Trans. Nonferrous Met. Soc. China 23(2013) 3758-3762
Bioleaching of Pb-Zn-Sn chalcopyrite concentrate in tank bioreactor and microbial community succession analysis
Jun WANG1,2, Hong-bo ZHAO1,2, Tian ZHUANG1,2, Wen-qing QIN1,2, Shan ZHU1,2, Guan-zhou QIU1,2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biohydrometallurgy of Ministry of Education, Central South University, Changsha 410083, China
Received 20 March 2013; accepted 13 June 2013
Abstract: The variation of microbial community structure was investigated for the tank bioleaching process of Pb-Zn-Sn chalcopyrite concentrate in the presence of mixed moderately thermophilic bacteria. The parameters, such as pH value, solution potential and concentrations of metal ions, were determined by the method of polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) to analyze the succession of microbial community. The results showed that a final copper extraction rate of 85.6% could be obtained after tank bioleaching for 30 d. The Acidithiobacillus caldus was the dominant population with abundance of about 73.80% in the initial stage, then Sulfobacillus thermosulfidooxidans dominated from the 18th day to the end of bioleaching, while the abundance of Leptospirillum ferriphilum changed slightly. A higher solution potential within a certain range and appropriate concentration of ferric ions were essential for this tank bioleaching of chalcopyrite.
Key words: chalcopyrite; tank bioleaching; microbial community; PCR-RFLP technique
1 Introduction
Chalcopyrite (CuFeS2) accounts for about 70% of copper reserves in the world [1-3], but due to its special crystal structure, the extraction rate of copper using traditional hydrometallurgy process is too low. Compared with the conventional processes, bioleaching technology possesses many advantages, such as mild reaction conditions, environmental benefits, low energy consumption, low cost and short flow process [4,5]. Therefore, bioleaching technology capable of extracting copper from low-grade chalcopyrite is becoming increasingly important.
Tank leaching is one of the commonly used processes in the industrial bioleaching, due to its advantages of mass transfer effect (stirring, ventilatory, etc). This process can be effectively controlled, the parameters of leaching in the process can be regulated, and then accurate experimental data can be obtained [6]. Large amounts of elemental sulfur, polysulfides, jarosite and other substances would form on the surface of chalcopyrite, which could cause passivation phenomenon in the leaching process [7,8], especially by mesophilic microorganisms [9,10]. Using moderately thermophilic bacteria for leaching chalcopyrite, not only reaction rate can be accelerated, but also excessive chalcopyrite passivation can be avoided to some extent [11-13]. More and more researchers are interested in applying moderately thermophilic bacteria to the bioleaching of chalcopyrite due to their resistance to high pulp density and high metal concentration [14]. And many researchers found that mixed culture could accelerate the rate of bioleaching process and increase the copper extraction rate of chalcopyrite [15-17]. However, the variation of microbial community structure and the corresponding influence in tank bioleaching of chalcopyrite are not explicit enough as well as the relationship between microbial community structure and bioleaching process.
In this work, a mixed culture of three kinds of moderately thermophilic bacteria (Acidithiobacillus caldus, Leptospirillum ferriphilum, Sulfobacillus thermosulfidooxidans) was used for the bioleaching of chalcopyrite, PCR-RFLP technique was used for the research of community succession, and the parameters, such as pH, solution potential (φh) and concentrations of metal ions were determined to analyze the bioleaching process.
2 Experimental
2.1 Minerals
The minerals used in the test were collected from Meizhou of Guangdong province in China. The samples were crushed, ground and screened to be less than 75 μm. X-ray diffraction (XRD) and chemical elements analyses showed that the mineral samples consisted of 61.7% chalcopyrite, 29.7% Zn0.825Fe0.175S, 3.9% blende, 3.7% lead sulfate, and 1.1% seligmannite.
2.2 Microorganisms
Three strains of bacteria used in this work were obtained from the Key Laboratory of Biometallurgy of Ministry of Education, Central South University, China. The mixed culture was composed of Acidithiobacillus caldus, Leptospirillum ferriphilum and Sulfobacillus thermosulfidooxidans. The medium for the domestication of mixed microorganisms was composed of (NH4)2SO4 (3.0 g/L), MgSO4·7H2O (0.5 g/L), K2HPO4 (0.5 g/L), KCl (0.1 g/L), Ca(NO3)2 (0.01 g/L) and 5% chalcopyrite concentrate. The domestication process was conducted in a stirred tank at 45 °C, pH value was adjusted to1.5-1.7, and stirring rate was adjusted to 300 r/min.
2.3 Bioleaching
The leaching experiment was conducted in a stirred tank with a volume of 10 L, which was connected with an inflatable equipment, and water of 45 °C was used for heat preservation (Fig. 1). Strains were inoculated into the stirred tank with 10% pulp density, and the concentration of inoculated strains was 2.5×108 mL-1, which was domesticated before. The whole bioleaching time was 30 d. Leaching conditions were as follows: the temperature 45 °C, pH value 1.5-1.7 and stirring speed 300 r/min. During the bioleaching process, the variation of pH values and potentials was recorded regularly, as well as the concentration of copper, iron ions and the bacteria concentration in the bioleaching solution. All the potential values were expressed against the Ag/AgCl electrode (3 mol/L KCl).
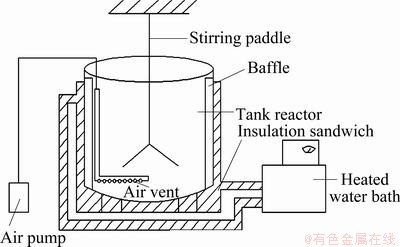
Fig. 1 Tank bioreactor used for bioleaching of chalcopyrite concentrate
2.4 Restriction fragment length polymorphism analysis (RFLP)
Total DNA of the bacteria was extracted from 2 mL leaching solution every 6-8 d, and the primers 27f and 1492r were used in 16S-rDNA amplification system: ddH2O 36 μL, 10×PCR buffer 5 μL, dNTPs 4 μL, primers 27f 1 μL and 1492r 1 μL, TaqDNA polymerase 1 μL, DNA template 2 μL, total 50 μL. The amplification program was as follows: 94 °C (5 min); 94 °C (5 s), 55 °C (45 s), 72 °C (90 s, 32 cycles); 72 °C (10 min). Plastic cutting recovery kit was used on 16S-rDNA. The pGEM-T vector connection was in a water bath at 16 °C overnight, and then transformed into E. coli Top10 competent cells. Coated tablet by a blue-white screening method was used to pick out the positive clones, amplify gene fragment after transferring tablet, then the restriction enzymes Msp I and Rsa I digestion of the amplified fragment was digested at 37 °C for 18 h. Finally, 3% agarose gel for restriction analysis was used to obtain the macrorestriction map, sequencing to show the different types of clones (OTU), and the identification of bacterial species.
3 Results and discussion
The mechanism of chalcopyrite bioleaching is mainly to oxidize insoluble chalcopyrite and form metal ions of Cu2+ and Fe2+ dissolved into the leaching solution [18]. The oxidation process begins with the oxidation of chalcopyrite by Fe3+ and Fe3+ accepts electrons released by S2- as rupture of chemical bonds. In this process, the role of bacteria in the bioleaching process is to oxidize Fe2+ to Fe3+ (Eq. (3)), and S0 to 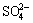 (Eq. (4)). The dissolution of chalcopyrite during bioleaching can be represented as the following reactions [19]:
(Eq. (4)). The dissolution of chalcopyrite during bioleaching can be represented as the following reactions [19]:
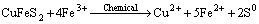 (1)
(1)
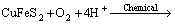
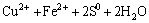 (2)
(2)
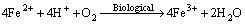 (3)
(3)
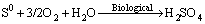 (4)
(4)
3.1 Bioleaching
The growth curve of bacteria is shown in Fig. 2. The bacteria concentration grew steadily from the 1st to the 13th day, and reached a stable state of 109 mL-1 in the subsequent leaching time. The bacteria grew rapidly to the logarithmic growth phase, and no obvious lag phase could be found. Bacteria with high activity were obtained after a long period of domestication before bioleaching. The bacteria concentration then steadily increased to 109 mL-1 after 13 d. In the subsequent bioleaching process, a large number of metabolites and metal ions were obtained because of consumption of energy substances, which led the bacteria to be stable.
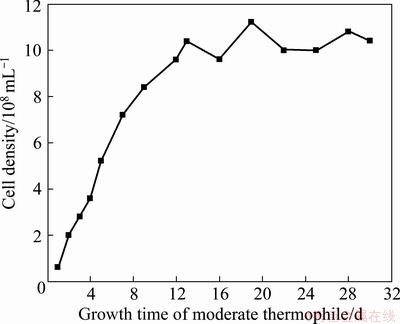
Fig. 2 Growth curve of moderate thermophile in tank bioleaching
The variation of potentials is shown in Fig. 3. The potential increased sharply from the 1st to 3rd day, and reached a peak of 540 mV, and then sharply decreased to 390 mV in the following 4 d. Finally, a steady trend of growth was obtained in the subsequent leaching process. In the initial stage of bioleaching, ferrous ions were rapidly oxidized, so the ferric ions concentration in the leaching solution increased (Eq. (3)), resulting in a sharp rise of potentials. Then the ferric ions were reduced to ferrous ions (Eq. (1)), and a large number of ferrous ions were released from eroded chalcopyrite surface (Eq. (2)), resulting in a sharply decline of potentials in a short time. In the subsequent bioleaching process, with the increase of the bacteria concentration, the oxidation rate of ferrous ions gradually accelerated, and potential gradually increased, accompanied by a decline of pH values with the hydrogen ions produced in the process of bioleaching (Eq. (4)). Potential was mainly determined by c(Fe3+)/c(Fe2+), and c(H+)/c(OH-).
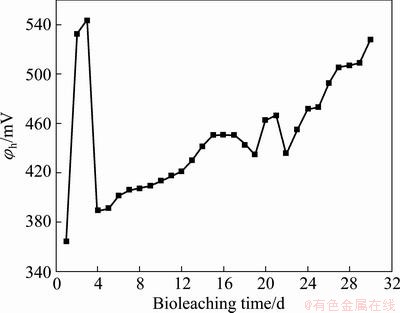
Fig. 3 Variation of solution potential (vs Ag/AgCl) during tank bioleaching
Figure 4 shows the variation of ferrous ion, ferric ion and total iron concentrations in the chalcopyrite bioleaching. In the bioleaching process, the iron ion concentration of the bioleaching solution is an important leaching parameter, and ferrous iron concentration and ferric iron concentration can directly affect the bioleaching of chalcopyrite. Figure 4 shows that the total iron concentration decreased significantly from the 6th to 14th day, which may mainly attribute to the formation of precipitates in this period [20]:

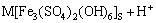 (5)
(5)
where M is a monovalent cation, such as H3O+, Na+, K+ and 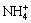 . The passivation phenomenon is a major affecting factor in the chalcopyrite bioleaching. Passivation layer coated on the surface of chalcopyrite can inhibit the leaching reactions, and most of the precipitate appears in the form of jarosite [21]. PINCHES et al [22] considered that jarosite layer formed in the leaching process had a compact structure, slowing down the chalcopyrite bioleaching rate. KINNUNEN et al [23] discovered that jarosite mainly formed on the surface of sulfur-rich layer.
. The passivation phenomenon is a major affecting factor in the chalcopyrite bioleaching. Passivation layer coated on the surface of chalcopyrite can inhibit the leaching reactions, and most of the precipitate appears in the form of jarosite [21]. PINCHES et al [22] considered that jarosite layer formed in the leaching process had a compact structure, slowing down the chalcopyrite bioleaching rate. KINNUNEN et al [23] discovered that jarosite mainly formed on the surface of sulfur-rich layer.
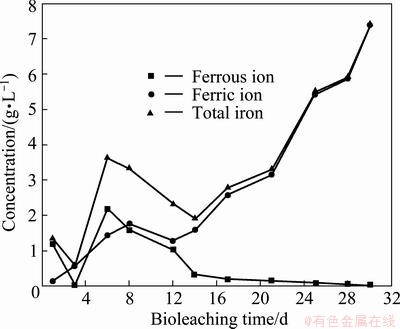
Fig. 4 Variation of ferrous iron, ferric iron and total iron concentration during tank bioleaching
Figure 5 shows the variation of copper ions concentration in 30 d. It increased to 9.20 g/L in the first 8 d, and then remained at a stable state of about 9.00 g/L from the 8th to 16th day. The concentration of copper started to increase again along with the increase of ferric ions concentration and solution potential, and reached 17.36 g/L finally, with a final copper extraction rate of 85.6%. The results indicated that a relatively higher potential within a certain potential range was beneficial for chalcopyrite bioleaching, and appropriate concentration of ferric ions was also essential for the bioleaching of chalcopyrite in this work.
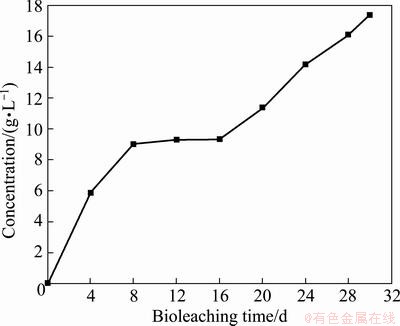
Fig. 5 Copper extraction during tank bioleaching
3.2 Microbial community succession
RFLP technique [24] was used to analyze the proportion of various bacteria in the tank bioleaching. The results showed that the Acidithiobacillus caldus (At. caldus) was the dominant population with an abundance of 73.80% in the initial stage, the Sulfobacillus thermosulfidooxidans became the dominant population with an abundance of 54.2% from the 18th day, and gradually increasing to the abundance of 66.70% on the 28th day, while the abundance of Leptospirillum ferriphilum just changed slightly, which always kept at a low value (Fig. 6). Equations (1) and (2) show that S0 can form on chalcopyrite surface in the initial stage of bioleaching [25], which might prevent the contact between chalcopyrite and leaching solution, and then inhibit the leaching process. Acidithiobacillus caldus could oxidize elemental sulfur to form sulfuric acid (Eq. (4)), so it dominated in the initial stage of bioleaching. When sulfur layer was dissolved, the ferric ions could erode chalcopyrite, and were reduced to be ferrous ions, which could provide energy substances for Sulfobacillus thermosulfidooxidans and Leptospirillum ferriphilum. Additionally, with the low resistance of Acidithiobacillus caldus to the metal ions like ferric ions and zinc ions, the proportion of Acidithiobacillus caldus gradually decreased in the subsequent bioleaching process, while Sulfobacillus thermosulfidooxidans dominated in the final stage of bioleaching.
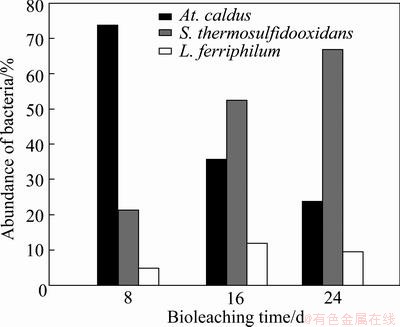
Fig. 6 Microbial community succession during tank bioleaching
4 Conclusions
1) A high leaching efficiency with copper extraction rate of 85.6% could be obtained in tank bioleaching of Pb-Zn-Sn chalcopyrite concentrate in the presence of mixed moderately thermophiles.
2) In the tank bioleaching process, the Acidithiobacillus caldus was the dominant population in the initial stage. The Sulfobacillus thermosulfidooxidans dominated from the 18th day, while the abundance of Leptospirillum ferriphilum just changed slightly.
3) The total iron concentration decreased significantly from the 6th to 14th day, which may be mainly attributed to the formation of iron precipitates in this period.
4) A relative high potential within a certain potential range was beneficial for chalcopyrite bioleaching, and appropriate concentration of ferric ions was essential for chalcopyrite bioleaching.
References
[1] WANG Yu-guang, SU Li-jun, ZHANG Li-juan, ZENG Wei-min, WU Jun-zi, WAN Li-li, QIU Guan-zhou, CHEN Xin-hua, ZHOU Hong-bo. Bioleaching of chalcopyrite by defined mixed moderately thermophilic consortium including a marine acidophilic halotolerant bacterium [J]. Bioresource Technology, 2012, 121: 348-354.
[2] WANG Shi-jie. Copper leaching from chalcopyrite concentrates [J]. JOM Journal of the Minerals, Metals and Materials Society, 2005, 57(7): 48-51.
[3] ZHANG Li-min, PENG Juan-juan, WEI Man-man, DING Jian-nan, ZHOU Hong-bo. Bioleaching of chalcopyrite with Acidianus manzaensis YN25 under contact and non-contact conditions [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 1981-1986.
[4] MARHUAL N P, PRADHAN N, KAR R N, SUKLA L B, MISHRA B K. Differential bioleaching of copper by mesophilic and moderately thermophilic acidophilic consortium enriched from same copper mine water sample [J]. Bioresource Technology, 2008, 99(17): 8331-8336.
[5] XIA Jin-lan, YANG Yi, HE Huan, LIANG Chang-li, ZHAO Xiao-juan, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong, NIE Zhen-yuan, QIU Guan-zhou. Investigation of the sulfur speciation during chalcopyrite leaching by moderate thermophile Sulfobacillus thermosulfidooxidans [J]. International Journal of Mineral Processing, 2010, 94(1-2): 52-57.
[6] BRIERLEY C L. Bacterial succession in bioheap leaching [J]. Hydrometallurgy, 2001, 59(2): 249-255.
[7] HE Huan, ZHANG Cheng-gui, XIA Jin-lan, PENG An-an, YANG Yi, JIANG Hong-chen, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong, NIE Zhen-yuan, QIU Guan-zhou. Investigation of elemental sulfur speciation transformation mediated by Acidithiobacillus ferrooxidans [J]. Current Microbiology, 2009, 58(4): 300-307.
[8] XIA Jin-lan, YANG Yi, HE Huan, LIANG Chang-li, ZHAO Xiao-juan, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong, NIE Zhen-yuan, QIU Guan-zhou. Investigation of the sulfur speciation during chalcopyrite leaching by moderate thermophile Sulfobacillus thermosulfidooxidans [J]. International Journal of Mineral Processing, 2010, 94(1): 52-57.
[9] DEVECI H, JORDAN M A, POWELL N, ALP I. Effect of salinity and acidity on bioleaching activity of mesophilic and extremely thermophilic bacteria [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(3): 714-721.
[10] ZENG Wei-min, WU Chang-bin, ZHANG Ru-bing, HU Pei-lei, QIU Guan-zhou, GU Guo-hua, ZHOU Hong-bo. Isolation and identification of moderately thermophilic acidophilic iron-oxidizing bacterium and its bioleaching characterization [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 222-227.
[11] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M L, GONZALEZ F, MUNOZ J A. New information on the chalcopyrite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71(1): 47-56.
[12] KONISHI Y, ASAI S, TOKUSHIGE M, SUZUKI T. Kinetics of the bioleaching of chalcopyrite concentrate by acidophilic thermophile Acidianus brierleyi [J]. Biotechnology Progress, 1999, 15(4): 681-688.
[13] ZHOU Hong-bo, ZENG Wei-min, YANG Zhi-feng, XIE Ying-jian, QIU Guan-zhou. Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor [J]. Bioresource Technology, 2009, 100(2): 515-520.
[14] GERICKE M, GOVENDER Y, PINCHES A. Tank bioleaching of low-grade chalcopyrite concentrates using redox control [J]. Hydrometallurgy, 2010, 104(3-4): 414-419.
[15] D'HUGUES P, FOUCHER S, GALLE-CAVALLONI P, MORIN D. Continuous bioleaching of chalcopyrite using a novel extremely thermophilic mixed culture [J]. International Journal of Mineral Processing, 2002, 66(1): 107-119.
[16] QIU M Q, XIONG S Y, ZHANG W M, WANG G X. A comparison of bioleaching of chalcopyrite using pure culture or a mixed culture [J]. Minerals Engineering, 2005, 18(9): 987-990.
[17] ZHANG Yan-sheng, QIN Wen-qing, WANG Jun, ZHEN Shi-jie, YANG Cong-ren, ZHANG Jian-wen, NAI Shao-shi, QIU Guan-zhou. Bioleaching of chalcopyrite by pure and mixed culture [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1491-1496.
[18] TRIBUTSCH H. Direct versus indirect bioleaching [J]. Hydrometallurgy, 2001, 59(2): 177-185.
[19] SHU Rong-bo, RUAN Ren-man, WEN Jian-kang. Review on passivation of chalcopyrite during bioleaching process [J]. Chinese Journal of Rare Metals, 2006, 30(3): 395-400. (in Chinese)
[20] ZHEN Shi-jie, QIN Wen-qing, YAN Zhong-qiang, ZHANG Yan-sheng, WANG Jun, REN Liu-yi. Bioleaching of low grade nickel sulfide mineral in column reactor [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1480-1484.
[21] MERUANE G, VARGAS T. Bacterial oxidation of ferrous iron by Acidithiobacillus ferrooxidans in the pH range 2.5–7.0 [J]. Hydrometallurgy, 2003, 71(1): 149-158.
[22] PINCHES A, ALJAID F O, WILLIAMS D J A, ATKINSON B. Leaching of chalcopyrite concentrates with thiobacillus ferrooxidans in batch culture [J]. Hydrometallurgy, 1976, 2(2): 87-103.
[23] KINNUNEN P M, HEIMALA S, RIEKKOLA-VANHANEN M L, PUHAKKA J. Chalcopyrite concentrate leaching with biologically produced ferric sulphate [J]. Bioresource Technology, 2006, 97(14): 1727-1734.
[24] HE Zhi-guo, XIAO Sheng-mu, XIE Xue-hui, HU Yue-hua. Microbial diversity in acid mineral bioleaching systems of dongxiang copper mine and Yinshan lead–zinc mine [J]. Extremophiles, 2008, 12(2): 225-234.
[25] ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, LIU Xue-duan, CHEN Miao, CHAO Wei-liang, ZHANG Cheng-gui, PENG Juan-hua. Characterization of extracellular polymeric substances extracted during the bioleaching of chalcopyrite concentrate [J]. Hydrometallurgy, 2010, 100(3-4): 177-180.
微生物浸出Pb-Zn-Sn黄铜矿及其生物群落的演替分析
王 军1,2,赵红波1,2,庄 田1,2,覃文庆1,2,朱 珊1,2,邱冠周1,2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083
摘 要:研究了Pb-Zn-Sn黄铜矿精矿在混合中度嗜热微生物槽浸过程中的细菌群落结构变化,并监测浸出体系中金属离子浓度、溶液电位、溶液pH值变化,通过聚合酶链式反应-限制性片段长度多态性(PCR-RFLP)技术分析微生物群落的结构变化。结果表明,最终铜浸出率高达85.6%,在浸出前期,Acidithiobacillus caldus为优势群落,从第18天开始到浸出结束,Sulfobacillus thermosulfidooxidans为优势群落,但Leptospirillum ferriphilum丰度变化较小。试验结果表明,适当较高的溶液电位和合适的铁离子浓度对黄铜矿精矿的生物浸出作用很关键。
关键词:黄铜矿;微生物槽浸;微生物群落;聚合酶链式反应-限制性片段长度多态性技术PCR-RFLP
(Edited by Hua YANG)
Foundation item: Project (51374248) supported by the National Natural Science Foundation of China; Project (NCET-13-0595) supported by the Program for New Century Excellent Talents in University, China; Project (2012AA061501) supported by the High-tech Research and Development Program of China; Project (2010CB630905) supported by the National Basic Research Program of China; Project (20120162120010) supported by the Research Fund for the Doctoral Program of Higher Education of China; Project (CSUZC2012020) supported by the Open-End Fund for the Valuable in Central South University, China.
Corresponding author: Jun WANG; Tel: +86-731-88876557; E-mail: wjwq2000@126.com
DOI: 10.1016/S1003-6326(13)62926-X