Effects of low-intensity pulsed ultrasound stimulation on porous hydroxyapatite blocks for posterolateral fusion of lumbar spine in rabbits
来源期刊:中国有色金属学报(英文版)2010年第10期
论文作者:卓祥龙 吕红斌 徐大启 刘彬 王锡阳 张莹 胡建中
文章页码:1921 - 1927
Key words:low-intensity pulsed ultrasound stimulation; lumbar posterolateral fusion; porous hydroxyapatite blocks
Abstract: The effects of porous hydroxyapatite blocks (PHABs) and an adjunct low-intensity pulsed ultrasound stimulation (LIPUS) on the fusion rate in a rabbit spinal posterolateral fusion (PLF) model were evaluated. Twenty rabbits underwent PLF with autograft and PHABs were randomly assigned to two groups: treated group with 20 min LIPUS daily and untreated control group for 4 weeks until euthanasia. The fused motion segments were subjected to manual palpation, gross observation, and radiographic investigation before histomorphologic and scanning electron microscopic analyses. Statistical differences between the LIPUS group and the control group are found in the fusion rate, bone density gray scale, trabecular bone formation, osteoblast-like cells, chondrocytes and positive expression of BMP-2 and TGF-β1 in the junction zone (significance level p<0.05). The results suggest that LIPUS can increase fusion rates and accelerate bone in-growth into PHAB. Hence, PHAB and LIPUS may be used together to increase fusion rates in a rabbit spinal fusion model with a promising extension to human application.

ZHUO Xiang-long(卓祥龙)1, 2, L? Hong-bin(吕红斌)3, 4, XU Da-qi(徐大启) 1, 2, LIU Bin(刘 彬)5,
WANG Xi-yang(王锡阳)1, 2, ZHANG Ying(张 莹)5, HU Jian-zhong(胡建中)1, 2
1. Department of Spinal Surgery, Xiangya Hospital, Central South University, Changsha 410008, China;
2. Orthopedic Institute of Central South University, Changsha 410008, China;
3. Department of Sports Medicine, Xiangya Hospital, Central South University, Changsha 410008, China;
4. Research Center of Sports Medicine, Central South University, Changsha 410008, China;
5. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 3 December 2009; accepted 23 April 2010
Abstract: The effects of porous hydroxyapatite blocks (PHABs) and an adjunct low-intensity pulsed ultrasound stimulation (LIPUS) on the fusion rate in a rabbit spinal posterolateral fusion (PLF) model were evaluated. Twenty rabbits underwent PLF with autograft and PHABs were randomly assigned to two groups: treated group with 20 min LIPUS daily and untreated control group for 4 weeks until euthanasia. The fused motion segments were subjected to manual palpation, gross observation, and radiographic investigation before histomorphologic and scanning electron microscopic analyses. Statistical differences between the LIPUS group and the control group are found in the fusion rate, bone density gray scale, trabecular bone formation, osteoblast-like cells, chondrocytes and positive expression of BMP-2 and TGF-β1 in the junction zone (significance level p<0.05). The results suggest that LIPUS can increase fusion rates and accelerate bone in-growth into PHAB. Hence, PHAB and LIPUS may be used together to increase fusion rates in a rabbit spinal fusion model with a promising extension to human application.
Key words: low-intensity pulsed ultrasound stimulation; lumbar posterolateral fusion; porous hydroxyapatite blocks
1 Introduction
Lumbar posterolateral fusion (PLF) is a common surgical procedure for the treatment of several spinal diseases. For one reason or another, the extensive utilization of autologous bone and allograft was limited[1]. Hence, one of the synthetic materials used as substitutes for bone graft is the porous hydroxyapatite (HA) which has been used as a bone graft material in clinical practice for decades[1]. HA ceramics were widely used as bone graft substitutes because of their biocompatibility, osteoconductivity, ability to integrate with the bone tissue, and the absence of immune response[2]. Synthetic HA has been used to coat various materials to enhance bone formation, and mineralization adjacent to the implant surface[3-4], and to achieve osseointegration between the host bone and the implant similar to autograft[5]. CHANG et al[6] stated that although HA graft as a cage filler is an osteoconductive rather than osteoinductive material, it is a safe and effective substitute for cancellous bone graft. The interconnected porous structure of HA ceramics encourages host bone in-growth deep into pores when implanted in a bone defect, which eventually increases the mechanical strength of the reconstruction[7].
For the lack of osteoinductive property, the porous HA used alone or in combination with bone graft or other materials yielded mixed results in spine PLF in human[8-10]. To achieve fusion, improved methods combining both osteoconductive and osteoinductive strategies are required. Low-intensity pulsed ultrasound stimulation (LIPUS) accelerates bone mineralization and regeneration by increasing alkaline phosphatase activity and osteogenesis-related gene expression in vitro[11-12]. In vivo, LIPUS significantly accelerates fresh fracture healing in animal studies and in randomized controlled clinical trials. LIPUS also appears as an effective and safe non-operative treatment of aseptic and septic delayed-unions and nonunions with a healing rate ranging from 70% to 93% in different nonrandomized studies[13].
Although LIPUS may be used safely together with HA ceramics to increase bone in-growth into the pores of the ceramics[14] and it can also accelerate the process of bone fracture repair, the healing of delayed-union or nonunion, the effects of a combined use of LIPUS with HA graft on spinal fusion have not been reported. The goal of this exploratory study was to examine the potential enhancement effect of daily LIPUS stimulation on the PHAB grafted junctions in a spine fusion model using rabbit transverse process and vertebral lamina. The hypothesis of this investigation was that LIPUS could improve and accelerate osteointegration between PHAB and the host bone, consequently the rate of spine fusion would be enhanced and the time of fusion would be shortened.
2 Experimental
2.1 Preparation of porous HA blocks
The HA ceramics (Hunan Gongchuang Biofunctional Materials Ltd. Co, Hengyang, China) used in the animal experiment were prepared into rectangular block of 5 mm in width and height and 30 mm in length. The interconnected porous structure of HA ceramic contained pores ranging from 200 to 500 μm in diameter. The microstructure of this material enhances bone ingrowth while the material itself allows good osteoconductivity[7].
2.2 Study design
Twenty adult New Zealand white rabbits with mass between 2.0 kg and 2.5 kg were obtained from the Department of Animal Experiment, Xiangya School of Medicine, Central South University, Changsha, China. These rabbits were randomly divided into two groups (Group A treated with LIPUS and untreated Group B used as the control). The surgeons and evaluators were blinded to the group assignment. After being acclimated at our facility for one week, all rabbits then underwent an L5–L6 inter-transverse process fusion using a muscle-splitting approach, as described by BODEN et al[15]. The approximate 2.0 cm×1.0 cm×0.5 cm autografts harvested from the superior ilium and PHAB were randomly implanted either on the right side or left side of each rabbit’s spinal processes. Peri-operative antibiotics were given (penicillin 40 KIU/kg through intramuscular injection).
2.3 LIPUS treatment
After a 7 d latent period, the LIPUS group rabbits were anesthetized with an intravenous injection of pentobarbital by ear vein (15 mg/kg) and the spinal fusion field was subjected to LIPUS 20 min daily for 4 weeks until euthanasia. The ultrasound energy was provided by a sonic accelerated fracture healing system (SAFHS; Smith & Nephew, Memphis, TN, USA). The treatment head module delivered an ultrasound signal comprising a burst width of 200 μs containing 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm2. All the surgical procedures and postoperative treatments were approved by the Animal Experimentation Ethics Committee of the Central South University, Changsha, China. Except for a daily 20 min period for the ultrasound therapy and wound care, the rabbits were kept in cages with free access to food pellets and water, and allowed to ambulate freely during the experiment. The single-cage occupancy was applied to all rabbits.
2.4 Fusion evaluation
The animals were euthanized at 5 weeks after surgery. The fusion segments were first evaluated radiographically. The lumbar spine of each rabbit was harvested en bloc. Following gross observation and manual palpation test, the specimens were prepared for histomorphology and scanning electron microscopy (SEM).
2.4.1 Manual palpation/gross observation
After en bloc excision, the lumbar spines were observed and palpated manually by two observers in a blinded fashion through flexion and extension loading test at the level of the fused motion segment. The manual palpation has been proved to be more accurate than plain radiographs and correlated closely with biomechanical testing data[15]. Motion segments were graded as “solid” without detectable motion which was classified as fused or “not solid”. By gross observation, the fusion mass by the amount of “bridging” was defined as continuous or discontinuous callus. Bridging was defined as the percentage of graft surface area exhibiting continuity of the graft integrated with the adjacent transverse processes and vertebral lamina.
2.4.2 Radiographic analysis
Antero-posterior view radiographs using a microradiographic machine (Model 43855C, Faxitron X-ray Systems, Wheeling, IL, USA) were taken preoperatively, immediately after surgery and euthanasia. The X-ray output voltage was set at 35 kV, 30 mA for 1 s. The pixel intensity of aluminum stepped wedge (ASW) on each radiograph was standardized using the Image J version 1.29 software (NIH, Bethesda, MD, USA)[16]. The gray scale value of the plain callus image was then measured to represent its projected bone mineral density (BMD). The difference value (D-value) of gray scale between pre- and post-treatment of each group represented the projected BMD change. Radiographs were evaluated blindly by two authors, based on the presence of a contiguous trabecular pattern within the inter-transverse process fusion mass. Specimens were graded as fusion only when both observers agreed in their reading results.
2.4.3 Histomorphologic analysis
The routine histology of harvested specimens was observed using hemotoxylin and eosin staining (H&E). Histologic fusion was defined as bony trabeculae bridging from one transverse process to the next. Sections were placed on saline-coated slides for protein expression using immuno-histochemistry (IHC). IHC was performed for BMP-2 and TGF-β1 expression according to the lab test kit specification (Boster Biological Technology Limited company, Wuhan, China). Two observers independently assessed the histology and immuno-histochemistry data for new bone formation, tissue reaction and protein expression.
2.4.4 Scanning electron microscope (SEM) analysis
Specimens approximately of 5 mm×1 mm×1 mm in dimension were harvested from fusion field and dealt with routine preparation for SEM analysis. The images of these specimens were magnified and viewed by JSM5600-LV scanning electron microscope. The cells adhering to or entering into pores of porous HA block, new bone trabeculae incorporation, and degradation of graft substitute were evaluated.
2.5 Statistical analysis
The data were presented as “Mean±Standard”. The treatment effects for all continuous response variables were evaluated using a two-way ANOVA followed by a factorial analysis procedure. All statistical analyses were conducted using SPSS 14.0 statistical software (IBM Acquires SPSS Inc, USA) at a significance level of P < 0.05.
3 Results
3.1 Manual palpation/gross observation
No adverse events are encountered during experiment. The fusion rates according to manual palpation and gross observation are higher in the LIPUS group than the control group. By manipulation, the fusion rates of the LIPUS group and the control group on the HA grafted sides are 60% and 20%, respectively (P< 0.05). The same result also occurs when comparing the iliac bone grafted sides of the LIPUS group and the control group animals (100% vs 50%, P<0.05) (Fig.1(a)). By observation, six out of ten animals of the LIPUS group (HA) have continuous callus bridging in the grafted field, but only one animal in the control group (HA) has the same result (60% vs 10%, P<0.05). The same result also occurs when comparing the iliac bone grafted sides of the LIPUS group and the control group animals (100% vs 50%, P<0.05) (Fig.1(b)). From these data, a conclusion may be drawn that LIPUS can accelerate spinal fusion with either PHAB or autograft. However, there are no difference between auto ilium and LIPUS+HA(50% vs 60%, P<0.05).
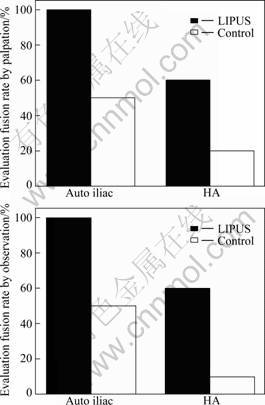
Fig.1 Fusion rate evaluated by palpation (a) and observation (b) (P<0.05)
3.2 Radiograph
Faxitron radiographs show some softening of the edges of the HA graft after 5 weeks postoperatively in both the LIPUS treated and the control specimens although no major loss of material is observed. The D-value of gray scale of fusion zone between pre- and post-treatment is higher in the LIPUS group when compared with the control group (Fig.2). Factor analysis shows that the main effect of factor A (factor A: LIPUS) is 0.075 (P = 0.00 < 0.01), which suggests that LIPUS increases the D-value of gray scale, namely the hard callus volume is increased due to stimulation. The main effect of factor B (factor B: graft) is 0.115 (P = 0.005 < 0.01), which means that the use of graft also increases D-value gray scale. However, the interaction between factor A and B is 0.025 (P = 0.331 > 0.05), which means that there is no interaction between LIPUS and the type of graft used.
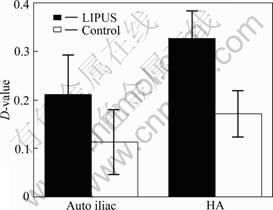
Fig.2 D-value of gray scale of fusion zone by radiograph (P< 0.05)
3.3 Histomorphology
Histomorphological analysis shows that new bone is formed into the pores of the PHAB with or without LIPUS treatment after 5 weeks postoperatively (Fig.3). There are more chondrocytes, osteoblast-like cells and osteocyte-like cells in the LIPUS group when compared with the control group after 5 weeks postoperatively (Fig.4). Factor analysis shows that the main effect of factor A is 13 (P = 0.000 < 0.01), which suggests that LIPUS can accelerate cell differentiation, proliferation and entochondrostosis. The main effect of factor B is 4.4 (P = 0.761 > 0.05), which means that graft alone may not accelerate cell differentiation and proliferation. Interaction of factor A and B is 0.025 (P = 0.816 > 0.05), which means that there is no interaction between factor A and B. Higher magnification of sagittal sections of junction of PHAB and host bone show new bone formation after 4 weeks of LIPUS treatment. After 4 weeks LIPUS treatment, a composite of reactive bone and the new regeneration fibrous callous is formed at the interface between PHAB and the host bone. With LIPUS treatment, there are chodrocytes arranged in columns, new bone trabeculai, calcified cartilage coated by a thin layer of new bone containing osteoblasts and osteocytes. Cavum medullare ossium forms among new bone trabeculae with numerous mesenchymal cells and micrangium filling in it. With respect to the control group, fewer chodrocytes are observed in the junction tissue, and disorganized with uncertain trabecular bone structure and cavum medullare ossium formation. Compared with the LIPUS group, the bone area is significantly smaller in the control group. For immunohistochemical staining analysis in the junction region of PHAB and host bone, both TGF-β1 and BMP-2 strongly express in osteoblast, mature chondrocyte and the interstitial substance in the LIPUS group after 5 weeks postoperatively, but strong expression of TGF-β1 and BMP-2 is only found in the interstitial substance in the control group. As for the index of positive expression of TGF-β1 and BMP-2, there are significant differences between the two groups in positive index (4.543±0.431 vs 3.125±0.462, P < 0.05).
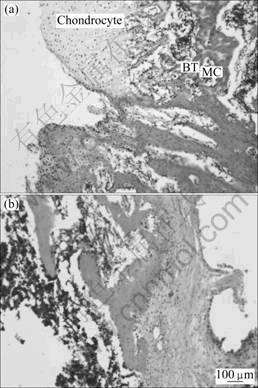
Fig.3 High magnification photomicrograph of sagittal sections of junction of PHAB and host bone after 5 weeks postoperatively (BT—Bone trabeculae; MC—Medullae cavum
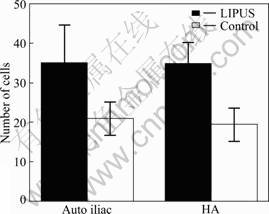
Fig.4 Number of cells of photomicrograph of sagittal sections of junction of PHAB and host bone after 5 weeks postoperatively (P< 0.05)
3.4 SEM images
There are more osteoblast-like and chondroblast- like cells on the surface of the PHAB adjacent to the transverse processes in the LIPUS group when compared with the control group (Fig.5). With the effect of LIUPUS, the surface of porous HA block is softened and attached and coupled with network of fibrous callus. In the control group, less fibrous callus is found except on the softened surface of PHAB. Many osteoblast-like and chondroblast-like cells are attached and coupled on the surface of PHAB in the LIPUS group, but only a few of such cells are observed on the PHAB surface in the control group. Under further magnification of the field of vision, osteoblast-like and chondroblast-like cells are found to adhere to the PHAB with tight integration. A very long cell projection extends out through a pore.
4 Discussion
The objective of the current study was to elucidate the effects of LIPUS on PHAB in a rabbit spine PLF model. The results of this study confirmed our initial hypothesis and indicated that LIPUS might play a positive role in enhancing HA integration with spine and thus improving fusion outcome.
New bone formation is often limited to the areas where the bone graft substitute is in contact with host bone, reflecting its osteoconductive but not osteoinductive property. Synthetic porous HA ceramic has been used as a bone graft substitute[17]. The interconnected porous structure of HA ceramics encourages new bone ingrowth deep into the pores, which will eventually increase the mechanical strength of the implants and the reconstruction[7]. Osteoconductive capacity of artificial bone substitute mainly depends on interconnectivity of the porous structure. However, there is not yet agreement on the suitable pore size. ZHU et al[18] proposed that the suitable pore size of porous nano-HA for bone formation was 100-150 μm, which may provide a good guideline for further improving the material structure and creating an ideal bone graft substitute. Some studies even suggested that 500 μm was a more suitable pore size and the porosity of HA graft less than 30% would seriously limit bone ingrowth [19-20]. The bigger the pore size of bone graft substitute
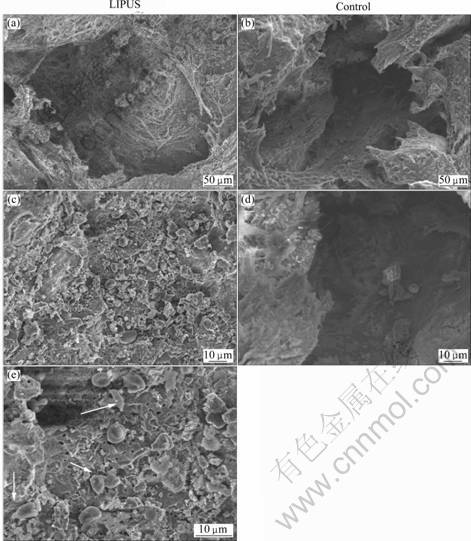
Fig.5 SEM images of porous HA block in bone of rabbit after 5 weeks postoperatively (There are more osteoblast-like and chondroblast-like cells (indicated by arrows) ingrown and coupled on HA block pore in LIPUS treated group than control specimens)
material, the higher the bone conductibility. However, the more the pore size and connectivity of bone graft substitute material, the lower the mechanical strength will result. Hence, an optimal combination of pore size, pore connectivity and mechanical strength of a HA material needs to be found to achieve the demands of biological activity and mechanical strength on different conditions. In this study, the PHAB was opted for larger pore size ranging from 200 to 500 μm. Although the initial compressive strength might be decreased, eventual osteointegration would strengthen the fusion. The reason for such success was that the graft would not be significantly compressed in PLF, and bone graft substitutes were mainly required for bone formation rather than for the initial mechanical strength requirement[21]. In this study, no breakage of porous HA block in PLF is observed. It is therefore concluded that the interconnected large and highly porous HA block would be an acceptable material for a bone graft extender in PLF in a rabbit model. However, the anatomical structure and biomechanical loading will be significantly different in human, so clinical trial study must be conducted to support its safe and effective application.In addition, ultrasound at intensities ranging from 1 to 50 mW/cm2 has been demonstrated to act through certain cellular reactions involved in each phase of the healing process such as inflammatory reaction, angiogenesis, chondrogenesis, intramembranous ossification, endochondral ossification and bone remodeling to accelerate skeletal connective tissue healing in animal[22-24] and in human[25-26]. In vitro cell-culture experiments have shown that LIPUS mediates osteoblastic production of IL-8, basic-FGF, VEGF, TGF-β, alkaline phosphatase, the non- collagenous bone proteins[27-28], and the expression of osteoblast-related genes[29]. These may be accounted for the biologic mechanisms in accelerating bone fracture healing with LIPUS therapy.
Radiographs and histology demonstrated a significant improvement in bone formation when LIPUS was applied to the PLF sites with the PHAB when compared with no LIPUS treatment after 5 weeks postoperatively. At the 5th week, histology reveals significantly more new mature bone trabecula, cartilaginous tissue, osteoblasts and chondrocytes in PHAB pores and junction zone in the LIPUS group when compared with the control group. SEM images also demonstrate significantly more osteoblast-like and chondroblast-like cells ingrown into the HA pores, more osteoid substance aggregates on the surface of HA pores in LIPUS group when compared with control group. These results show that LIPUS has accelerated endochondral ossification, bone callus remodeling, and bone in-growth into porous HA block, which corroborated with the previous finding[14]. The expressions of both TGF-β1 and BMP-2 significantly increase in the LIPUS group than in the control group. TGF-β1 is a cluster of multifunctional protein peptides and belongs to TGF superfamily. These peptides are abundant in bone tissue and platelets which promote osteoblast proliferation and differentiation, and accelerate secretion of fibroblasts, induce intramembranous ossification and endochondral osteogenesis and inhibit the activity of osteoclasts[30], thus playing important regulatory role in bone cell growth, differentiation and immune function. BMP, one member of TGF-β superfamily members, is a multifunctional growth factor, and plays an important role in bone formation, in which BMP-2 is known so far to have the most osteoinductive activity of bone morphogenetic regulator[31]. The higher expression of TGF-β1 and BMP-2 in the LIPUS group reflects the increased healing response and degree of new bone generation. This shows that LIPUS appears to involve the stimulation of osteoblast differentiation and calcified matrix production along with increased expression levels of TGF-β1 and BMP-2.
Through radiographic analysis, BMD of fusion mass is significantly higher in the LIPUS group when compared with the control group. That is to say that LIPUS may have accelerated ossification. Based on the results of spinal fusion by palpation and observation, the rate of fusion improves with LIPUS therapy.
The present study had several technical limitations. The X-ray quantification was a 2D-based technique and its data was dependent on both the size and the placement of the bone. The 3D-based technique using micro-CT or p-QCT may be a better method to evaluate the spinal PLF. In addition, only one time point after 4 weeks postoperative treatment was used, and 4 weeks was not long enough to allow sufficient implant resorption. Finally, a certain biomechanical testing method on the specimens would be more desirable to substantiate the fusion results.
5 Conclusions
1) LIPUS can accelerate endochondral ossification, bone callus remodeling and bone ingrowth into PHAB.
2) LIPUS enhances the rates of PLF. The use of LIPUS in combination with a porous HA block bone graft substitutes may further improve the treatment results in PLF, which will avoid the morbidity associated with the use of autograft bone and not limited by the supply of allograft bone.
Acknowledgments
This study was supported in part by the Open Fund of the State Key Laboratory of Powder Metallurgy, Central South University, China.
References
ZHU W, XIAO J, WANG D, LIU J, XIONG J, LIU L, ZHANG X, ZENG Y. Experimental study of nano—HA artificial bone with different pore sizes for repairing the radial defect [J]. Int Orthop, 2009, 33(2): 567-571.
LI S H. A ReviewTGF-β1 carrier in bone defect remodeling [J]. Journal of Modern Stomatology. 2007, 21(2): 200-202. (in Chinese)
Corresponding author: L? Hong-bin; Tel: +86-731-84327331; E-mail: hongbinlu@hotmail.com
DOI: 10.1016/S1003-6326(09)60396-4

