Catalytic cracking mechanisms of tar model compounds
来源期刊:中南大学学报(英文版)2016年第12期
论文作者:蒋绍坚 陈波 时章明 田红
文章页码:3100 - 3107
Key words:biomass; tar model compounds; catalytic cracking; mechanisms; quantum chemistry
Abstract: B3LYP/6-31G(d, p) method was used to investigate the catalytic cracking mechanism of biomass tar model compound. Phenol, toluene and benzene were selected as the tar model compounds and CaO was selected as the catalyst. The pathways of tar compound radical absorbed by CaO were determined firstly through comparing enthalpy changes of the absorption, and then Mulliken population changes were analyzed. The results show that the absorption of tar model compound radical and CaO is an exothermic reaction. Formation of C—O—Ca is more easily than that of C—Ca—O and formation of Caromatic—Caromatic—Ca—O is more easily than that of Caromatic—C(O)—Ca—O. The C—C bond Mulliken populations in tar model compound radicals are reduced by 11.9%, 10.5% and 15.5% in the case of a hydrogen atom removed, and those are 15.7%, 14.3% and 16.3% in the case of two hydrogen atoms removed through the absorption of CaO. Catalytic ability of CaO acting on the tar model compound is in an order of phenol>benzene>toluene.
J. Cent. South Univ. (2016) 23: 3100-3107
DOI: 10.1007/s11771-016-3375-7
CHEN Bo(陈波)1, SHI Zhang-ming(时章明)1, JIANG Shao-jian(蒋绍坚)1, TIAN Hong(田红)2
1. School of Energy Science and Engineering, Central South University, Changsha 410083, China;
2. Institute of Energy & Power Engineering, Changsha University of Science and Technology, Changsha 410076, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: B3LYP/6-31G(d, p) method was used to investigate the catalytic cracking mechanism of biomass tar model compound. Phenol, toluene and benzene were selected as the tar model compounds and CaO was selected as the catalyst. The pathways of tar compound radical absorbed by CaO were determined firstly through comparing enthalpy changes of the absorption, and then Mulliken population changes were analyzed. The results show that the absorption of tar model compound radical and CaO is an exothermic reaction. Formation of C—O—Ca is more easily than that of C—Ca—O and formation of Caromatic—Caromatic—Ca—O is more easily than that of Caromatic—C(O)—Ca—O. The C—C bond Mulliken populations in tar model compound radicals are reduced by 11.9%, 10.5% and 15.5% in the case of a hydrogen atom removed, and those are 15.7%, 14.3% and 16.3% in the case of two hydrogen atoms removed through the absorption of CaO. Catalytic ability of CaO acting on the tar model compound is in an order of phenol>benzene>toluene.
Key words: biomass; tar model compounds; catalytic cracking; mechanisms; quantum chemistry
1 Introduction
As by-products of the biomass gasification process, the tars not only cause corrosion to the gasifier, but also reduce the efficiency of the gasification system. Massive studies on the characteristics of the tars and methods to deal with the tars have been done by scholars home and abroad [1-3]. The composition of the tars is complex. Hundreds of compounds have been detected, most of which are aromatic compounds [4-5]. With the increase of the temperature, the aromatization of tar will increase [6-7]. So, the study on aromatic tar model compounds is an important way to study the properties of the tars and deal with the tars. Rich in the tars, benzene, toluene and phenol are often treated as tar model compounds [8-9]. Presently, physical treatment, thermal cracking and catalytic cracking are the major methods to deal with the tars, and the catalytic cracking process is the most effective one [10]. On one hand, the method diminishes the tars in the biomass gasification process; on the other hand, the tars can be converted to gas. CaO is widely used catalyst in the catalytic cracking process for its low price and good catalytic efficiency. Substantial catalytic cracking experiments using CaO as catalyst were employed [11-13] and the results show that CaO can reduce the tars cracking activation energy and improve the conversion of the tars. The study results of LIU et al [14] show that CaO catalyzes and ameliorates the cracking of heavy hydrocarbon rich in the aromatic compounds during the biomass gasification process. The investigation results of ZHOU [15] show that CaO has significant impacts on aromatic hydrocarbon cracking, and it prompts the crack of the ring structures.
The investigation on the catalytic cracking characteristics of the biomass tars is based on macro- experiments mostly. Those experiments reveal only the superficial laws. Microscopic studies on the catalytic cracking characteristics of the biomass tars have great significance on establishing theoretical systems, conduct experiments and offering solutions in engineering designs. However, studies about the molecular level are rarely, and even less than the catalytic mechanisms studies by quantum chemistry. MEERWEIN and EMSTER [16] proposed and developed the concept of positive carbon ions. WHITMORE [17] further proposed that catalytic action was associated with active center of the catalyst and believed that the C—H rupture expedited the generation of the positive carbon ion, which would benefit the activation of the catalyst. ZHOU et al [18] found that there were many active centers in the catalyst, C—C and C—H weakened in bond energies and became easier to break after the adsorption reactions. Although C—H bond energy is larger than that of C—C in the catalytic reaction, the C—H bonds are easier to break, which expedites the rupture of C—C bonds [19]. L [20] studied the catalytic cracking of the toluene by quantum chemistry, and came up with the conclusion that it was feasible to analyze the catalytic mechanisms by the enthalpy changes and the Mulliken population in the adsorption reaction of the catalysts and the free radicals generated in the toluene dehydrogenation process.
[20] studied the catalytic cracking of the toluene by quantum chemistry, and came up with the conclusion that it was feasible to analyze the catalytic mechanisms by the enthalpy changes and the Mulliken population in the adsorption reaction of the catalysts and the free radicals generated in the toluene dehydrogenation process.
By analyzing the enthalpy changes and the Mulliken population in the adsorption reaction, the catalytic cracking mechanisms of toluene, benzene and phenol under the catalytic action of CaO were analyzed using the quantum chemistry method.
2 Analytical method
2.1 Enthalpy change of absorption
By using the B3LYP/6-31G(d, p) method based on the density functional theory of the Gaussion 09 software, the equilibrium geometries of the reactants and products were optimized, and their thermodynamic parameters were calculated. The relationships of those parameters are as follows. The calculations in this work were under the condition of 750 °C and 1.01×105 Pa due to the tar formation high temperature atmosphere (temperature in biomass gasification furnace is generally higher than 600 °C).
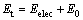 (1)
(1)
where Et is the sum of zero point energy and the electron energy; Eelec is the electron energy; E0 is the zero point energy.
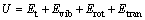 (2)
(2)
where U is the thermodynamic energy; Evib is the vibrational energy; Erot is the rotational energy; Etran is the translational energy.
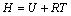 (3)
(3)
where H is the enthalpy; R is the mole gas constant, 8.314 J/(mol·K); T is the thermodynamic temperature.
Thus, the enthalpy change of the adsorption process, △H, can be calculated as follows:
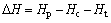 (4)
(4)
where Hp is the enthalpy of the adsorption products; Hc is the enthalpy of the catalysts; Ht is the enthalpy of the tar model compounds radical.
Since there are many absorption ways between the tar model compounds and the catalyst, massive structure optimizations and frequency calculations are implemented. Thus, the enthalpy changes of every adsorption process can be obtained. Thermodynamically, absorption process with greater enthalpy changes is more likely to take place.
2.2 Mulliken population
In the field of quantum chemistry calculations, Mulliken population indicates the chemical bond strength [21] that chemical bonds with greater Mulliken population are more stable, and the reverse is also true.
In summary, the pathways of tar compound radical absorbed by CaO were determined based on the enthalpy changes. By comparing those Mulliken population changes of the C—C bonds after absorption, it is feasible to analyze the catalytic capability of CaO on the tar model compounds cracking process. Tar model compounds exist in radical form at high temperature, and any C—C bond breaking can be considered a benzene ring fracture. So, it is practicable to treat the minimum change of the Mulliken population as an indicator to judge the catalytic performance of CaO.
3 Enthalpy change of absorption
According to Refs. [17-20], the tar model compounds tend to involve in the absorption reaction after they lose one or more hydrogen atoms and formulate the free radicals. In order to simplify the research work, absorption process of CaO and compounds of a atom or two atoms removed was analyzed. Other cases can be analyzed through combining the two cases above.
3.1 Enthalpy changes of absorption between benzene and CaO
There are three reaction pathways in the absorption process of benzene with the catalyst (see Fig. 1). Pathway 1 represents that the dehydrogenated C(1) reacts with CaO, resulting in C(1)—O—Ca. Pathway 2 represents that the dehydrogenated C(1) reacts with CaO, resulting in C(1)—Ca—O. Pathway 3 represents that the dehydrogenated C(1) and C(2) react with CaO, resulting in C(1)—C(2)—Ca—O.
The gross energy values of CaO, benzene radicals and the absorption products were calculated, then, the enthalpy changes of different reaction pathways were obtained, as shown in Fig. 2.
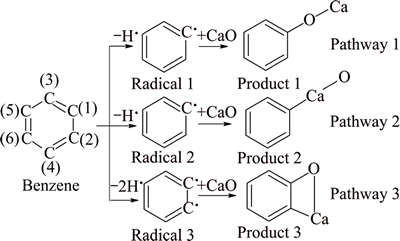
Fig. 1 Three reaction pathways of benzene and CaO
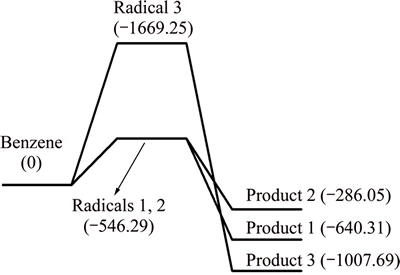
Fig. 2 Potential energy profiles along reaction pathway of benzene and CaO (unit: kJ/mol)
The enthalpy change of dehydrogenation reaction of benzene is bigger than 0, and the process is endothermic. All the enthalpy changes of the three absorption reactions are less than 0, and those processes are exothermic. In the circumstance of a hydrogen atom removed, the enthalpy change of Pathway 1 is larger than that of Pathway 2. Thus, it can be thermodynamically deduced that Product 1 is easier to formulate and it also indicates that enthalpy change of C—O—Ca pathway is larger than that of C—Ca—O pathway, and C—O—Ca is easier to generate. Due to the symmetry of benzene, there is only one pathway between CaO and dehydrogenation benzene radicals of two hydrogen atoms removed. In conclusion, it is deducible that the absorption reaction between benzene and CaO occurs in pathways 1 and 3.
3.2 Enthalpy changes of absorption between toluene and CaO
There are 14 reaction pathways in the absorption process of benzene with the catalyst. For Pathways 1, 3, 5, 7, the benzene radicals formed after hydrogen atoms of C(1), C(3), C(2) and C(7) removed react with the CaO to formulate C-O-Ca products. If Ca exchanges site with O, Pathways 2, 4, 6, 8 will be acquired, and those reaction pathways will result in C—Ca—O products. For Pathways 9-14, C—C—Ca—O adsorption products will be obtained, as shown in Fig. 3 (in the case of a hydrogen atom removed) and Fig. 4 (in the case of two hydrogen atoms removed).
The gross energy of CaO, toluene radicals and the absorption products are calculated, then the enthalpy changes of different reaction pathways are obtained, as shown in Fig. 5.
The enthalpy change of dehydrogenation reaction of toluene is bigger than 0, and the process is endothermic. The dehydrogenation process on methyl is easier to occur compared with benzene ring. All the enthalpy changes of the fourteen absorption reactions are less than 0, and those processes are exothermic. The dehydrogenated toluene radicals proceed absorption reaction with CaO. C—Ca—O corresponds with pathways 1, 3, 5, 7 and C—O—Ca corresponds with pathways 2, 4, 6, 8. The enthalpy changes of the C—O—Ca absorption reaction are larger than that of C—Ca—O, which indicates that C—O—Ca absorption reaction is easier to happen. The enthalpy changes of pathways 1, 3, 5 are same, while they are larger than that of pathway 7. Thermodynamically, it is practicable to believe that toluene radicals formed after a hydrogen atom removed react with CaO in pathways 1, 3, 5.
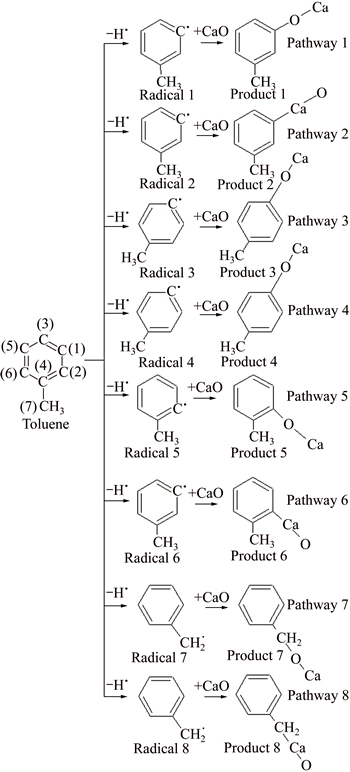
Fig. 3 Reaction pathway of toluene and CaO in the case of one hydrogen atom removed
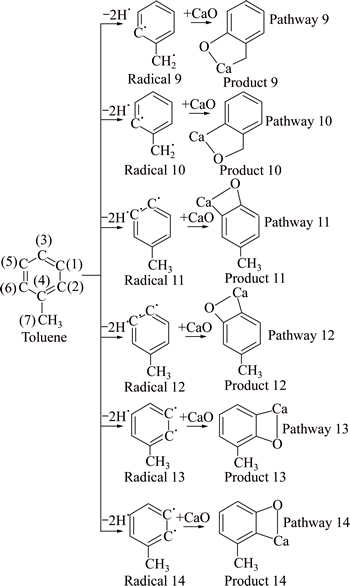
Fig. 4 Reaction pathway of toluene and CaO (in the case of two hydrogen atoms removed)
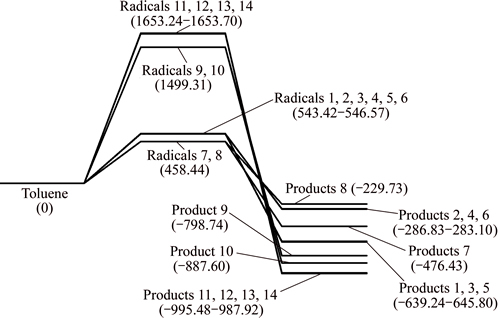
Fig. 5 Potential energy profiles along reaction pathway of toluene and CaO (unit: kJ/mol)
Similarly, for reaction of CaO with toluene radicals formed after two hydrogen atoms are removed, the enthalpy changes of pathways 11, 12, 13, 14 are same, while they are larger than that of pathways 9, 10. Thus, it is believed that toluene radicals formed after two hydrogen atoms are removed react with CaO in pathways 11, 12, 13, 14. In conclusion, it is deducible that the absorption reactions between benzene and CaO occur in pathways 1, 3, 5, 11, 12, 13, 14.
3.3 Enthalpy changes of absorption between phenol and CaO
All the atoms of phenol are on the same plane, but the two sides of the phenolic hydroxyl groups are not completely symmetric due to the influence of phenolic hydroxyl groups. So, the chemical environments of C(6), C(2), C(5) and C(1) are different though they are at the similar position of phenol carbon ring [22]. Compared with toluene, there are more absorption reaction pathways, as shown in Fig. 6 (in the case of one hydrogen atom removed) and Fig. 7 (in the case of two hydrogen atoms removed).
The enthalpy changes of different reaction pathways are obtained, as shown in Fig. 8.
Enthalpy change of phenol dehydrogenation reaction is bigger than 0, and the process is endothermic. The dehydrogenation process of phenol is easier to occur compared with benzene. All the enthalpy changes of the 22 absorption reactions are less than 0, and those processes are exothermic. For dehydrogenated radicals formed after a hydrogen atom is removed take absorption reaction with CaO. C—Ca—O corresponds with pathways 1, 3, 5, 7, 9, 11 and C—O—Ca corresponds with pathways 2, 4, 6, 8, 10, 12. The enthalpy changes of the C—Ca—O absorption reaction are larger than that of C—O—Ca, which indicates that absorption reaction of C—O—Ca is easier to happen. The enthalpy changes of pathways 1, 3, 5, 11 are same, while they are larger than that of pathways 7, 9. For absorption reaction of CaO with phenol radicals formed after two hydrogen atoms are removed, the enthalpy changes of pathways 13, 14, 19, 20, 21 are same, and they are larger than those of the other pathways. In conclusion, it is deducible that the absorption reactions between phenol and CaO occur in pathways 1, 3, 5, 11, 13, 14, 19, 20, 21.
In summary, hydrogen atom on the benzene ring substituent groups (methyl and phenol hydroxyl) is easier
to break compared with hydrogen atom on benzene ring thermodynamically. And the enthalpy changes of corresponding dehydrogenation reaction are in an order of phenol
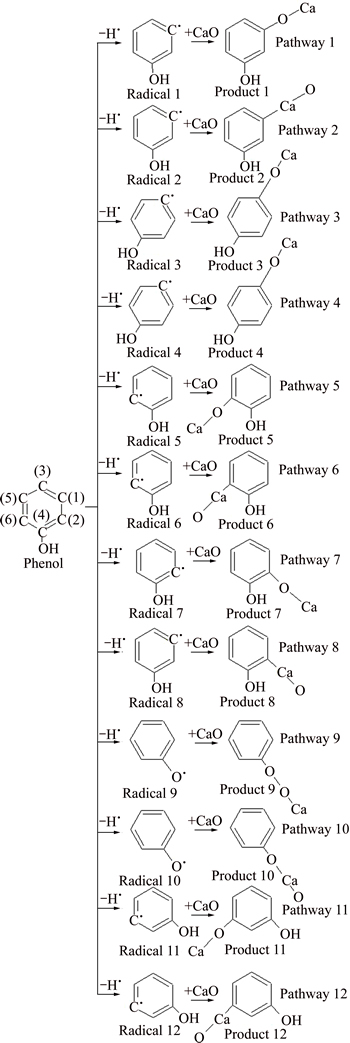
Fig. 6 Reaction pathway of phenol and CaO in the case of a hydrogen atom removed
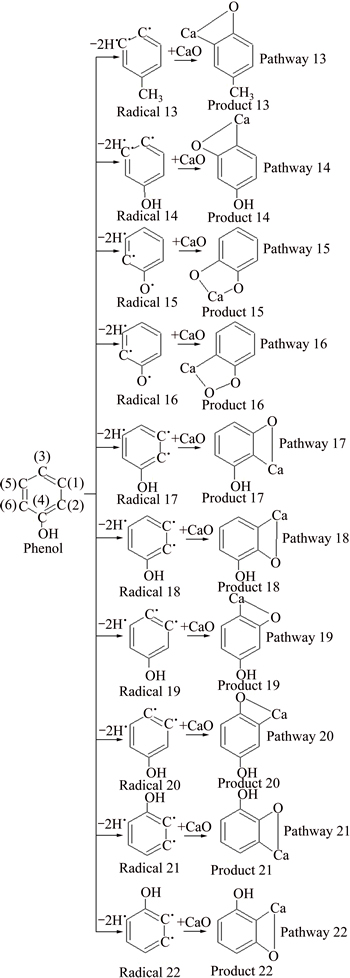
Fig. 7 Reaction pathway of phenol and CaO in the case of two hydrogen atoms removed
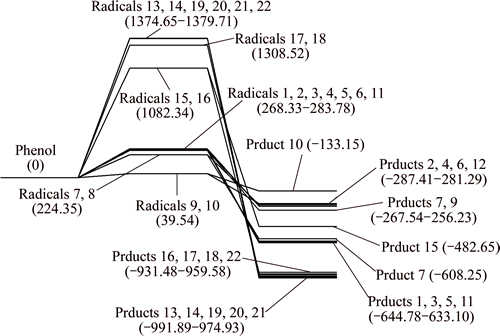
Fig. 8 Potential energy profiles along reaction pathway of phenol and CaO (unit: kJ/mol)
4 C—C Mulliken population changes
4.1 C—C Mulliken population changes of benzene with CaO catalyst
As is shown in Table 1, the minimum C—C Mulliken population of benzene radical with a hydrogen atom removed decreases from 0.540 to 0.476 of C(2)—C(4), a drop of 11.9%. The minimum C—C Mulliken population of benzene radical with two hydrogen atoms dehydrogenated decreases from 0.494 to 0.416 of C(1)—C(3), a drop of 15.7%.
4.2 C—C Mulliken population changes of toluene with CaO catalyst
As shown in Table 2, toluene radical with a hydrogen atom removed reacts with CaO in pathways 1, 3, 5. The minimum C—C Mulliken populations decrease from 0.514 of C(1)—C(2) to 0.451 of C(5)—C(6), from 0.515 of C(5)—C(6) to 0.473 of C(2)—C(4) and from 0.534 of C(5)—C(6) to 0.474 of C(1)—C(2), and the average drop is 10.5%. Toluene radical with two hydrogen atoms removed reacts with CaO in pathways 11, 12, 13, 14. The minimum C—C Mulliken populations decrease from 0.460 to 0.412 of C(3)—C(5), from 0.460 to 0.405 of C(3)—C(5), from 0.483 of C(5)—C(6) to 0.400 of C(1)—C(2) and from 0.483 of C(5)—C(6) to 0.400 of C(1)—C(2), and the average drop is 14.3%.
4.3 C—C Mulliken population changes of phenol with CaO catalyst
As shown in Table 3, phenol radical with a hydrogen atom removed reacts with CaO in pathways 1,3, 5, 11. The minimum C—C Mulliken populations decrease from 0.481 to 0.417 of C(5)—C(6), from 0.508 to 0.427 of C(4)—C(6), from 0.517 of C(1)—C(2) to 0.385 of C(4)—C(6) and from 0.488 of C(5)—C(6) to 0.456 of C(2)—C(4), and the average drop is 15.5%. Phenol radical with two hydrogen atoms removed reacts with CaO in pathways 13, 14, 19, 20, 21. The minimum C—C Mulliken populations decrease from 0.472 of C(1)—C(2) to 0.404 of C(3)—C(5), from 0.472 of C(1)—C(2) to 0.402 of C(3)—C(5), from 0.474 to 0.394 of C(1)—C(3), from 0.474 to 0.384 of C(1)—C(3), and from 0.470 to 0.394 of C(5)—C(6), and the average drop is 16.3%.
Table 1 C—C bond Mulliken population change of benzene by CaO catalyst
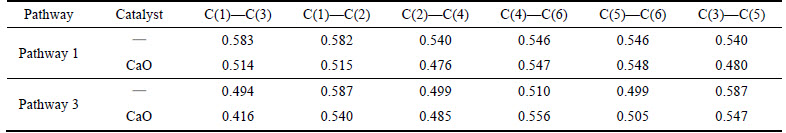
Table 2 C—C bond Mulliken population change of toluene by CaO catalyst
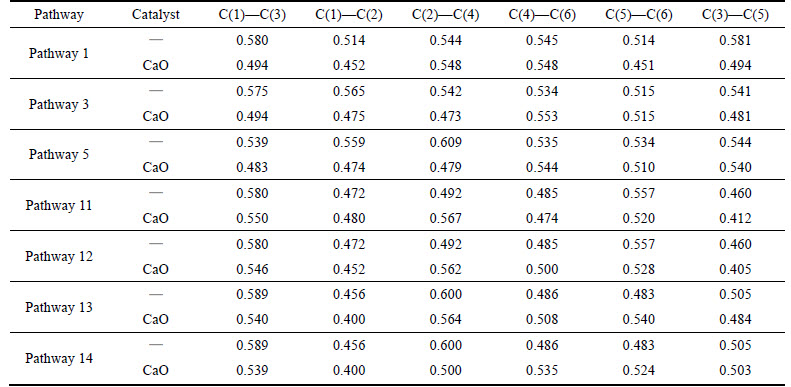
Table 3 C—C bond Mulliken population change of phenol by CaO catalyst
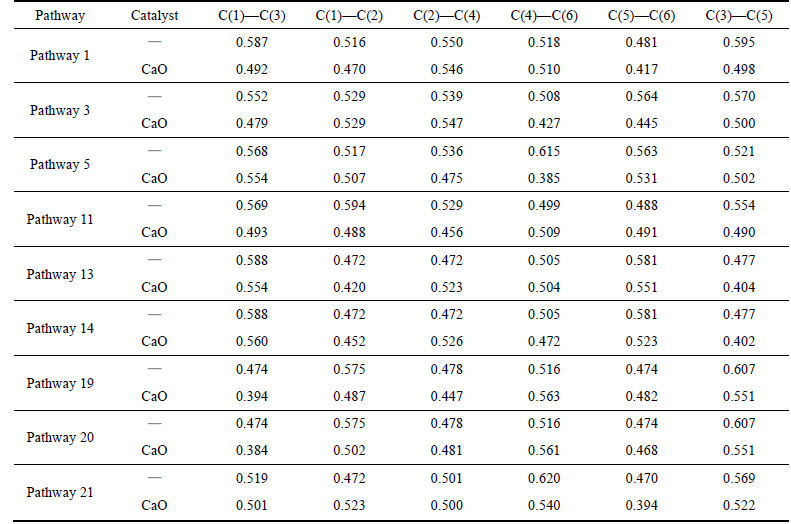
In summary, CaO is capable of decreasing the minimum C—C Mulliken populations of benzene radicals, toluene radicals and phenol radicals, indicating that CaO can lower the activation energy of cracking and it agrees well with the experimental result [14]. By comparing the minimum Mulliken populations of benzene ring with one or two hydrogen atoms dehydrogenated, the falling rate of C—C Mulliken population of the tar model compounds with CaO catalyst is in an order of phenol>benzene>toluene. Thus, it can be concluded that the catalytic performance of CaO on the tar model compounds at 750 °C is in an order of phenol>benzene>toluene, which fits well with the experimental results at 650-850 °C [24].
5 Conclusions
1) Thermodynamically, hydrogen atom on the benzene ring substituent groups (methyl and phenol hydroxyl) is easier to break compared with hydrogen atom on benzene ring. And the enthalpy changes of corresponding dehydrogenation reaction are in an order of phenol
3) For reaction between CaO and tar model compound radicals with a hydrogen atom removed, formation of C—O—Ca is more easily than that of C—Ca—O. For reaction between CaO and tar model compound radicals with two hydrogen atoms removed, formation of Caromatic—Caromatic—Ca—O is more easily than that of Caromatic—C(O)—Ca—O.
4) By analyzing the minimum C—C Mulliken populations changes of the benzene ring radicals, CaO has a notable catalytic performance to the cracking of the tar model compound, and the catalytic performance is in an order of phenol>benzene>toluene.
References
[1] KUMAR A, KUMAR N, BAREDAR P, SHUKLA A. A review on biomass energy resources, potential, conversion and policy in India [J]. Renewable and Sustainable Energy Reviews, 2015, 45: 530-539.
[2] AHMED I I, GUPTA A K. Kinetics of woodchips char gasification with steam and carbon dioxide [J]. Applied Energy, 2011, 88(5): 1613-1619.
[3] UMEKI K, NAMIOKA T, YOSHIKAWA K. Analysis of an updraft biomass gasifier with high temperature steam using a numerical model [J]. Applied Energy, 2012, 90(1): 38-45.
[4] L P M, XIONG Z H, CHANG J, WU C Z, CHEN Y, ZHU J X. An experimental study on biomass air-steam gasification in a fluidized bed [J]. Bioresource Technol, 2004, 95(1): 95-101.
P M, XIONG Z H, CHANG J, WU C Z, CHEN Y, ZHU J X. An experimental study on biomass air-steam gasification in a fluidized bed [J]. Bioresource Technol, 2004, 95(1): 95-101.
[5] LI Ji-hong, LEI Ting-zhou, SONG Hua-min, FENG ZONG-yu, HU Jian-jun, ZHANG Quan-guo. Analysis of chemical component of biomass-based tar by GC/MS [J]. Henan Science, 2005, 23(1): 42-43. (in Chinese)
[6] WU Zheng-shun, MI Tie, CHEN Yi-feng, LI Xue-hui. The research of tar variation mechanism for biomass gasification [J]. Acta Energlae Solaris Sinica, 2010, 31(2): 233-235. (in Chinese)
[7] MI Tie, XU Ling-na, YUAN Yu-shu, DU Nin-yuan, XIAO Shao-fei, WU Zheng-shun. Study of tar formation and variation mechanism for biomass pyrolysis gasification [J]. Acta Energlae Solaris Sinica, 2013, 47(5): 671-674. (in Chinese)
[8] KINOSHITA C M, WANG Y, ZHOU J. Tar formation under different biomass gasification conditions [J]. Journal of Analytical and Applied Pyrolysis, 1994, 29(2): 169-181.
[9] ZHANG Yun-liang, LUO Yong-hao, WU Wen-guang. Heterogeneous cracking reaction of tar over biomass char, using naphthalene as model biomass tar [J]. Energy & Fuels, 2014, 28(5): 3129-3132.
[10] ANIS S, ZAINAL Z A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review [J]. Renewable & Sustainable Energy Reviews, 2011, 15(5): 2355-2377.
[11] GRACIA X A, ALARCON N A, GORDON A L. Steam gasification of tars using a CaO catalyst [J]. Fuel Processing Technology, 1999, 58 (2): 83-102.
[12] CHENG Xiao-han, HE Xuan-ming, CHEN Cheng. Influence of Fe2O3/CaO catalysts on the pyrolysis products of low-rank coal [J]. Energy Technology, 2015, 3(10): 1068-1071.
[13] MI Tie, WU Zheng-shun, YU Xin-ming, WU Xue-jun. The experimental study on biomass tar-cracking by CaO catalyst [J]. Acta Energlae Solaris Sinica, 2011, 32(5): 724-728.
[14] LIU Bo, SHI Zhang-ming, HE Jin-qiao, XIAO Bo. Decomposition and combustion characteristics of dry distillation pine tar with calcium-based catalyst [J]. Journal of Central South University: Science and Technology, 2013, 44(3): 1235-1237. (in Chinese)
[15] ZHOU Kai-hua. Catalytic steam reforming of phenol as model compound of tar in biomass gasification over a novel palygorskite [D]. Hefei: Hefei University of Technology, 2009. (in Chinese)
[16] MEERWEIN H, EMSTER K V. About the equilibrium isomerism between bornyl chloride isobornyl chloride and camphene chlorohydrate [J]. Chem Ber, 1922, 55: 2500-2505.
[17] WHITMORE F C. The common basis of intramolecular rearrangements [J]. J Am Chem Soc, 1932, 54(8): 3274-3281.
[18] ZHOU Jing-song, LIU Ya-jun, LUO Zhong-yang, CEN Ke-fa. Effects of solid acid and alkali catalysts on catalytic cracking of biomass tar [J]. Journal of Zhejiang University: Engineering Science, 2005, 39(7): 1047-1049. (in Chinese)
[19] HUANG Kai-hui, WAN Hui-lin. Catalytic principles [M]. Beijing: Science Press, 1983. (in Chinese)
[20] L Bo. Study on catalytic cracking of tar in biomass gasification with steam in fluidized bed [D]. Shanghai: Shanghai Jiao Tong University, 2014. (in Chinese)
Bo. Study on catalytic cracking of tar in biomass gasification with steam in fluidized bed [D]. Shanghai: Shanghai Jiao Tong University, 2014. (in Chinese)
[21] MULLIKEN R S. Electronic population analysis on LCAO[single bond] MO molecular wave functions [J]. The Journal of Chemical Physics, 1995, 23(10): 1833-1840.
[22] LIU Mei, LIAO Xian-wei. Quantum chemistry study on luminescent spectra of phenol, 1-and 2-naphthol [J]. Journal of Southwest University for Nationalities Natural Science Edition, 2010, 33(3): 433-436. (in Chinese)
[23] HUANG Jin-bao, LIU Chao, REN Li-rong, TONG Hong, LI Wei-min, WU Dan. Studies on pyrolysis mechanism of syringol as lignin model compound by quantum chemistry [J]. Journal of Fuel Chemistry and Technology, 2013, 41(6): 657-665.
[24] SUN Hai-quan. Catalytic conversion of biomass pyrolysis tar [D]. Zibo: Shandong University of Technology, 2009. (in Chinese)
(Edited by YANG Hua)
Foundation item: Project(51276023) supported by the National Natural Science Foundation of China
Received date: 2016-09-06; Accepted date: 2016-10-24
Corresponding author: JIANG Shao-jian, Professor; Tel: +86-731-88830269; E-mail: sjjiang@mail.csu.edu.cn

