DOI:10.19476/j.ysxb.1004.0609.2018.08.21
沉铂钯后液中碲铋的分离与回收工艺
张林宝,郑雅杰,安小凯
(中南大学 冶金与环境学院,长沙 410083)
摘 要:以铜阳极泥处理中的沉铂钯后液为原料,经过氢氧化钠沉淀、酸浸沉淀渣、SO2还原后,得到碲粉和还原碲后液,在还原碲后液中加入氢氧化钠沉淀后过滤得到氯氧铋,在氯氧铋中加入氢氧化钠溶液脱氯制得氧化铋。结果表明:加入氢氧化钠调节沉铂钯后液pH为6、反应温度20~25 ℃、反应时间为1 h时,沉铂钯后液中碲和铋沉淀率分别达到99.91%和99.96%;沉铂钯后液得到的沉淀渣混酸浸出适宜条件是3 mol/L盐酸和1.5 mol/L硫酸体积比为2:1,H+浓度为3 mol/L,反应温度为50 ℃,反应时间为2 h,铋和碲的浸出率分别为99.93%和98.21%;在富集碲铋的浸出液中通入SO2还原,当SO2流量为0.25 L/min、反应温度为70 ℃、反应时间为50 min时,碲的还原率为96.59%,还原碲粉中碲含量达到79.45%,砷和铋含量仅为0.003%和0.067%(质量分数);在SO2还原碲后液中加入氢氧化钠调节溶液pH值为2,过滤后得到氯氧铋;在氯氧铋中加入6 mol/L氢氧化钠溶液,当液固比为3:1、反应温度为80 ℃、反应时间为2 h时,所得氧化铋产物中氧化铋含量达到93.80%。
关键词:碲;铋;沉淀;酸溶;还原
文章编号:1004-0609(2018)-08-1660-09 中图分类号:TF803.2 文献标志码:A
碲、铋具有很多优良的性能,在冶金、光敏材料、制冷、太阳能、化工、医药等领域具有广泛用途[1-3],其需求量与日剧增。由于碲、铋的资源量有限,碲、铋的回收具有重要价值。由于碲矿物资源少,直接从矿物中提取碲研究甚少,碲主要来源于铜阳极泥和铅阳极泥。铋以与多金属伴生,可采用矿浆电解法从低品位的多金属铋矿中提取铋[4],铜和铅冶炼的副产物也是提取铋的主要原料。铜阳极泥和铅阳极泥是含碲铋的物料,从其阳极泥中回收碲和铋的方法因原料成分不同而各异,回收碲主要采用的方法有碱溶电解和铜粉还原法等[5-7],采用工艺有碱浸碲-氧化焙烧-酸浸或氧化浸出-还原-置换等[8-11]。回收铋主要采用火法工艺,火法处理所需的矿量较大,不适宜小规模的碲铋的回收。从含碲铋的溶液中回收碲铋的处理方法较多,有溶剂萃取法、SO2还原法、沉淀法、置换法等[12],这些回收碲和铋的方法具有生产成本高、流程繁琐等缺点。铜阳极泥经过氯化分金-还原金-锌粉还原铂钯得到的沉铂钯后液中含有一定量的碲、铋,具备一定的回收价值,工业上处理沉铂钯后液一般采用NaOH沉淀后加入过量的碱溶解得到Na2TeO3溶液,Na2TeO3溶液经过反复沉淀与碱浸提纯后,电解制备单质碲,该工艺流程繁琐且生产成本高[14-15]。本文作者以铜阳极泥的沉铂钯后液为原料,采用氢氧化钠使沉铂钯后液中碲和铋共沉淀,利用混酸溶解碲和铋,碲和铋分别从1.40 g/L、6.72 g/L提高到12.05 g/L、56.33 g/L,在溶液中碲和铋分别富集了8.59倍和8.39倍。然后采用二氧化硫还原使碲铋分离,氢氧化钠沉淀和脱氯分别得到含量为79.45%粗碲粉和纯度为93.80%氧化铋,碲和铋总回收率分别达到96.44%和93.41%[16]。该工艺与其他分离回收碲、铋方法相比具有富集效果好、回收率高、工艺简单,适合于工业生产。
1 实验
1.1 实验原料
实验原料为铜阳极泥氯化分金后锌粉置换铂钯后得到的沉铂钯后液, pH值为0.02,经ICP检测其成分如表1所列。
由表1可知,沉铂钯后液中主要成分为碲、铋和砷,其含量分别为1402 mg/L,6731 mg/L、1588 mg/L。
表1 沉铂钯后液元素含量
Table 1 Element components of precipitating platinum and palladium solution (mg/L)

1.2 实验步骤
取一定量的沉铂钯后液置于三颈瓶中,启动搅拌并加热,加入浓度为2.5 mol/L氢氧化钠溶液调节pH,反应时间为1 h,过滤、烘干得到沉淀渣。取上述沉淀渣,放入三颈瓶中,按液固比(mL:g)为6:1加入所需的混酸或者盐酸,启动搅拌,在一定的反应温度与反应时间下,反应完成后过滤得到富集碲铋的浸出液。
将得到的富集碲铋的浸出液倒入三颈瓶中加热并启动搅拌,通入SO2,在一定温度下反应后过滤、洗涤并烘干得到碲粉和还原碲后液。将还原碲后液倒入三颈瓶中,加入浓度为5 mol/L的氢氧化钠溶液调节pH值,在反应时间为2 h、反应温度为50 ℃条件下沉淀铋后过滤得到氯氧铋,将所得到的氯氧铋进行脱氯后过滤、烘干得到三氧化二铋。
1.3 工艺流程
根据上述实验过程,绘制其工艺流程如图1所示。
1.4 分析与检测
采用美国热电元素公司的Intrepid II XSP型电感耦合等离子体发射光谱仪(ICP)分析溶液成分,X射线荧光光谱仪(XRF)定性半定量分析固体物质成分,日本理学D/max-TTR III型X射线衍射仪(XRD)分析固体物质物相,日本电子株式会社JSM-6300型扫描电镜(SEM)观察固体产物微观形貌。
2 结果与讨论
沉铂钯后液中回收碲铋,除主要研究碲和铋的分离和回收规律外,由于沉铂钯后液中砷为主要成分之一,因此砷的沉淀与分离也进行了研究。
2.1 pH值对沉铂钯后液中砷、碲、铋沉淀率的影响
将200 mL沉铂钯后液加入三颈瓶中,启动搅拌后加入浓度为2.5 mol/L的氢氧化钠溶液调节pH值,反应温度为25 ℃,反应时间为1 h, pH值对沉铂钯后液中砷、碲、铋沉淀率的影响如图2所示。
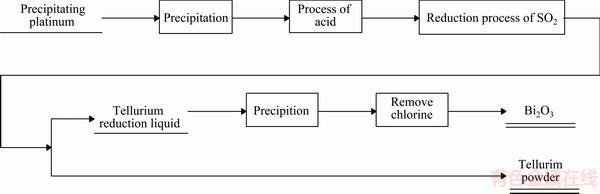
图1 沉铂钯后液碲铋的分离与回收工艺流程图
Fig. 1 Separation and recovery process of tellurium and bismuth flow diagram from precipitating platinum and palladium solution
由图2可知,pH值从2增加到13时,铋沉淀率基本不变。pH值为2~6时,碲的沉淀率随着pH值的增加而增加,pH值为6~9时碲的沉淀率随着pH值的增加基本不变,pH值>9时碲的沉淀率随着pH值的增加而逐渐降低。由于碲水解生成氢氧化碲沉淀,继续加入氢氧化钠时,使氢氧化碲沉淀发生溶解生成Na2TeO3,因此碲的沉淀率下降[15]。pH值为2~9时,砷的沉淀率随着pH值的增加而增加;pH值>9时砷的沉淀率随着pH值的增加而降低,其沉淀规律与碲相似。沉铂钯后液中,砷主要以 As(Ⅴ)价态存在,以H3AsO4、H2AsO4-、HAsO42- 、AsO43-等形态存在[17]。除产生砷酸铋沉淀外,从砷与碲沉淀规律来,砷的沉淀主要是由于氢氧化碲对砷的各种形态吸附产生的作用,当pH值大于9时随着氢氧化碲溶解砷沉淀率发生下降。其相关反应如下:
BiCl3+3NaOH=Bi(OH)3↓+3NaCl (1)
H2TeCl6+6NaOH=Te(OH)4↓+6NaCl+2H2O (2)
Te(OH)4+2NaOH=Na2TeO3+3H2O (3)
Bi3++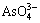 =BiAsO4↓ (4)
=BiAsO4↓ (4)
在pH值为6~9时,碲和铋的沉淀率都达到99.10%,As沉淀率从33.53%增加到53.21%。取5 L沉铂钯后液进行放大实验,pH值对沉铂钯后液中砷、碲、铋沉淀率的影响如图3所示。
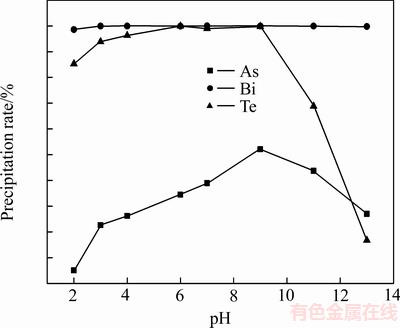
图2 pH值对砷、碲、铋沉淀率的影响
Fig. 2 Effects of pH value on precipitation rate of As,Te and Bi
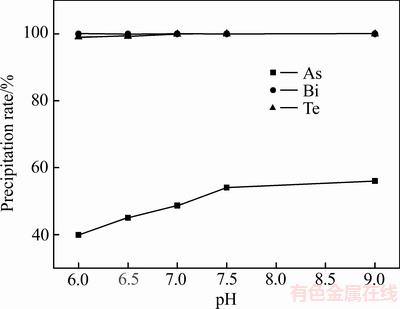
图3 放大实验pH值对砷、碲、铋沉淀率的影响
Fig. 3 Effects of amplification experiment pH value on precipitation rate of As,Te and Bi
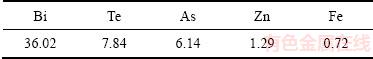
由图3可知,当pH值从6增加到7时,碲沉淀率从99.08%增加到99.91%,铋沉淀率从99.92%增加到99.96%,砷沉淀率从39.99%增加到48.63%。pH值继续增加时,碲和铋的沉淀率不变,而砷沉淀率继续增加,放大实验结果与小实验结果一致。实验取pH值为7的沉淀渣经过60℃真空干燥,XRD实验结果如图4所示,沉淀渣成分如表2所列。
由图4可知,经过60 ℃真空干燥后沉淀渣中铋以氧化铋存在。由表2可知,沉淀渣中主要含有铋、碲、砷和锌。
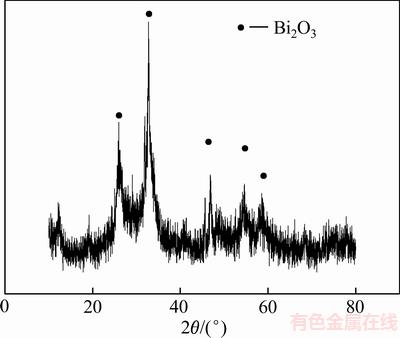
图4 pH=7时沉淀渣的XRD谱
Fig. 4 XRD patterns of precipitate at pH=7
表2 沉淀渣化学成分(质量分数,%)
Table 2 Chemical components of precipitate (mass fraction, %)
2.2 沉淀渣酸浸溶解与碲铋的富集
2.2.1 盐酸浓度对碲铋溶解率的影响
取上述沉淀渣100 g,按液固比(mL:g)为6:1加入盐酸600 mL,启动搅拌,当反应温度为60 ℃、反应时间为0.5 h时,盐酸浓度对砷碲铋浸出率的影响如图5所示。
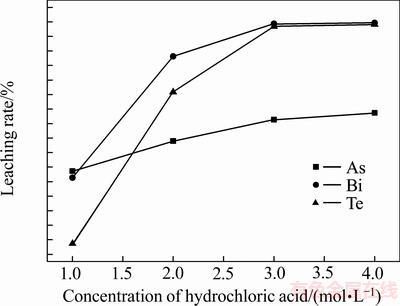
图5 盐酸浓度对沉淀渣中砷、碲、铋浸出率的影响
Fig. 5 Effect of concentration of hydrochloric acid on leaching rates of As, Te and Bi in precipitate
由图5可知,沉淀渣中碲、铋和砷的浸出率随着盐酸浓度的增加而增加,当盐酸浓度为3 mol/L时,碲铋的浸出率分别为98.43%、99.35%,砷浸出率仅为66.32%,浸出液中碲、铋和砷的浓度分别为12.05 g/L、56.33 g/L、6.341 g/L,与沉铂钯后液相比较,碲、铋分别富集了8.59倍和8.39倍。但是,当盐酸浓度继续增加,盐酸的挥发性逐渐增加[18],导致操作环境差,且对碲铋的浸出率影响不大,盐酸浸出过程的反应如下:
Bi2O3+6HCl=2BiCl3+3H2O (5)
TeO2+4HCl=TeCl4+2H2O (6)
采用盐酸浸出时,H+浓度越高,碲和铋浸出率越高。当碲铋接近完全浸出时,盐酸浓度为3 mol/L,当液固比为6:1时,H+浓度为浸出碲和铋理论量的2.25倍。
2.2.2 不同体积比的混酸对浸出率的影响
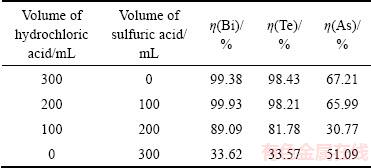
由以上实验可知,使用盐酸溶解沉淀渣,其适宜的H+用量为浸出碲铋理论用量的2.25倍。在H+用量不变的条件下,选取3 mol/L盐酸和1.5 mol/L的硫酸在不同体积比下进行浸出。当反应温度为50℃,液固比(mL:g)为6:1,反应时间为2 h时,混酸浸出结果如表3所列。
由表3可知,完全使用盐酸浸出时,铋、碲、砷的浸出率分别为99.38%、98.43%、67.21%;完全使用硫酸溶解时铋、碲、砷的浸出率分别仅为33.62%、33.57%、51.09%;当盐酸与硫酸体积比分别为2:1和1:2时铋、碲、砷的浸出率分别为99.93%、98.21%、65.99%和89%、81.78%、30.77%。实验结果表明铋、碲、砷的浸出率随着硫酸用量增加而降低,当盐酸和硫酸体积比为2:1时,其浸出效果与完全使用盐酸浸出效果相当,其碲铋浓度分别为12.01 g/L、56.46 g/L。盐酸有利于沉淀渣中碲铋的浸出,这是由于盐酸浸出时铋与氯离子可以形成络合物 (x为1~6),碲与氯离子可以形成络合物
(x为1~6),碲与氯离子可以形成络合物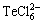 等[19]。
等[19]。
表3 不同体积比的混酸对渣中砷、碲、铋浸出率的影响
Table 3 Effect of different volume ratio of mixed acid on leaching rate of As, Te and Bi in precipitate
2.3 SO2还原分离碲和铋
将上述酸浸液混合,其溶液成分如表4所列。溶液中含有Cl-,采用SO2还原有利于碲、铋的分离[20]。
表4 富集碲铋的浸出液中砷、碲、铋浓度
Table 4 Concentration of As, Te and Bi in solution of enriching Bi and Te (g/L)

2.3.1 反应温度对碲、铋、砷还原沉淀率的影响
实验取浸出液300 mL,通入流量为0.25L/min的SO2,当反应时间为0.5 h时,反应温度对还原后碲铋砷沉淀率的影响如图6所示。
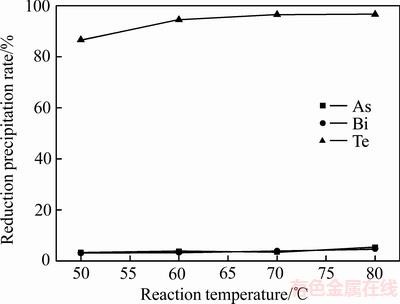
图6 反应温度对砷、碲、铋还原沉淀率的影响
Fig. 6 Effect of on reduction precipitation rate of As, Bi and Te
由图6可知,碲还原沉淀率随着反应温度的增加而增加,当温度为50℃增加到70℃时碲还原沉淀率从86.66%增加到96.56%,继续增加反应温度碲还原沉淀率没有明显增加。因此,适宜的反应温度为70℃。
2.3.2 反应时间对砷、碲、铋还原沉淀率的影响
控制反应温度为70 ℃,其他实验条件不变,反应时间对砷、碲、铋还原沉淀率的影响如图7所示。
由图7可知,当反应时间达到50 min后,碲的还原沉淀率为96.59%,继续增加反应时间碲的还原沉淀率不再增加。实验取SO2流量为0.25 L/min、反应温度为70℃、反应时间为50 min的条件下得到的还原渣烘干后进行XRD和SEM实验,实验结果如图8和9所示。

图7 反应时间对砷、碲、铋还原沉淀率的影响
Fig. 7 Effect of reaction time on reduction precipitation rate of As, Te and Bi

图8 还原沉淀渣的XRD谱
Fig. 8 XRD pattern of reduction precipitate

图9 还原沉淀渣SEM像
Fig. 9 SEM image of reduction precipitate
由图8可知,采用SO2还原得到的产物为单质碲;由图9可知,还原产物为细小粉末状。当SO2流量为0.25 L/min、反应温度为70 ℃、反应时间为50 min的实验条件下得到的还原产物为单质碲,其中碲含量为79.95%,铋和砷含量仅为0.067%和0.003%。经过沉淀,溶解与还原,碲总回收率为96.44%。
沉铂钯后液经过氢氧化钠沉淀和酸浸后得到富集碲铋的浸出液,浸出液中砷、碲、铋主要以H3AsO4、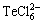 、
、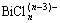 等形式存在[21-23]。SO2还原体系中,相关离子及其化合物标准电极电位如表5所列。
等形式存在[21-23]。SO2还原体系中,相关离子及其化合物标准电极电位如表5所列。
表5 相关电极反应及标准电极电势[24]
Table 5 Related electrode reaction and standard electrode potential
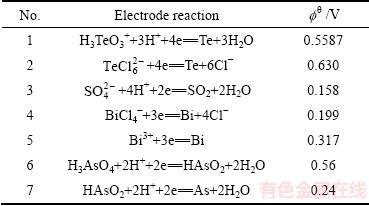
由表5可知,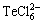 的氧化电位最高,易被SO2还原。而
的氧化电位最高,易被SO2还原。而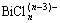 的氧化电位与SO2还原电位相差不大,因此
的氧化电位与SO2还原电位相差不大,因此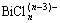 不被还原留在溶液中实现碲铋的分离,H3AsO4的氧化电位比SO2的还原正,H3AsO4易被还原为HAsO2。SO2还原反应如下:
不被还原留在溶液中实现碲铋的分离,H3AsO4的氧化电位比SO2的还原正,H3AsO4易被还原为HAsO2。SO2还原反应如下:
2SO2+4H2O+H2TeCl6=Te↓+6HCl+2H2SO4 (7)
H3AsO4+SO2=HAsO2+H2SO4 (8)
2.4 还原碲后液中铋的提取与回收
经过SO2还原,碲和铋得到分离,还原碲后液铋以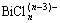 形式存在。通过氢氧化钠调节pH值沉淀后得到氯氧铋,然后氢氧化钠脱氯得到氧化铋,实现铋的回收[25]。
形式存在。通过氢氧化钠调节pH值沉淀后得到氯氧铋,然后氢氧化钠脱氯得到氧化铋,实现铋的回收[25]。
2.4.1 pH值对铋的沉淀率的影响
实验取上述还原碲后液的混合溶液300 mL,其中砷、碲、铋浓度分别为5.778 g/L、0.3955 g/L、52.336 g/L,倒入三颈瓶中,加入5 mol/L的氢氧化钠溶液调节溶液的pH值,当反应温度为50 ℃,反应时间为 1.0 h时,pH值对砷、碲、铋沉淀率的影响如图10所示。
由图10可知,砷、碲、铋沉淀率随着pH值的升高而增加,当pH值≥5时,铋的沉淀率达99.98%,碲的沉淀率≥99.94%;pH值从1增加到7,砷沉淀率从6.15%增加到63.28%。还原碲后液的砷主要以As(Ⅲ)存在,pH值较低时As(Ⅲ)主要以AsO+和HAsO2形态存在,随着pH值的升高,主要As(Ⅲ)转化为AsO2-形式,AsO2-与相关金属离子形成亚砷酸盐沉淀[17]。
为了验证小实验结果,取5 L还原碲后液进行放大实验。用氢氧化钠调节pH值为2,反应温度为50 ℃,反应时间0.5 h后过滤得到沉淀渣。经过120 ℃烘干后进行XRF和XRD分析,其实验结果分别如表6和如图11所示,砷、碲、铋沉淀率和滤液残留浓度如表7所列。

图10 pH值对砷、碲、铋沉淀率的影响
Fig. 10 Effect of pH value on precipitation rate of As, Te and Bi

图11 沉淀渣的XRD谱
Fig. 11 XRD pattern of precipitate
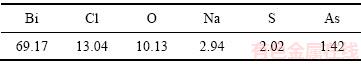
表6 氢氧化钠沉淀产物化学成分(质量分数,%)
Table 6 Chemical components of bismuth chloride (mass fraction, %)
由表7可知,pH值为2时铋沉淀率达到99.93%。由图11可知,在pH值为2的实验条件得到的产物为氯氧铋,其实验结果与盐酸溶解漂浮阳极泥后NaOH沉淀结果一致[25]。
由表6可知,沉淀产物中氯、铋、氧的含量分别为13.04%、10.13%、69.17%。整个流程中,则铋的总回收率为93.41%。
实验采用氢氧化钠进行脱氯处理,当氢氧化钠浓度为6 mol/L、液固比为3:1、反应温度为80 ℃、反应时间为2 h后过滤,洗涤,烘干后,XRD、SEM和XRF实验结果分别如图12、图13和表8所示。
表7 放大实验砷、碲、铋沉淀率和滤液中残留浓度
Table 7 Precipitation rates of As, Te and Bi and residual concentration in filtrate
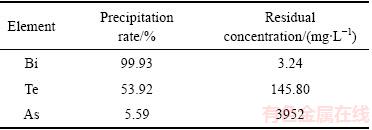
表8 脱氯渣化学成分(质量分数,%)
Table 8 Chemical components of dechlorination precipitate (mass fraction, %)
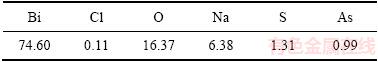
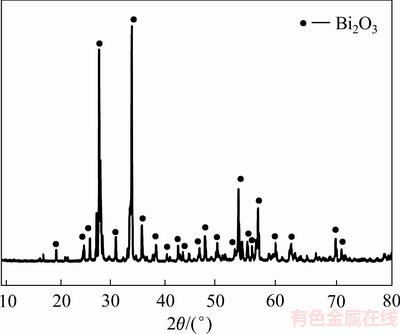
图12 脱氯渣XRD谱
Fig. 12 XRD pattern of dechlorination precipitate
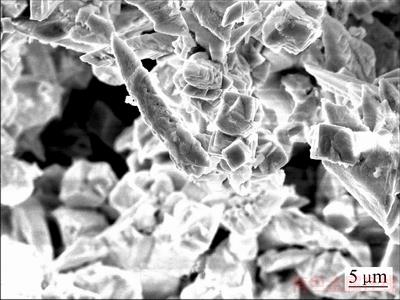
图13 脱氯渣SEM像
Fig. 13 SEM image of dechlorination precipitate
由表8可知,氢氧化钠转化脱氯后,脱氯渣中铋含量为74.60%,氯含量仅为0.11%,实验表明氢氧化钠能够有效脱去BiOCl中的氯。图12可知,晶型为单斜的α-Bi2O3[24]。由图13可知,烘干得到氧化铋形貌为纤维状。其反应原理如下:
2BiOCl+2NaOH=Bi2O3+2NaCl+H2O (9)
还原碲后液回收铋,在298.15 K下Bi-Cl-H2O系中相关物质的吉布斯自由能如表9所列。
表9 298.15 K时Bi-Cl-H2O系中相关物质的标准吉布斯自由能[26]
Table 9 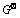 values of the relaitive substances in Bi-Cl-H2O system at 298.15 K[26]
values of the relaitive substances in Bi-Cl-H2O system at 298.15 K[26]
根据表9计算,相关氢氧化钠沉淀与脱氯转化相关反应自由能如下:
BiCl3+2NaOH=BiOCl↓+2NaCl+H2O
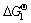 =-174.04 kJ/mol (10)
=-174.04 kJ/mol (10)
2BiOCl+2NaOH=Bi2O3+2NaCl+H2O
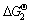 =-16.24 kJ/mol (11)
=-16.24 kJ/mol (11)
上述两个反应的 均小于0,表明还原碲后液中BiCl3转化为氯氧铋和氯氧铋转化为氧化铋在热力学上是可行的。
均小于0,表明还原碲后液中BiCl3转化为氯氧铋和氯氧铋转化为氧化铋在热力学上是可行的。
3 结论
1) 用NaOH溶液调节沉铂钯后液的pH值沉淀碲铋,当pH值从2增加到13时铋沉淀率不变,碲铋共沉淀适宜的pH值为6~9,碲和铋的沉淀率都高于99.10%,碲铋沉淀渣中碲、铋含量分别为7.84%、36.02%。
2) 采用盐酸或盐酸和硫酸的混合液浸出碲铋沉淀渣,当H+浓度为3 mol/L,反应温度为50 ℃,反应时间为2 h时,碲和铋浸出率分别达到98.43%和99.35%,浸出液中碲和铋浓度分别为12.05 g/L和56.33 g/L,与沉铂钯后液中碲铋浓度比较,碲铋在溶液中分别富集了8.59倍和8.39倍。
3) 采用SO2还原富集碲铋的浸出液中的碲,当反应温度为70 ℃、反应50 min后过滤得到碲粉和还原碲后液,碲还原沉淀率为96.59%,还原产物中碲含量达到79.45%,其中砷、铋含量仅为0.003%和0.067%。经过沉淀,溶解与还原,碲总回收率为96.44%。
4) 氢氧化钠溶液调节还原碲后液pH值,当pH≥5时,铋沉淀率大于99.94%,水解产物为BiOCl。在所得BiOCl中加入6 mol/L的氢氧化钠溶液,液固比为3:1,反应温度为80 ℃时,反应2 h后过滤、烘干得到氧化铋,氧化铋含量达到74.60%,纯度为93.80%,整个流程铋的总回收率为93.41%。
REFERENCES
[1] SARGAR B M, ANUSE M A. Liquid-liquid extraction study of tellurium(Ⅳ) with N-n-octylaniline in halide medium and its separation from real samples[J]. Tatanta, 2001, 55(3): 469-478.
[2] JEON S J, OH M, JEON H, HYUN S, LEE H J. Effects of post-annealing on thermoelectric properties of bismuth–tellurium thin films deposited by co-sputtering[J]. Microelectronic Engineering, 2011, 88(55): 541-544.
[3] SHIM M, KIM Y M, LEE H H, HONG S J, LEE H J. Separation behavior of impurities and selenium reduction by the reactive zone refining process using high-frequency induction heating to purify Te[J]. Journal of Crystal Growth, 2016, 455: 6-12.
[4] 王成彦, 邱定蕃, 张寅生. 矿浆电解法处理铋精矿的研究[J]. 有色金属, 1992, 36(2): 8-12.
WANG Cheng-yan, QIU Ding-fan, ZHANG Yin-sheng. Treatment of bismuth concentrate slurry electrolysis research[J]. Nonferrous metals, 1992, 36(2): 8-12.
[5] SHIBASAKI T, ABE K, TAKEUCHI H. Recovery of tellurium from decopperizing leach solution of copper refinery slimes by a fixed bed reactor[J]. Hydrometallurgy, 1992, 29(1/3): 399-412.
[6] GUO X, XU Z, LI D, TIAN Q, XU R, ZHANG Z. Recovery of tellurium from high tellurium-bearing materials by alkaline sulfide leaching followed by sodium sulfite precipitation[J]. Hydrometallurgy, 2017, 171: 355-361.
[7] HA Y C, SOHN H J, JEONG G J, LEE C K, RHEE K I. Lectrowinning of tellurium from alkaline leach liquor of emented Te [J]. Applied Electrochemistry, 2000, 30(3): 315-322.
[8] ZHANG F Y, ZHENG Y J, PENG G M. Selection of reductants for extracting selenium and tellurium from degoldized solution of copper anode slimes[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(4): 917-924.
[9] RHEE K, LEE C K, HA Y C, JEONG G J, KIM H S, SOHN H J. Tellurium recovery from cemented tellurium with minimum aste disposal [J]. Hydrometallurgy, 1999, 53(2): 189-201.
[10] YANG J G, TANG M T, TANG C B, LIU W. The solvent extraction separation of bismuth and molybdenum from a low grade bismuth glance flotation concentrate[J]. Hydrometallurgy, 2009, 96 (4): 342-348.
[11] CHEN S L, GUO X Y, LIANG Y X. Remove of arsenic, antimony and bismuth from crude crystal copper sulfate[J]. Journal of Central South University, 2010, 41(4): 1251-1255.
[12] 马 辉. 从碲渣中浸出和分离碲的工艺研究[D]. 长沙: 中南大学, 2009: 7-14.
MA Hui. Studies on the technics for leaching and separation of tellurium from tellurium slag[D]. Changsha: Central South University, 2009: 7-14..
[13] 乐红春. 中和渣中碲的提取及电解制备高纯碲研究[D]. 长沙: 中南大学, 2012: 28-39.
LE Hong-chun. Research on extraction of tellurium from neutralizationsludge and electrolytic preparation of high puritytellurium[D]. Changsha: Central South University, 2012: 28-39.
[14] 赖建林, 钱 勇, 蔡春秀. 碲粉电解精炼过程中面积电流对电碲质量的影响[J]. 湿法冶金, 2004, 23(3): 162.
LAI Jian-lin, QIAN Yong, CAI Chun-xiu. The influence of area current on the quality of electric Te in the process of electrolytic refining of Te powder[J]. Hydrometallurgy, 2004, 23(3): 162.
[15] 马玉天, 龚竹青, 武 俊, 陈文汨. 从高铅碲渣中浸出碲的热力学分析及实验[J]. 中南大学学报(自然科学版), 2011, 37(3): 499-503.
MA Yu-tian, GONG Zhu-qing, WU Jun, CHEN Wen-mi. Thermodynamic analysis and experimental studies of leaching lead-rich tellurium slag[J]. Journal of Central South University (Science and Technology), 2011, 37(3): 499-503.
[16] 郑雅杰, 张林宝, 李 伟, 安小凯, 吕重安. 一种从溶液中富集与分离回收碲铋的方法: 中国, 201610813922.3[P]. 2016-09-12.
ZHENG Ya-jie, ZHANG Lin-bao, LI Wei, AN Xiao-kai, Lü Zhong-ang. A method for the enrichment and separation of tellurium and bismuth from solution:China, 201610813922.3[P]. 2016-09-12.
[17] 王 勇. 铜冶炼含砷废水处理新工艺及其基础理论研究[D].长沙: 中南大学, 2009: 44-49.
WANG Yong. Study on new treatment technology and fundamental theory of arsenic waste water produced in copper metallurgy[D]. Changsha: Central South University, 2009: 43-49.
[18] 孙召明. 铜阳极泥中碲的回收与提纯及其基础理论研究[D]. 长沙: 中南大学, 2012: 27-29.
SUN Zhao-ming. Study on recovery and purification of tellurium from copper anode slime and relative fundamental theory[D]. Changsha: Central South University, 2012: 27-29.
[19] 吴绍华, 刘春艳, 兰尧中. 铁粉置换沉淀海绵铋的动力学研究[J]. 湿法冶金, 2007, 29(3): 140-142.
WU Shao-hua, LIU Chun-yan, LAN Yao-zhong. Kinetic study on cementation of bismuth from solution using iron powder[J]. Hydrometallurgy, 2007, 29(3): 140-142.
[20] 马亚赟, 郑雅杰, 丁光月, 王俊文, 董俊斐, 张福元. 卤素离子催化作用下SO2还原沉金后液及其热力学特征[J]. 中国有色金属学报, 2016, 26(4): 903-905.
MA Ya-yun, ZHENG Ya-jie, DING Guang-yue, WANG Jun-wen, DONG Jun-fei, ZHANG Fu-yuan. Precipitated gold solution reduced by SO2 under halogen ion composite catalyst andits thermodynamic characteristics[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 903-905.
[21] SEBY F, POTIN-GAUTIER M, GIFFAUT E, BORGE G, DONARD O F X. A critical review of thermodynamic data for selenium species at 25 ℃[J]. Chemical Geology, 2001, 171(3/4): 173-194.
[22] GRUNDLER P, BRUGGER J, ETSCHMANN B E. Speciation of aqueoustellurium(Ⅳ) in hydrothermal solutions and vapors and the role of oxidized tellurium species in Te transport and gold deposition[J]. Geochimica et Cosmochimica Acta, 2013, 120: 298-325.
[23] MOKMELI M, DREISINGER D, WASSINK B. Thermodynamics and kinetics study of tellurium removal with cuprous ion[J]. Hydrometallurgy, 2014, s147/148(8): 20-29.
[24] 吴维昌, 冯洪清, 吴开冶. 标准电极电位数据手册[M]. 北京: 科学出版社, 1991.
WU Wei-chang, FENG Hong-qing, WU Kai-ye. Standard electrode potential datasheet[M]. Beijing: Science Press, 1991.
[25] 郑雅杰, 洪 波. 漂浮阳极泥富集金银及回收锑铋工艺[J]. 中南大学学报(自然科学版), 2011, 42(8): 2220-2226.
ZHENG Ya-jie, HONG Bo. Enrichment of Au and Ag and recovery of Sb and Bi from floating anode slime[J]. Journal of Central South University (Science and Technology), 2011, 42(8): 2220-2226.
[26] J.A.迪安. 兰氏化学手册[M]. 北京: 科学出版社, 1998.
DEAN J A. Lang’s handbook of chemistry[M]. Beijing: Science Press, 1998.
Separation and recovery process of tellurium and bismuth from precipitating platinum and palladium solution
ZHANG Lin-bao, ZHENG Ya-jie, AN Xiao-kai
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: Tellurium powder and tellurium reduction liquid were obtained through a sets of steps including precipitation process of NaOH, HCl leaching process of slag producing in the previous process, and the reduction process of SO2. The BiOCl was obtained after reduction tellurium liquid was precipitated using sodium hydroxide and then the slurry was filtered. The Bi2O3 was obtained by using NaOH to remove chlorine from BiOCl. The results show that the precipitation rates of Te and Bi are 99.91% and 99.96%, respectively, using NaOH as pH regulator, when the pH value is 6, temperature is 20-25 ℃ and the reaction time is 1 h. The leaching rates of Te and Bi are 98.21% and 99.93%, respectively, when the volume ratio of 3 mol/L HCl to 1.5 mol/L H2SO4 is 2:1, the concentration of H+ is 3 mol/L, the reaction temperature is 50 ℃ and the reaction time is 2 h. The reduction rate of Te is 96.59%, and the contents of Te, As and Bi in the tellurium powder are 79.45%, 0.003% and 0.067% (mass fraction), respectively. The BiOCl is obtained by filtering when the pH of Te reduction solution is 2. Finally, the Bismuth content reaches 93.80% after adding 6 mol/L NaOH to the BiOCl when the ratio of liquid to solid is 3:1, the reaction temperature is 80 ℃ and the reaction time is 2 h.
Key words: tellurium; bismuth; precipitation; acid dissolution; reduction
Foundation item: Project(2013A090100013) supported by the Teaching and Research Program of Guangdong Province, China
Received date: 2017-06-12; Accepted date: 2017-10-08
Corresponding author: ZHENG Ya-jie; Tel: +86-731-88836285; E-mail: ZYJ@csu.edu.cn
(编辑 王 超)
基金项目:广东省教育部产学研重大项目(2013A090100013)
收稿日期:2017-06-12;修订日期:2017-10-08
通信作者:郑雅杰,教授,博士;电话:0731-88836285;E-mail: ZYJ@csu.edu.cn