesium (mass fraction, %)

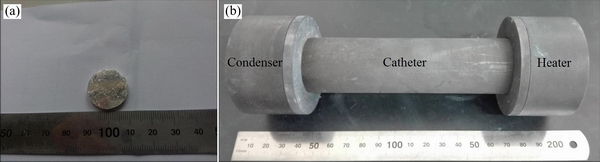
图1 样品实物图和反应装置实物图
Fig. 1 Photograph of magnesium ingot(a) and schematic diagram(b) of reaction device
进行镁的挥发冷凝实验时,在加热温度分别达到590、615、640、665、690 ℃后保温30 min停电降温,直至炉温降至室温时关闭真空泵,取出加热器中残余物和冷凝器中冷凝物,称量记录后保存,并适当取样送分析。从图2可以看出,在真空度30 Pa、保温30 min及不同加热温度条件下,镁的挥发率随加热温度升高而升高;当加热温度为690 ℃时,镁挥发率达到95.9%。因此,控制挥发温度高于690 ℃,有利于镁的挥发,从而保证CO2和CO通气实验的进行。

图2 体系压力30Pa条件下金属镁的挥发率随温度的变化关系
Fig. 2 Relationship between volatilization rate of metal magnesium and temperature under system pressure of 30 Pa
进行通气实验时,在加热温度分别为700、750、800 ℃(对应的冷凝区温度分别为375、398、421 ℃)条件下保温30 min,关闭真空泵后通入CO2/CO气体,待管内压强达到大气压后停止通气,然后停电降温,管式炉冷却至室温后取出残余物和冷凝产物,称量记录后保存,并取样送分析。借助扫描电子显微镜(SEM)、能谱仪(EDS)和背散射(BSE)分析冷凝物结晶形貌及冷凝物表面元素含量。
2 结果与讨论
2.1 镁蒸气的冷凝
图3所示为系统压力30 Pa、加热温度为650 ℃下保温30 min的镁挥发冷凝实验获得的冷凝物实物图和SEM像。从图3可看出,冷凝物结晶呈致密块状,且无明显裂缝。EDS分析显示,Mg含量达91.85%(质量分数),少量的氧元素是样品受到氧化所致。结合冷凝物的形貌特征和能谱分析的结果可知,实验条件下获得的冷凝物为结晶良好的金属镁块。
由热力学分析可知,在真空碳热还原法炼镁过程中镁蒸气冷凝时发生的逆反应以气-固相态的转变为主[15]。当压强为333 Pa、温度高于650 ℃时,镁蒸气出现气态-液态-固态的转变;当温度低于650 ℃时,镁蒸气直接由气态冷凝为固态[16],不排除镁蒸气直接冷凝成固态粉末的可能性[17]。图4所示为镁与CO2/ CO反应的吉布斯自由能与温度的关系图,可看出在30 Pa的系统压力下,镁只有以固态和气态形式出现的拟合线。这是因为在温度达到923 K(大气压下镁的熔点)之前镁就变成了气态,在重力的作用下,冷凝物结晶形貌没有凝固的迹象。结合热力学分析和实验可知,系统压力30 Pa下镁蒸气冷凝是直接从气态到固态的凝华过程。
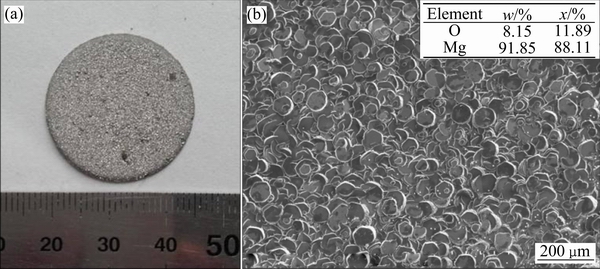
图3 镁的挥发冷凝产物实物图及SEM像
Fig. 3 Photograph(a) and SEM image(b) of magnesium condensate
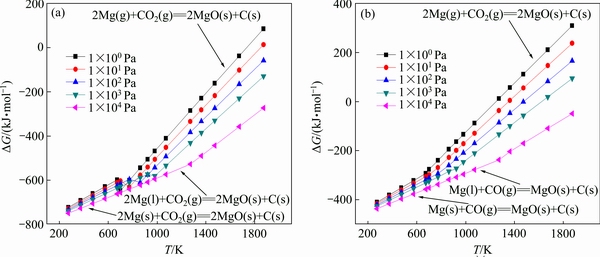
图4 不同压力条件下各反应的吉布斯自由能与温度的关系
Fig. 4 Relationship between free energy and temperature of reactions under different pressures
2.2 CO2对镁蒸气冷凝的影响
图5(a)、(b)和(c)所示分别为冷凝温度为375、398和421 ℃通入CO2气体实验所得冷凝物的实物图和SEM像。由图5可以看出,冷凝物都是直径25 mm、厚度约3mm的带有金属光泽的固体,表面呈淡黄色且凹凸不平,不同于具有银白色金属光泽的镁。这是由于实验过程中CO2分子与镁原子发生碰撞造成冷凝结晶的表面凹凸不平,投射在粗糙表面上的光向各个方向反射导致肉眼观察到的颜色不同。其SEM像显示晶粒呈块状,有明显裂缝。对比图3和图5可知,在通入CO2的条件下获得的镁蒸气冷凝物表面结晶不平整。由于金属表面挥发出的金属气体原子要经过传递才能到达冷凝面,所以会与空间中存在的分子发生碰撞[16],冷凝过程中镁原子与CO2分子相互碰撞会影响镁蒸气冷凝速率,进而造成冷凝物结晶表面不平整。
由EDS分析可知,不同温度条件下得到冷凝物的Mg含量高达90%以上,剩下的微量元素为氧,镁和氧的含量占比与图3(b)中的EDS分析结果一致,微量氧元素是检测样品接触空气受到氧化所致。结合上述分析可知,实验条件下CO2与镁蒸气不发生化学反应。所以在实验条件下通入CO2对镁蒸气的冷凝整体影响不大。
2.3 CO对镁蒸气冷凝的影响
图6(a)、(b)和(c)所示分别为在冷凝温度为375、398和421 ℃通入CO实验获得的冷凝物实物图和SEM像。从图6可以看出,实验获得的冷凝物都为深色块状固体,在冷凝温度为375和398 ℃下收集的冷凝物破碎成小块,在421 ℃下获得的冷凝物完整,但是触碰易碎。镁挥发得到的冷凝物SEM像(见图3)显示晶体表面平整无裂缝,颗粒大小均匀。对比发现图6中通入CO后得到的冷凝物晶体表面凹凸不平,晶粒有明显裂缝,颗粒尺寸存在巨大差异,因此冷凝物不稳定易破碎。
对比图5和图6可知,CO2作用下获得的冷凝物表面结晶光滑,而CO作用下获得的冷凝物表面结晶不光滑且成色不均匀,需要进一步研究其形貌特征,所以分别对冷凝温度为375、398和421 ℃下通CO实验获得的冷凝物做BSE成像和EDS分析,如图7所示。由图7可知,能谱分析显示碳含量分别为17.92%、81.64%和88.80%,随着颜色变深,碳含量增高,表明BSE图中深色区域含有碳元素,CO与镁发生化学反应生成碳。图7(a)中氧含量达到14.78%,对比图3中镁蒸气的冷凝实验检测到的的氧含量占比,考虑到冷凝物检测过程受到的氧化,结合热力学可知在实验条件下通入的CO与镁发生化学反应并生成氧。
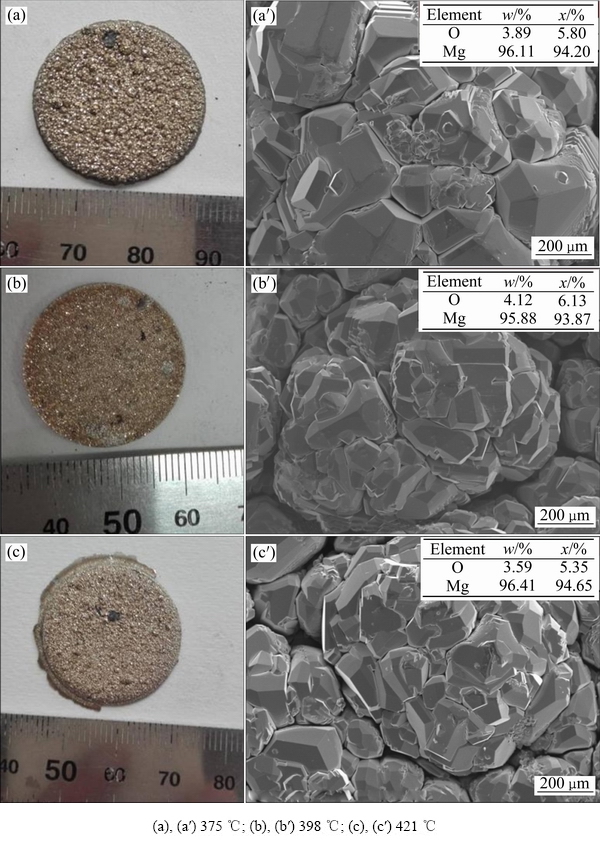
图5 不同温度下CO2与镁蒸气作用得到的冷凝物实物图及SEM像
Fig. 5 Material photos((a), (b), (c)) and SEM images((a′), (b′), (c′)) of condensates of CO2 reaction with magnesium vapor at different temperatures of condensation region
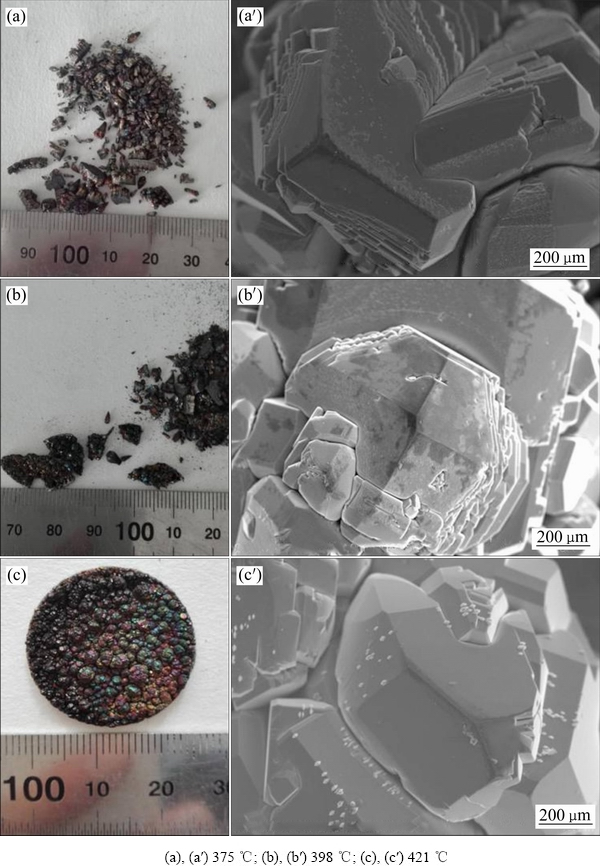
图6 不同温度下CO与镁蒸气作用得到的冷凝物实物图及SEM像
Fig. 6 Physical photos((a), (b), (c)) and SEM image((a′), (b′), (c′)) of condensates of CO reaction with magnesium vapor at different temperatures of condensation region
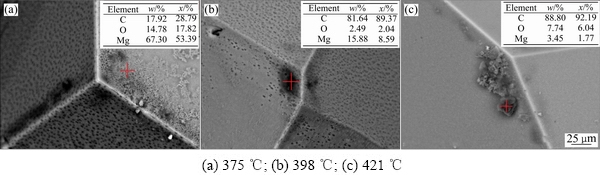
图7 不同温度下CO与镁蒸气作用得到的冷凝物BSE像
Fig. 7 BSE images of condensates of CO reaction with magnesium vapor at different temperatures of condensation region
通入CO后得到的冷凝物的表面为深色,完全不同于镁金属本身具有的银白色,是因为CO与镁发生了化学反应生成氧化镁和碳,不同物质表面自由电子数不同,自由电子对可见光选择性吸收和反射使得金属晶体具有金属光泽和一定颜色。CO一方面降低了系统内镁蒸气的分压及冷凝表面镁蒸气的浓度,缩短了镁蒸气原子的自由程,在降低镁原子之间碰撞几率的同时也减低了形核率;另一方面CO与镁蒸气发生反应的产物包裹在核和团簇的表面,严重阻碍了团簇之间的相互融合[12]。图5和图7中EDS分析表明实验条件下,CO2与镁不发生化学反应,而CO与镁发生化学反应并伴随碳、氧元素的生成。故在实验条件下通入CO会阻碍镁蒸气的有效冷凝,即对镁蒸气的冷凝造成不可逆的不利影响。
3 结论
1) 30 Pa下镁蒸气的冷凝是直接由气态变为固态的凝华过程。在系统压力30 Pa、加热温度为700~800 ℃保温30 min的条件下,通入的CO2会使冷凝物表面结晶不平整,但不会与镁蒸气发生化学反应,冷凝物仍然为镁。
2) 在系统压力30 Pa、加热温度为700~800 ℃保温30 min的条件下,通入CO会与镁发生化学反应,冷凝物含镁、氧、碳3种元素。
3) 对比通入CO2和通入CO的实验可知,在系统压强为30 Pa,加热温度为700~800 ℃保温30 min的条件下,CO2对镁蒸气的冷凝整体影响不大,而CO会对镁蒸气的冷凝造成不利影响。
REFERENCES
[1] 马鸿文, 曹 瑛, 蒋 芸, 吴秀文, 刘玉芹. 中国金属镁工业的环境效应与可持续发展[J]. 现代地质, 2008, 22(5): 829-837.
MA Hong-wen, CAO Ying, JIANG Yun, WU Xiu-wen, LIU Yu-qin. Environmental Impacts and sustainable developments of magnesium smelting industry in China: A Review[J]. Geoscience, 2008, 22(5): 829-837.
[2] 刘艳飞, 张 艳, 于汶加, 刘 璇. 全球原镁需求预测及中国合理产能分析[J]. 资源科学, 2015, 37(5): 1047-1058.
LIU Yan-fei, ZHANG Yan, YU Wen-jia, LIU Xuan. Analysis and forecast of world primary magnesium demand and reasonable productivity for China[J]. Resources Science, 2015, 37(5): 1047-1058.
[3] MORDIKE B L, EBERT T. Magnesium properties application-potential[J]. Materials Science and Engineering A, 2001, 302(1): 37-45.
[4] 钟 胜. 氧化镁真空碳热还原研究[D]. 昆明: 昆明理工大学, 1998.
ZHONG Sheng. Vacuum carbothermic reduction of magnesium oxide[D]. Kunming: Kunming University of Science and Technology, 1998.
[5] DAS S. Primary magnesium production cost for automotive application[J]. JOM, 2008, 60(11): 63-69.
[6] TIAN Y, QU T, YANG B, DAI Y N, XU B Q, GENG S. Behavior analysis of CaF2 in magnesia carbothermic reduction process in vacuum[J]. Metallurgical and Materials Transactions B, 2012, 43(3): 657-661.
[7] 田 阳, 杨 斌, 刘红湘, 杨成博, 曲 涛, 戴永年, 彭 鹏. 氧化镁在真空碳热还原法炼镁过程中行为研究[J]. 真空科学与技术学报, 2013, 33(9): 920-925.
TIAN Yang, YANG Bin, LIU Hong-xiang, YANG Cheng-bo, QU Tao, DAI Yong-nian, PENG Peng. Behavior of magnesia in Mg extraction by carbothermic reduction in vacuum[J]. Chinese Journal of Vacuum Science and Technology, 2013, 33(9): 920-925.
[8] BROOKS G, NAGLE M, TASSIOS S, TRANG S, HASAN N. The physical chemistry of the carbothermic route to magnesium[J]. JOM, 2006, 58(5): 25-31.
[9] BROOKS G, TRANG S, WITT P J, KHAN M N H, NAGLE M. The carbothermic route to magnesium[J]. JOM, 2006, 58(5): 51-55.
[10] PRENTICE L H, NAGLE M, BARTON T R D, TASSIOS S, KUAN B T, WITT P J, CONSTANTI-CAREY K K. Carbothermal production of magnesium: CSIRO'S MagSonicTM process[C]// Essential Readings in Magnesium Technology, TMS, 2012: 127-131.
[11] YANG C B, TIAN Y, QU T, YANG B, XU B Q, DAI Y N. Magnesium vapor nucleation in phase transitions and condensation under vacuum conditions[J]. Transaction of Nonferrous Metals Society of China, 2014, 24(2): 561-569.
[12] YANG C B, TIAN Y, QU T, YANG B, XU B Q, DAI Y N. Analysis of the behavior of magnesium and CO vapor in the carbothermic reduction of magnesia in a vacuum[J]. Journal of Magnesium and Alloys, 2014, 2(1): 50-58.
[13] TIAN Y, XU B Q, YANG B, Yang C B, LIU D C, DAI Y N. Magnesium production by carbothermic Reduction in Vacuum[J]. Journal of Magnesium and Alloys, 2015, 3(2): 149-154.
[14] 刘 海, 田 阳, 杨 斌, 刘大春, 徐宝强, 曲 涛, 戴永年. 真空碳热还原氧化镁制取金属镁的冷凝实验研究[J]. 真空科学与技术学报, 2015, 35(7): 867-871.
LIU Hai, TIAN Yang, YANG Bin, LIU Da-chun, XU Bao-qiang, QU Tao, DAI Yong-nian. Condensation of Mg-vapor in vacuum carbothermic reduction of magnesia[J]. Chinese Journal of Vacuum Science and Technology, 2015, 35(7): 867-871.
[15] 曲 涛, 戴永年, 杨 斌, 田 阳, 刘大春, 徐宝强. 真空碳热还原炼镁的研究[J]. 真空, 2014(4): 11-18.
QU Tao, DAI Yong-nian, YANG Bin, TIAN Yang, LIU Da-chun, XU Bao-qiang. Research of magnesium extraction by carbothermic reduction in vacuum[J]. Vacuum, 2014(4): 11-18.
[16] 杨成博. 真空碳热法炼镁过程中镁蒸气冷凝的实验研 究[D]. 昆明: 昆明理工大学, 2013.
YANG Cheng-bo. Experimental investigation on vapor condensation of magnesium by vacuum carbothermal method[D]. Kunming: Kunming University of Science and Technology, 2013.
[17] 夏绍龙. 金属蒸气冷凝法制取高纯镁粉[J]. 轻金属, 1987(6): 44-49.
XIA Shao-long. Preparation of high pure magnesium powder by vapor condensation of metals[J]. Light Metals, 2014, 2(1): 50-58.
Influence of CO2 and CO on magnesium vapor condensation in vacuum
XIONG Neng1, 2, 3, 4, TIAN Yang1, 2, 3, 4, YANG Bin1, 2, 3, 4, LIU Da-chun1, 2, 3, 4, XU Bao-qiang1, 2, 3, 4, QU tao1, 2, 3, 4, DAI Yong-nian1, 2, 3, 4, LIU Hai1, 2, 3
(1. State Key Laboratory of Complex Non-ferrous Metal Resources Clear Utilization, Kunming University of Science and Technology, Kunming 650093, China;
2. National Engineering Laboratory for Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China;
3. Key Laboratory of Vacuum Metallurgy for Nonferrous Metal of Yunnan Province, Kunming 650093, China;
4. School of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: Aiming at the key problem of reverse reaction of magnesium vapor in vacuum carbothermal reduction method, the effects of CO2/CO on magnesium vapor condensation under vacuum conditions were studied, which is to provide theoretical basis for safe and efficient condensation and collection of magnesium vapor in the process. The results show that the condensation of magnesium vapor is a desublimation process from gas to solid at 30 Pa. In addition, when the system pressure is 30 Pa, the heating temperature is 700-800 ℃ and is kept for 30 min, CO2 can make the crystalline morphology of the condensate uneven, but cannot react with magnesium vapor. And under the same experimental conditions, CO can chemically react with magnesium. The obtained condensate contains magnesium, oxygen and carbon. CO can hinder the magnesium vapor to condensate into the regular crystal.
Key words: magnesium vapor; CO; CO2; vacuum; condensation
Foundation item: Project (51734006) supported by the National Natural Science Foundation of China; Project (2017HB009) supported by the Science and Technological Talent Cultivation Plan of Yunnan Province, China
Received date: 2018-01-20; Accepted date: 2018-11-05
Corresponding author: TIAN Yang; Tel: +86-13888805314; E-mail: emontian@hotmail.com
(编辑 龙怀中)
基金项目:国家自然科学基金重点项目(51734006);云南省中青年学术科技带头人后备人才项目(2017HB009)
收稿日期:2018-01-20;修订日期:2018-11-05
通信作者:田 阳,副教授,博士;电话:13888805314;邮箱:emontian@hotmail.com