沉淀法合成的纳米CeO2前驱体的热分解动力学
宋晓岚,何 希,杨海平,徐大余,江 楠,邱冠周
(中南大学 资源加工与生物工程学院,湖南 长沙,410083)
摘 要:以Ce(NO3)3?6H2O为铈源,(NH4)2 CO3?H2O为沉淀剂,加入少量PEG4000作为分散剂,采用化学沉淀法并经水洗、超声波醇洗,70 ℃干燥后得到CeO2的前驱体Ce2(CO3)3?H2 O。对Ce2(CO3)3?H2 O样品运用差示/热重分析(DSC/TG)和X射线衍射(XRD)方法进行其热分解过程研究,并通过多重速率扫描法记录样品在不同升温速率下的DSC/TG曲线, 采用Kissinger-Akahira-Sunose(KAS)法和Coats-Redfern法进一步研究Ce2(CO3)3?H2 O的热分解动力学。研究结果表明:Ce2(CO3)3?H2 O热分解反应过程分2步进行,主要反应阶段的反应动力学参数是:反应活化能为105.51 kJ/mol,反应级数为2,频率因子为3.61;由此推断出可能的Ce2(CO3)3?H2 O热分解机理函数为 Anti-Jander方程,受三维扩散机制控制。
关键词:纳米CeO2;热分解动力学;化学沉淀法
中图分类号:TB383 文献标识码:A 文章编号:1672-7207(2007)03-0428-05
Thermal decomposition kinetics of precursor of CeO2 nanocrystalline
prepared by precipitation method
SONG Xiao-lan, HE Xi, YANG Hai-ping, XU Da-yu, JIANG Nan, QIU Guan-zhou
(School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: With Ce(NO3)3?6H2O and (NH4)2 CO3?H2O as raw materials, the precursor Ce2(CO3)3?H2O of CeO2 nanocrystalline was prepared by chemical precipitation method using a little amount of PEG4000 as surfactant. The technique of DSC/TG and XRD were used to study the process of thermal decomposition. In particular, the differential thermal analysis curves were measured at different temperature raising-rate in air atmosphere for decomposition of the precursor of CeO2 nanocrystalline by thermal analysis apparatus. The kinetics process was studied with the method of Kissinger-Akahira-Sunose(KAS) and Coats-Redfern. The results show that the decomposition of Ce2(CO3)3?H2O consists of two steps. The active energy of the main step is 105.51 kJ/mol, the reaction order is 2, and the frequency factor is 3.61. The possible conversion functions for the decomposition of the precursor of CeO2 nanocrystalline is Anti-Jander equation and the mechanism of reaction is three-dimensional diffusion.
Key words: CeO2 nanocrystalline; thermal decomposition kinetics; chemical precipitation method
纳米CeO2因具有优越的储、放氧功能及高温快速氧空位扩散能力而被广泛应用于氧化还原反应, 成为极具应用前景的汽车尾气净化催化材料[1-2]、高温氧敏材料[3-4]、pH传感材料[5]、燃料电池尤其是固体氧化物燃料电池(SOFC)的电极材料[6-8]、电化学反应促进材料[9]、化学机械抛光(CMP)研磨材料[10-11]以及金属抗氧化及抗腐蚀的涂层材料和添加剂[12]等,在现代高科技领域有着巨大的应用潜力。
进行热分析动力学(TAK)的研究目的在于定量表征反应(或相变)过程,确定其遵循的最概然机理函数f(α),求出动力学参数:活化能E、频率因子lnA,算出速率常数k,提出模拟TA曲线的反应速率dα/dt表达式,为新材料的热稳定性、反应过程的定量描述和反应机制的推断提供科学依据[13]。为此,本文作者通过程序控温记录多重扫描速率下纳米CeO2的前驱体试样的DSC/TG曲线,采用自编软件结合XRD对纳米CeO2前驱体的热分解过程进行研究。
1 实 验
以分析纯Ce(NO3)3?6H2O和(NH4)2CO3?H2O为原料,配制浓度均为0.1 mol/L的(NH4)2CO3?H2O溶液和Ce(NO3)3?6H2O溶液,按体积比2?3将(NH4)2CO3?H2O溶液快速倒入加有一定量表面活性剂PEG4000的Ce(NO3)3?6H2O混合溶液中,在温度为40 ℃,搅拌速率为800 r/min的条件下反应10 min,得到溶胶。溶胶经过滤、水洗和醇洗,同时加超声波分散,再经70 ℃干燥,研磨后得前驱体Ce2(CO3)3?H2 O粉末。
采用NETZSCH STA 499C热分析仪器在空气气氛下研究前驱体Ce2(CO3)3?H2O的热行为,分别采用 3,5,10,15和20 K/min的升温速率,在300~773 K范围内进行测试,得到DSC/TG曲线,然后,利用自编软件结合Matlab进行前驱体Ce2(CO3)3?H2O的热分解动力学分析。
2 结果与讨论
2.1 纳米CeO2前驱体的热分解过程
在5 K/min的升温速率下,纳米CeO2的前驱体的DSC/TG曲线如图1所示。由图1可知:纳米CeO2前驱体的热分解过程大致分2个阶段进行:在100 ℃之前出现1个吸热峰,这是脱去吸附水的过程,理论质量损失为3.77%;在250~350 ℃有1个放热峰,这是Ce2(CO3 )3? H2O分解生成CeO2的过程,理论质量损失为25.22%。整个热分解过程的理论质量损失为28.99%,而实际质量损失为29.12%,相对误差为0.45%。因而,根据DSC/TG结果推测纳米CeO2的前驱体的热分解过程为2步:
第1步:Ce2(CO3)3?H2O→Ce2(CO3)3+H2O; (1)
第2步:Ce2(CO3)3→2CeO2+2CO2↑+CO↑。 (2)

图1 前驱体Ce2(CO3)3?H2O在5 K/min升温速率下的DSC/TG曲线
Fig.1 DSC /TG curves of Ce2(CO3)3·H2O at 5 K/min raising temperature rates
在多重扫描速率下,纳米CeO2前驱体的DSC曲线如图2所示。由图2可知,在不同升温速率下,DSC曲线形状不同,峰顶温度Tm随升温速率的升高而出现后滞现象,峰面积随升温速率升高而增大。说明在不同升温速率下,样品的分解速率是不同的。
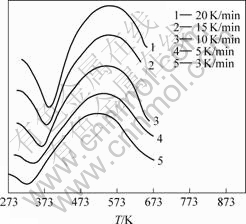
图2 前驱体Ce2(CO3)?H2O在不同升温速率下的DSC曲线
Fig.2 DSC curves of Ce2(CO3)?H2O at different raising temperature rates
前驱体经70 ℃干燥,再经200 ℃和300 ℃热处理 1 h后的XRD测试结果如图3所示。由图3可知,前驱体在70 ℃干燥后为Ce2(CO3)3·H2O;在200 ℃热处理1 h后,XRD图谱显示为Ce(CO3)2的特征峰,并且有微弱的CeO2特征峰出现。当前驱体经过300 ℃热处理1 h后,XRD图谱显示前驱体已全部转化为CeO2。由XRD测试结果可以推知前驱体Ce2(CO3)3?H2O在热分解过程中发生了氧化反应,最后全部分解为CeO2。这进一步验证了DSC/TG分析推测的结果,其氧化反应过程为:
Ce2(CO3)3+2O2→2Ce(CO3)2+CO↑。 (3)
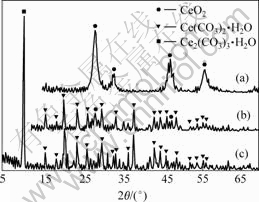
(a) 300 ℃, 1 h; (b) 200 ℃, 1 h; (c) 70 ℃, 干燥
图3 前驱体Ce2(CO3)?H2O经不同温度热处理后的XRD图谱
Fig.3 XRD patterns of precursor Ce2(CO3)?H2O treated at different temperatures
2.2 纳米CeO2前驱体的热分解反应动力学
2.2.1 微分法
对于固体的热分解反应动力学的计算,有基于微分方程的计算和基于积分方程的计算两类。根据文献[13],基于微分方程的计算方法至少有21种,本文采用多重扫描速率法中最常用的Kissinger-Akahira- Sunose(KAS)[14]法来进行微分方程的计算。热分析研究中最基本的非等温动力学关系式为[15]:

式(4)是动力学问题求解方式的微分式,式(5)是非等温条件下的动力学方程表达式。Kissinger[15]等经过一系列的变换,得到下式:

假设热分解过程的最大反应速率出现在DSC曲线的峰顶位置,与之对应的温度为Tp,转化率为αp,Kissinger[15]认为,n(1-αp)n-1与β(升温速率)无关,其值近似等于1,因此,从式(7)可以得到:
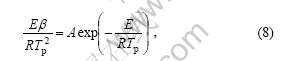
对式(8)两边取对数得:
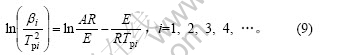
式(9)即为Kissinger方程。利用在不同升温速率下记录的DSC曲线,得到峰顶温度Tp(见表1),由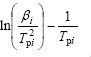 作图拟合得到直线(见图4),由斜率求得Ek,由截距求得Ak。通过计算得到放热峰的活化能Ek为108.99 kJ/mol,线性拟合的相关系数为0.994 16。
作图拟合得到直线(见图4),由斜率求得Ek,由截距求得Ak。通过计算得到放热峰的活化能Ek为108.99 kJ/mol,线性拟合的相关系数为0.994 16。
表1 不同升温速率下Ce2(CO3)?H2O的DSC曲线的峰顶温度
Table 1 Peak temperature at DSC curves of precursor Ce2(CO3)?H2O at different raising temperature rates

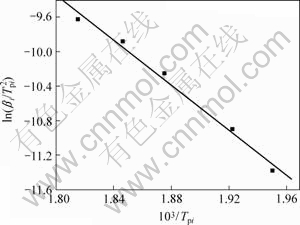
图4 使用Kissinger法所得的拟合直线
Fig.4 Fitting lines by Kissinger method
2.2.2 积分法
根据Coats-Redfern积分式[16],即:

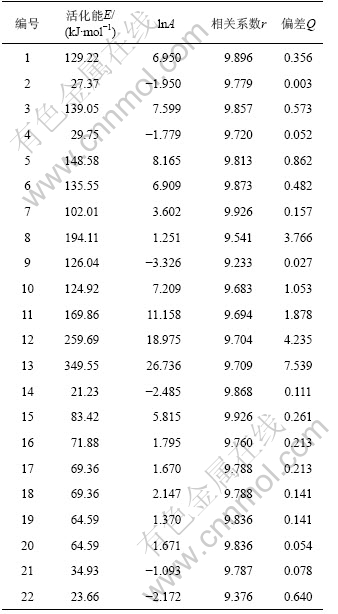
将文献[13]中所报道的22种典型的机理函数G(α)以及表2中的基础数据代入式(10),运用自编软件并结合Matlab数值分析工具,首先计算出ln[G(α)/T2]和1/T,然后以ln[G(α)/T2]对1/T进行线性回归,得到22种不同机理函数下的动力学参数E,lnA,相关系数r和偏差Q,结果见表3。
根据处理的数据结果和文献[13]的报道,比较Kissinger法和微分法求得的动力学参数E和lnA,选取E(108.99 kJ/mol)以及lnA值较为接近且相关系数比较好的一组,该组所对应的机理为该反应的热分解机 理[17]。依据上述数据,符合要求的是7号机理函数,故前驱体的热分解函数模型为:G(α)=[(1+α)1/3-1]2,属于Anti-Jander方程,是三维扩散机制,其积分式和微分式分别为[(1+α)1/3-1]2和3/2(1+α)2/3[(1+α)1/3-1]-1。通过计算得到:E=102.01 kJ/mol,lnA=3.602,n=2。
由于计算过程中采用的是数据逐点计算的方法,故不可避免地会产生累积误差,因而取2种方法所得结果的平均数作为热分解的活化能,即:E=105.5 kJ/mol。
表2 前驱体Ce2(CO3)?H2O的热分解过程DSC部分基础数据
Table 2 Basic data of DSC of precursors Ce2(CO3)?H2O during thermal decomposition
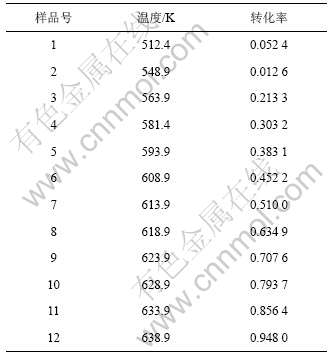
表3 不同反应机理的热分解反应动力学参数值
Table 3 Parameters of thermal decomposition kinetics by different mechanisms
3 结 论
a. 在采用沉淀法制备纳米Ce2O2的过程中,其前驱体的热分解反应分2步进行:首先前驱体Ce2(CO3)3?H2O在70 ℃时脱去吸附水;然后,在300 ℃时Ce2(CO3)3全部氧化分解为CeO2。
b. 根据微分法和积分法计算得到纳米Ce2O2的前驱体热分解的动力学参数为:E=105.5 kJ/mol,lnA=3.61,n=2。
c. 对于纳米Ce2O2前驱体的热分解反应,控制反应的机理函数属于Anti-Jander方程,为三维扩散机制。
参考文献:
[1] 董相廷, 刘桂霞, 孙 晶, 等. CeO2纳米水溶胶的制备[J].稀有金属材料与工程, 2002, 31(3): 229-231.
DONG Xiang-ting, LIU Gui-xia, SUN Jing, et al. Preparation of CeO2 nanoparticles hydrosol[J]. Rare Metal Materials and Engineering, 2002,31(3): 229-231.
[2] Kaspar J, Fornasiero M, Graziani M. Use of CeO2-based oxides in the three-way catalysis[J]. Catal Today, 2000, 50: 285-298.
[3] Noriya I, Woo S, Norimitsu M, et al. Resistive oxygen gas sensors based on CeO2 fine powder prepared using mist pyrolysis[J]. Sensors and Actuators B, 2002, 87: 95-98.
[4] 段文军,唐建成,唐志宏,等. ZrO2+CeO2涂层显微组织和抗热震性能[J]. 中南工业大学学报: 自然科学版,2001, 32(6): 604-607.
DUAN Wen-jun, TANG Jian-cheng, TANG Zhi-hong, et al. Microstructure and thermal shock resistance of ZrO2+CeO2[J]. Journal of Central South University of Technology: Natural Science, 2001, 32(6): 604-607.
[5] Shuk P, Ramanujachary K V, Greenblatt M. New metal-oxide-type pH sensors[J]. Solid State Ionics, 1996, 88(1): 1115-1120.
[6] Toshiyuki M, John D, Yarong W, et al. Influence of nano-structure on electrolytic properties in CeO2 based system[J]. Journal of Thermal Analysis and Calorimetry, 2002, 70(1): 309-319.
[7] Steele B C H. Appraisal of Ce1-yGdyO2-y/2 electrolytes for IT-SOFC operation at 500 ℃[J]. Solid State Ionics, 2000, 129: 95-110.
[8] Murray E P, Tsai T, Barnett S A. A direct methane fuel cell with a ceria based a node[J]. Nature, 1999, 400(3): 649-651.
[9] 董相廷. CeO2纳米晶的制备及其在电化学上的应用[J]. 科学通报, 1996, 41(9): 847-850.
DONG Xiang-ting. Preparation and application in electrochemistry of the CeO2 nanocrystalline[J]. Chinese Science Bulletin, 1996, 41(9): 847-850.
[10] Joseph M S, Shyam P M, Ronald J G. Chemical mechanical planarization of microelectronic materials[M]. New York: John Wiley & Sons Inc, 1997.
[11] 尹邦跃,王零森,林健凉,等. YCe-TZP陶瓷的低温效应[J]. 中南工业大学学报: 自然科学版,2000, 31(4): 335-338.
YIN Bang-yao, WANG Ling-sen, LIN Jian-liang, et al. Low-temperature aging of YCe-TZP ceramics[J]. Journal of Central South University of Technology: Natural Science, 2000, 31(4): 335-338.
[12] 马燕合. 我国稀土应用开发现状及其展望[J]. 材料导报, 2000, 14(1): 3-5.
MA Yan-he. Current status and prospects of rare earth applications in China[J]. Materials Review, 2000, 14(1): 3-5.
[13] 胡荣祖,史启祯. 热分析动力学[M]. 北京:科学出版社,2001.
HU Rong-zu, SHI Qi-zhen. Thermal analysis kinetic[M]. Beijing: Science Press, 2001.
[14] Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29(11): 1702-1706.
[15] Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures[J]. Thermochimica Acta, 1971, 3(1): 1-12.
[16] Coats A W, Redfern J P. Kinetic parameters from thermogravimetric data[J]. Nature, 1964, 201(4914): 68-69.
[17] Muraishi K, Suzuki Y, Kiruchi A. Kinetics of the thermal decomposition of dicarboxylic acids[J]. Thermochimica Acta, 1994, 239(1): 51-59.
收稿日期:2007-01-22
基金项目:科技部国际科技合作项目(2005DFBA028);国家自然科学基金资助项目(59925412)
作者简介:宋晓岚(1964-),女,湖南长沙人,副教授,从事无机功能材料及纳米材料研究
通讯作者:宋晓岚,女,副教授;电话:0731-8877203;E-mail:xlsong@hnu.cn