Electrochemical behavior of electroplated Zn-P alloy
来源期刊:中国有色金属学报(英文版)2001年第4期
论文作者:张昭
文章页码:603 - 605
Key words:Zn-P alloy; codeposition; electrochemical behavior
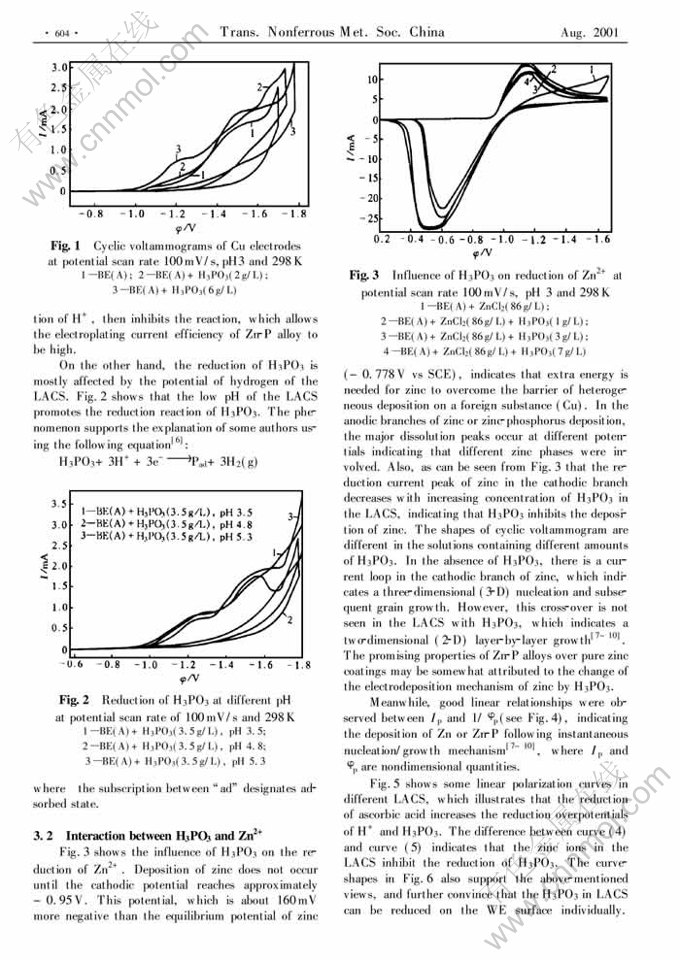
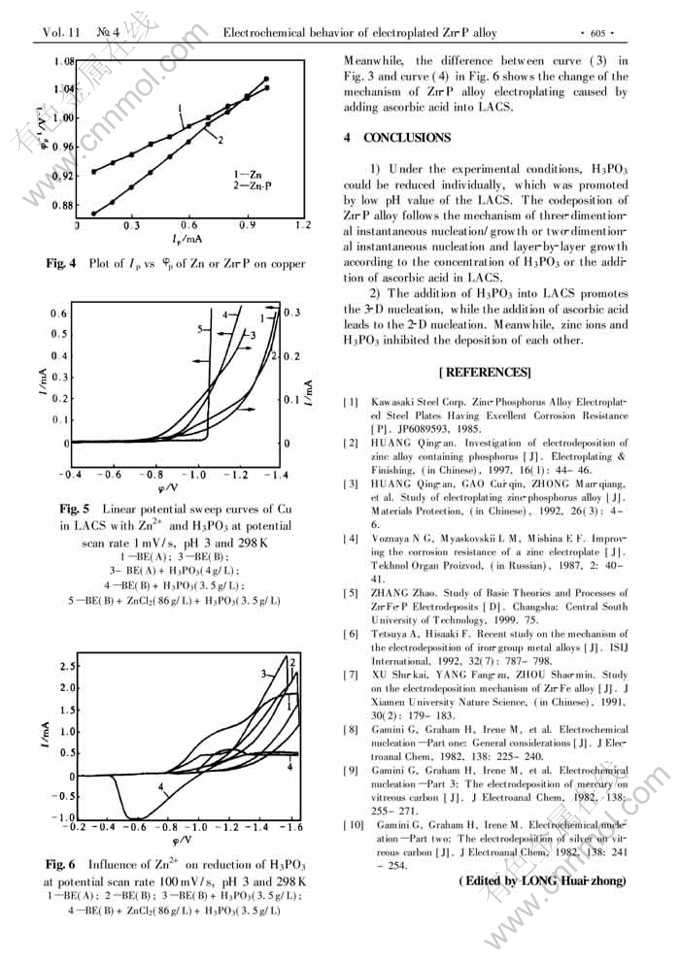
Abstract: The electroplating behavior of Zn-P alloy on copper from light acid chloride solutions (LACS) was investigated using cyclic voltammetry and linear potential sweep method. It is found that, under the experimental conditions, the concentrations of H3PO3 and ascorbic acid in LACS change the codeposition mechanism of Zn-P alloy, the addition of H3PO3 into LACS promotes the three-dimentional (3-D) instantaneous nucleation/growth, while the addition of ascorbic acid promotes the two-dimentional (2-D) instantaneous nucleation and layer-by-layer growth. H3PO3 and zinc ions inhibit the deposition each other. The experimental results also show that H3PO3 in the LACS can be reduced on the cathode individually, and low pH value of the LACS promotes the reduction of H3PO3.