文章编号:1004-0609(2010)05-1006-07
Sn-Cu合金的电沉积行为及添加剂的影响
胡 炜1, 2,谭澄宇1, 2,崔 航1, 2,郑子樵1, 2
(1. 中南大学 有色金属材料科学与工程教育部重点实验室,长沙 410083;
2. 中南大学 材料科学与工程学院,长沙 410083)
摘 要:利用循环伏安和计时安培研究Sn-Cu合金体系在玻碳电极上的电沉积行为,通过阴极极化曲线、SEM观察及EDS分析讨论柠檬酸和硫脲对Sn-Cu共沉积的影响。结果表明,Sn-Cu共沉积过程为扩散控制的不可逆过程;在沉积电位-600~-750 mV的范围内Sn-Cu共沉积初期结晶行为满足三维Scharifker-Hills瞬时成核模型,随着过电位的增大,形核活性点增多,形核弛豫时间缩短;当沉积过电位大于-700 mV时,Sn2+的扩散系数约为 6.435×10-6cm2/s;柠檬酸和硫脲的加入细化了镀层晶粒,使镀层表面更加平整和致密。此外,硫脲的加入降低了镀层中铜的含量,使合金镀层中铜的含量维持在0.5%~2.0%(质量分数)。
关键词:Sn-Cu合金;形核;循环伏安;电沉积;
中图分类号:TQ172.84 文献标志码:A
Electrodeposition behavior of Sn-Cu alloy and effect of
additives on deposition process
HU Wei1, 2, TAN Cheng-yu1, 2, CUI Hang1, 2, ZHENG Zi-qiao1, 2
(1. Educational Key Laboratory of Non-ferrous Metal Materials Science and Engineering,
Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The electrodeposition of Sn-Cu alloy system on the glass carbon electrode was investigated by cyclic voltammetry (CV) and chronoamperometry (CA). The effect of citric acid and thiourea additives on Sn-Cu alloy codeposition was discussed by cathodic polarization curve, SEM observation and EDS analysis. The results indicate that the electrodeposition process of Sn-Cu is an irreversible process controlled by diffusion, and the codeposition of Sn-Cu alloy system in the initial stage follows Scharifker-Hills (SH) instantaneous nucleation/growth mechanism in the potential range of -600- -750 mV. With increasing the overpotential, the activity-point for nucleation increases, and the nucleation relaxation time reduces. When the deposition overpotential is higher than -700 mV, the diffusion coefficient of Sn2 + is 6.435×10-6cm2/s. The addition of citric acid and thiourea refines grains in coating and the surface of coating becomes more even and compact. The presence of thiourea reduces clearly the copper content in coating and makes the copper content remain in the range of 0.5%-2.0%(mass fraction).
Key words: Sn-Cu alloy; nucleation; cyclic voltammetry; electrodeposition
电子产品中使用的印刷电路板和表面贴片元件表面通常都需要电镀可焊性镀层来实现二者的互联。Sn-Pb合金镀层是传统电子电镀中广泛使用的可焊性镀层[1],但是废旧电子产品中的铅对环境造成了严重污染。在欧盟WEE和RoHS 指令中,铅已经被明确禁止在电子产品中使用[2],我国也于2007年3月1日正式施行《电子信息产品污染控制管理办法》,确定了对电子信息产品中含有的铅等多种有毒、有害物质限制或禁止使用,因此,需要开发无铅可焊性镀层以实现电子封装行业的可持续发展。
由于Sn-Cu合金镀层的综合性能较好,因此,Sn-Cu合金镀层成为最有发展前途的可焊性镀层之一。其主要表现为低毒、沉积速度快、可焊性好、成本较低,且还具有在波峰焊过程中不污染焊料等优点。Sn-Cu合金镀层钎料既适宜于表面贴装的再流焊,也适用于接插型的波峰焊,是最具研究和应用价值的 Sn系镀层体系。OKADA等[3]研究表明,Sn-Cu(0.1%~ 0.2%)合金具有很强的抗锡须生长能力,即使在高温下也有很好的可焊性。日本上村工业公司开发的Soft Alloy GTC220 型电镀液,很好地解决了晶须的产生问题,已具备电镀作业性良好和成本低廉等优点。目前,报道有关Sn-Cu合金镀层研究的文献不多,并且大多数都集中在制备工艺、镀液组成及相关性能方面[4-12],有关电化学共沉积过程与电结晶机理的研究报道较少。TORRENT-BURGUES和GUAUS [13]对纯锡体系在酸性条件下的电结晶行为进行研究。结果表明:锡的电结晶行为遵循扩散控制的三维瞬时形核机制,添加剂的引入对其电结晶行为产生较大的影响。有关Sn-Cu镀液体系电沉积结晶,如镀液组成和添加剂等对合金镀层的形核、生长等结晶过程影响的研究则鲜见报道。然而,镀层的组成、形核/生长很大程度上影响了合金镀层的应用性能,研究Sn-Cu合金电沉积的初期行为显得尤为重要。
因此,本文作者在酸性柠檬酸-硫酸盐体系中,利用极化曲线、循环伏安及恒电位阶跃等电化学方法研究Sn-Cu合金电沉积结晶的初期行为;并结合SEM观察与EDS分析讨论柠檬酸和硫脲的加入对Sn-Cu合金共沉积的影响。
1 实验
1.1 电沉积实验
采用酸性柠檬酸-硫酸盐镀液体系,具体配方为40 g/L 硫酸亚锡,0.8 g/L 硫酸铜,1 g/L 对苯二酚,60 g/L 柠檬酸,100 g/L 硫酸,2 g/L明胶,0.8 g/L β-萘酚,0.04 g/L硫脲。采用纯锡板作阳极,镀液温度控制在(25±1)℃,使用WYJ 型直流稳流电源进行电镀,电流密度为2 A/dm2。使用经热处理的光亮紫铜片为阴极。电镀工艺流程为乙醇除油→蒸馏水洗→10%稀硫酸除氧化膜→蒸馏水洗→磷酸擦拭活化表面→电镀Sn-Cu合金→蒸馏水洗→干燥。利用Sirion200型场发射扫描电镜观察合金镀层的微观表面形貌及对镀层成分进行EDS分析。
1.2 电化学测试
实验采用CHI660C型电化学工作站(上海辰华)分别对Sn-Cu镀液体系进行阴极极化曲线、循环伏安及计时安培测试,研究Sn-Cu合金共沉积机理,探讨添加剂对合金沉积的影响。所有电化学测试都采用三电极体系,以玻碳电极为工作电极,辅助电极采用大面积光亮铂片,232型饱和甘汞电极为参比电极。极化曲线扫描速度为1 mV/s,循环伏安扫描速度为100 mV/s;溶液温度控制在(25±1)℃。
2 结果与分析
2.1 循环伏安分析
图1所示为不同扫描速度下Sn-Cu合金镀液的 循环伏安曲线。从图1(a)可以看出,随着扫描速度 的加快,正扫过程中的还原峰电流逐渐增大,还原电流峰的起峰电势向电势更负的方向移动。对于可逆 的电极反应过程,还原峰电势与扫描速度无关[14]。图1(a)所示的结果表明, Sn-Cu合金的电沉积过程是不可逆的。
当电极反应的控制步骤为扩散控制时,不同扫速度下循环伏安曲线的峰值电流( Im )与扫描速度(v)存在以下关系: 
由式(1)可以看出,当电极反应的控制步骤为扩散步骤控制时,峰电流(Im)与扫描速度的平方根(v1/2)呈线性关系。图1(a)中出现的还原峰是镀层Sn-Cu合金的共沉积峰,这个峰的峰值电流与相应的扫描速度的平方根的关系如图1(b)所示。由图1(b)可见,Im与v1/2呈线性关系,拟合后成一条直线,因此,可以推断Sn-Cu合金共沉积过程主要是受扩散控制的非可逆过程[15]。
2.2 Sn-Cu合金共沉积初期行为的分析
恒电位阶跃法测定的电沉积体系中的J— t曲线,
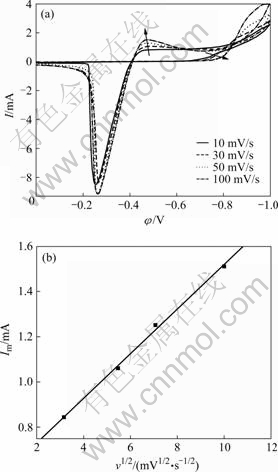
图1 不同扫描速度下Sn-Cu合金镀液的循环伏安曲线以及Im与v1/2的关系曲线
Fig.1 CV curves of Sn-Cu alloy electrodeposited at various scan rates (a) and linear relationship between Im and v1/2 from reductive current peak (b)
反映了有关金属电沉积结晶成核和生长的重要信息。在恒电位阶跃分析中,电位从开路电位开始阶跃到金属的沉积电位,在这样的条件下,体系从没有反应到稳态,由金属离子到电极表面的传质速度控制,得到的电流曲线总可以达到稳态,由Cottrell方程表述[16]:

因此,在电位阶跃的极短时间内,由于双电层充电导致电流先迅速上升随后下降,而后由于晶核的形成和新相的生长,电流再次逐渐上升并达到最大值,随后又出现衰减,此时整个电极表面表现为扩散控制。
电沉积初期阶段的成核经历诱导吸附,原子吸附聚集成簇而形成临界晶核。电结晶过程成核模型主要有Scharifker-Hill(SH)模型[17-20]。在电结晶初始阶段,当吸附原子进入晶核的过程为速度控制时,Scharifker和Hill基于假定在电极上随机分布的晶核为半球形,且在每个晶核周围逐渐扩展的扩展区内不能形成新晶核,并考虑到扩展区的重叠,晶核在扩散控制下长大,推导出恒电位暂态曲线的公式:
1) 瞬时成核

以上两式分别描述了瞬时成核和连续成核的暂态电流。每种状态都是电流先达到最大值,然后,接近平面电极的极限扩散电流。
根据Scharifker-Hills模型,不同过电位下的晶核密度可以用下式来计算:

在不同阶跃电位下,玻碳电极上酸性Sn-Cu镀液中的恒电位I—t曲线如图2(a)和(b)所示。由图2(a)和(b)可以看出,在电流的上升阶段,达到峰值电流后出现衰减,并没有出现由于双电层充电导致的电流迅速下降阶段。一般认为这与锡离子的电极反应速率常数较大有关。I—t曲线进入电流上升阶段,即形核与生长,达到峰值后,由于金属离子浓度的变化而又出现下降,再逐渐趋于稳定,此时,整个电极表面反应为扩散控制。随着阶跃电位的负移,即过电位增大,峰电流(Im)增大,其对应的tm值(出现峰电流所需时间)呈规律性的缩短,说明过电位升高导致成核速率加快,成核诱导时间缩短。
利用电位阶跃测定判断电结晶机理的常用方法之一是检验暂态初期的I—tn的线性关系,n取不同值代表
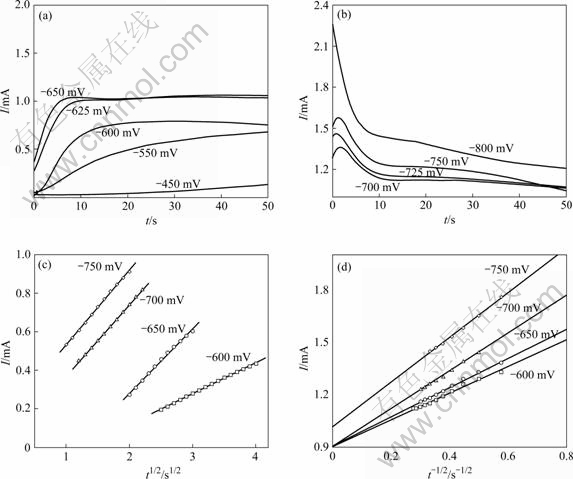
图2 不同阶跃电位下Sn-Cu合金在玻碳电极上共沉积的I—t暂态曲线以及对应的I—t1/2曲线和Cottrell曲线
Fig.2 I—t curves of Sn-Cu alloy deposited on vitreous carbon electrode ((a), (b)) and corresponding I—t1/2 curves (c) and Cottrell curves (d)
不同成核和生长机理。其中n为1/2时对应瞬时成核机理[19-24];这种成核过程只在脉冲的初期发生,由此可以估计成核数密度。由图2(a)得到图2(c),根据实验数据可以给出不同电位下的成核速率[20-21]。当t很小时,I与t1/2成正比,这预示着Sn与Cu共沉积的电结晶遵从形核和扩散控制的生长机理。根据Scharifker-Hill模型,I与t1/2的斜率与晶核数密度(N)成正比;由图2(c)可知,随着过电位的增大,曲线斜率随过电位的增大而增大,说明过电位越高,生成的晶核数密度越多。图2(d)是由式(2)得到的Cottrell曲线,在较高的过电位下,暂态曲线的下降部分所对应的I与t-1/2呈线性关系,一般认为此时整个电极表面反应表现为扩散控制。由图2(d)可看出,与简单的扩散过程相比,图2(d)中呈现了不同阶跃电位下残留的阴极电流,且直线的斜率随过 电位变化而略有不同。其他电结晶过程也有类似的结果[25]。
利用Scharifker和Hills[20]导出的扩散控制条件下三维多核生长的恒电位I—t的关系式,能更好地解释本实验结果:成核机理不同,无因次(I/Im)2—t/tm关系曲线的形状不同,并且它们不受电位的影响。将实验数据(I/Im)2对t/tm作图并分别与由式(3)和(4)计算得到的曲线进行对比,就可以推断出成核机理。
图3所示为酸性体系中不同阶跃电位下无因次(I/Imax)2-t/tm曲线和经典的SH(Scharifker-Hill)模型进行数据拟合而得到的拟合结果。图3中的实线1和2分别表示SH瞬时成核和连续成核机理的无因次(I/Im)2-t/tm理论计算曲线。显然,在实验的电位区内,Sn与Cu共沉积的成核机理遵从扩散控制下三维瞬时成核,这与前面的分析结果一致。
表1所列为不同阶跃电位下Sn-Cu镀液在玻碳电极上电结晶的实验结果。可以发现,随着过电位的增加,镀液电结晶形核的弛豫时间逐渐缩短;最大晶核
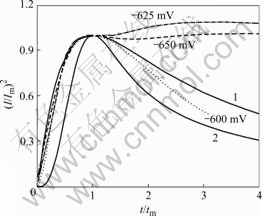
图3 Sn-Cu合金体系在不同电位下的无因次(I/Im)2—t/tm曲线
Fig.3 Non-dimensional (I/Im)2—t/tm plots for electrodeposition of Sn-Cu alloy system under different potentials: 1—SH model of instantaneous; 2—SH model of progressive
表1 Sn-Cu镀液在玻碳电极上电结晶的实验结果
Table 1 Experimental results of electro-crystallization on glass carbon electrode from Sn-Cu solutions

数密度随着过电位的增加迅速增大直到-700 mV后趋于稳定,这与图2(c)所示的结果一致,而峰值电流(Im)也呈现规律性的递增。从表1还可以看出,当阶跃电位到达-700 mV后,各项参数均趋于稳定,可以认为,由于铜的沉积电位较正,在较低的阶跃电位下,主要表现为铜离子的电结晶形核,且随着阶跃电位的增大,电极表面出现大量由铜的沉积而产生的活性点,促进了Sn的形核生长,N0值迅速增大;当电位较高时,沉积时间达到tm,电流表观上已经接近于平板电极的极限电流,抵达电极表面的金属离子立即被消耗掉,即电极表面的离子浓度趋于零,主要表现为扩散控制,与Sn2+相比,Cu2+的浓度可以忽略不计,由此时的Im2tm值可以近似求得Sn2+的扩散系数,D=6.435×10-6 cm2/s,这个结果与报道的相近[26]。
3 讨论
图4所示为玻碳电极在Sn-Cu复合溶液的极化曲
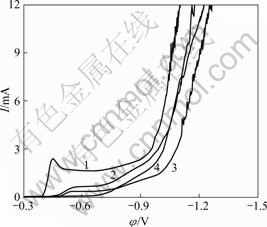
图4 Sn-Cu合金沉积的阴极极化曲线
Fig.4 Cathodic polarization curves for electrodeposition of Sn-Cu alloy: 1—Without additive; 2—With thiourea; 3—With citric acid; 4—With citric acid and thiourea
线。由图4可见,在不含添加剂的Sn-Cu复合溶液中,当线性扫描到电位为-0.4 V时,观察到阴极沉积电流明显增大,在-0.45 V附近出现明显的峰电流。当在电极上加线性扫描电压时,一方面,Sn与Cu共沉积反应使电极表面附近金属离子的浓度下降,促使电极反应速度有所减慢;另一方面,Sn与Cu共沉积反应随所加过电位的增大而讯速加快,反应电流也随之增大。这2个相反因素影响了电流峰值的产生,在峰值前,过电位起主导作用;在峰值后,反应物的扩散起主导作用。随着时间的延长,扩散层增厚,扩散电流降低,因反应受扩散控制,故电流下降,直到达稳态电流。随后,随着电位的增大,还原电流又增大到约-1 V,电流急剧增大,这有可能是析氢反应所致。
此外,从图4还可以看出,柠檬酸和硫脲对Sn与Cu共沉积有影响。在向溶液中单独加入柠檬酸或硫脲对Sn与Cu沉积有一定的极化作用,使沉积电位负移,同时,使析氢电位负移。添加剂的引入与Sn2+和Cu2+形成配合物,大大地降低了溶液中金属离子的实际 浓度,从而减慢了Sn2+与Cu2+的还原速率。从图4也 可以看出,加入添加剂后还原电流明显低于无添加剂时的。
图5所示为Sn-Cu合金镀层的SEM像。从这些SEM像可以明显地看出硫脲和柠檬酸对镀层微观形貌的影响。从图5(a)可以看出,镀层表面疏松、不平整,有大量孔洞。无添加剂时,镀液中的Sn2+极易被氧化成Sn4+而使镀液变混浊,EDS分析表明白色结晶物为锡的氧化产物,同时可以看出晶核周围有大量晶须生长(见图5(a))。柠檬酸和硫脲的加入使镀层表面变得平
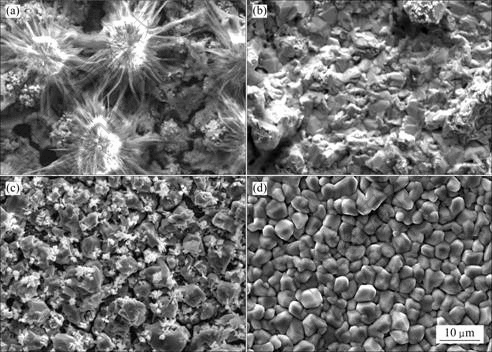
图5 Sn-Cu合金镀层的SEM像
Fig.5 SEM images of Sn-Cu films deposited with different additives: (a) Without additive; (b) Without citric acid; (c) With thiourea; (d) With citric acid and thiourea
整、均匀,晶粒细小呈半圆状,尺寸为3~5 μm。通过EDS分析可知硫脲的加入明显降低了镀层中Cu2+的含量,使镀层中Cu2+的含量维持在共晶点附近(0.5%~2.0%)(见图5(d))。当单独加硫脲或柠檬酸时,镀层表面仍有大量缺陷(见图5(b)和(c))。
从图5可以清楚地看出柠檬酸与硫脲对合金镀层微观形貌有影响。由于柠檬酸与硫脲对Sn2+和Cu2+的螯合作用,限制了镀液中自由金属离子的浓度,减慢了金属离子的还原速度,从而细化镀层晶粒,提高镀层表面质量,同时降低了镀层中Cu2+的含量。
4 结论
1) 循环伏安测试表明,Sn-Cu合金共沉积过程主要是受扩散控制的非可逆过程。
2) 共沉积初期行为分析表明,Sn-Cu合金电结晶初期行为遵从扩散控制下的三维Scharifker-Hill瞬时成核模型;随着过电位的增大,形核活性点增多,形核弛豫时间缩短;当电沉积趋于稳定,即阶跃电位大于-700 mV时,Sn2+的扩散系数约为6.435×10-6cm2/s。
3) 从酸性的柠檬酸-硫酸盐镀液体系中通过电沉积的方法获得了Sn-0.7%Cu合金镀层。极化曲线测试、SEM观察及EDS分析表明,柠檬酸和硫脲的加入降低了镀液中自由金属离子浓度,对Sn-Cu合金共沉积具有一定的阻碍作用,使沉积电位负移,细化了镀层晶粒,使镀层表面平整、致密,镀层中铜的含量维持在0.5%~2.0%。
REFERENCES
[1] 庄瑞舫. 电镀锡和可焊性锡合金发展概况[J]. 电镀与涂饰, 2000, 19(2): 38-43.
ZHUANG Rui-fang. Review of tin and solderable tin alloy electroplating[J]. Electroplating and Finishing, 2000, 19(2): 38-43.
[2] DAVIS B. Changing the rules of RoHS additive on hazardous substances[J]. Environmental Engineering, 2006, 19(2): 21-23.
[3] OKADA Y, HARADA H, SAKA K. Tin-copper alloy plated wire: Japan, 2000-087204[P]. 2000-03-27.
[4] 邓正平, 温 青. 锡及锡合金可焊性镀层电镀工艺[J]. 电镀与精饰, 2006, 28(5): 33-38.
DENG Zheng-ping, WEN Qing. Electroplating technology for tin and tin alloy solderable coating[J]. Electroplating and Finishing, 2006, 28(5): 33-38.
[5] 杜长华, 陈 方, 杜云飞. Sn-Cu、Sn-Ag-Cu 系无铅钎料的钎焊特性研究[J]. 电子元件与材料, 2004, 28(11): 34-36.
DU Chang-hua, CHEN Fang, DU Yun-fei. Investigation for solderability of Sn-Cu, Sn-Ag-Cu lead-free solders[J]. Electronic Components and Materials, 2004, 28(11): 34-36.
[6] KAO H J, WU W C, TSAI S T. Effect of Cu additives on Sn whisker formation of Sn(Cu) finishes[J]. Journal of Electronic Materials, 2006, 36(10): 1885-1891.
[7] MEDVEDEV G I, MAKRUSKIN N A, IVANOVA O V. Electrodeposition of copper-tin alloy from sulfate electrolyte[J]. Russian Journal of Applied Chemistry, 2004, 77(7): 1104-1107.
[8] PU Wei-hua, HE Xiang-ming, REN Jian-guo, WAN Chun-rong, JIANG Chang-yin. Electrodeposition of Sn-Cu alloy anodes for lithium batteries[J]. Electrochimica Acta, 2005, 50(20): 4140-4145.
[9] SHEN J, LIU Y C, GAO H X. Formation of bulk Cu6Sn5 intermetallic compounds in Sn-Cu lead-free solders during solidification[J]. Journal of Materials Science, 2007, 42(14): 5375-5380.
[10] LU M H, HSIEH K C. Sn-Cu intermetallic grain morphology related to Sn layer thickness[J]. Journal of Electronic Materials, 2007, 36(11): 1448-1454.
[11] WU C M, HUANG M L. Creep behavior of eutectic Sn-Cu lead-free solder alloy[J]. Journal of Electronic Materials, 2002, 31(5): 442-448.
[12] WILLIAMS M E, MOON K W, BOETTINGER W J, JOSELL D, DEAL A D. Hillock and whisker growth on Sn and SnCu electrodeposition a substrate not forming interfacial intermetallic compounds[J]. Journal of Electronic Materials, 2007, 36(7): 214-219.
[13] TORRENT-BURGUES J, GUAUS E. Electrodeposition of tin from tartrate solutions[J]. Portugaliae Electrochimica Acta, 2005, 23: 471-479.
[14] DOLATI A, AFSHAR A, GHASEMI H. A kinetic study on the electrodeposition of cadmium with the presence of organic agents in sulfate solutions[J]. Materials Chemistry and Physics, 2005, 94(1): 23-28.
[15] CHEN Z, ZHANG M L, HAN W, HOU Z Y, YAN Y D. Electrodeposition of Li and electrochemical formation of Mg-Li alloys from the eutectic LiCl-KCl[J]. Journal of Alloys and Compounds, 2008, 464(1/2): 174-178.
[16] 查全性. 电极过程动力学导论[M]. 北京: 科学出版社, 2004: 306-311.
CHA Quan-xing. Introduction of electrode process dynamics[M]. Beijing: Science Press, 2004: 306-311.
[17] FLEISCHMANN M, THIRSK H R. Kinetics of the growth of deposits on electrodes[J]. Eletrochimica Acta, 1959, 1(2/3): 146-160.
[18] HOLZLE M H, RETTER U, KOLB D M. The kinetics of structural changes in copper adlayers on Au(111)[J]. Journal of Electroanalytical Chemistry, 1994, 371(1): 101-109.
[19] ENRIQUE B, MANUEL P P, NIKOLA B, IGNACIO G. Formation mechanisms and characterization of black and white cobalt electrdeposition onto stainless steel[J]. Journal of Electrochemical Society, 2000, 147(5): 1787-1796.
[20] SCHARIFKER B, HILLS G. Theoretical and experimental studies of multiple nucleation[J]. Eletrochimica Acta, 1983, 28(7): 879-889.
[21] GUNAWARDENA G A, HILLS G J, MONTENEGRO J. Potentiostatic–galvanostatic–potentiostatic study of the deposition of mercury on graphite[J]. Faraday Symposia of the Chemical Society, 1977, 12: 90-100.
[22] BORT H, JUTTNER K, LORENZ W J, STAIKOV G. Underpotential alloy formation in the system Ag(hkl)/Cd2+[J]. Electrochimica Acta, 1983, 28(7): 993-1001.
[23] POTZSCHKE R T, GERVASI C A, VINZELBERG S, STAIKOV G, LORENZ W J. Nanoscale studies of Ag electrodeposition on HOPG (0001)[J]. Electrochimica Acta, 1995, 40(10): 1469-1474.
[24] HOLZLE M H, ZWING V, KOLB D M. The influence of steps on the deposition of Cu onto Au(111)[J]. Electrochimica Acta, 1995, 40(10): 1237-1247.
[25] ZEBIMENDDI J L, VAZQUEZ L, OCON P, VARA J M, TRIACA W E, SALVAREZZA R C, ARVIA A J. Early stages of platinum electrodeposition on highly oriented pyrolytic graphite: scanning tunneling[J]. The Journal of Physical Chemistry, 1993, 97: 5095-5101.
[26] GOMEZ E, GUAUS E, SANZ F, VALLES E. Tin electrodeposition on carbon electrodes. From nuclei to microcrystallites[J]. Journal of Electroanalytical Chemistry, 1999, 465(1): 63-71.
(编辑 杨 华)
基金项目:民口配套资助项目(MKPT-98-106)
收稿日期:2009-06-01;修订日期:2010-01-15
通信作者:谭澄宇,教授,博士;电话:0731-88830270;E-mail: tanchenyu@yahoo.com.cn