多孔钛植入体表层孔隙内TGF-β1
缓释明胶微球涂层的工艺优化
陈良建1, 2,袁剑鸣1,李益民2,李 挺2
(1. 中南大学 湘雅三医院,湖南 长沙,410013;
2. 中南大学 粉末冶金国家重点实验室,湖南 长沙,410083)
摘 要: 采用正交试验法优化载转化生长因子β1(transforming growth factor-β1,TGF-β1)缓释明胶微球多孔钛植入体制备工艺,探讨多孔钛植入体孔隙内微球涂层的载药、释药特性。采用粉末注射成形(Metal Injection Molding, MIM)技术制备多孔钛植入体,选用明胶为TGF-β1缓释载体材料,乳化冷凝聚合交联法制备明胶微球,检测微球粒径与形貌以及载TGF-β1微球的包封率、载药率,采用渗涂法制备多孔钛表层孔隙内载TGF-β1明胶微球涂层,释放试验检测涂层的释药特性。实验结果表明,MIM技术制备的多孔钛植入体的孔隙度为(62.02±1.82)%,孔径为50~300 μm,抗压缩强度为(63.23±12.81) MPa,弹性模量为(0.95±0.61) GPa。明胶微球粒径随明胶浓度的减小、搅拌速度和交联时间的增加而减小,交联剂用量对微球粒径影响无显著性差异。制备的TGF-β1明胶微球为球形,平均粒径为(21.42±3.67) ?m,载药量为(0.91±0.02) μg/g,包封率为(91.41±1.82)%。TGF-β1微球涂层体外14 d,时的TGF-β1释放率为(94.2±3.4)%;粒径为(21.42±3.67) ?m的明胶微球的最佳工艺参数如下:明胶浓度为10%,搅拌速度为800 r/min,交联剂用量为0.1 mL,交联时间为2 h。多孔钛植入经5%(质量分数)明胶溶液预处理后用20 g/L微球渗涂可在表层孔隙内形成均匀微球涂层,且不阻塞表层孔隙,微球涂层TGF-β1释放时间为14 d。
关键词:多孔钛;转化生长因子β1;明胶;缓释微球;正交实验
中图分类号:R783.1 文献标识码:A 文章编号:1672-7207(2009)05-1228-07
Optimization of porous titanium coated with TGF-β1
loaded gelatin microspheres process parameters
CHEN Liang-jian1, 2, YUAN Jian-ming1, LI Yi-min2, LI Ting2
(1. The Third Xiangya Hospital, Central South University, Changsha 410013, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract: The effects of process parameters on the preparation of porous titanium coated with TGF-β1 loaded gelatin microspheres were systematically studied by orthogonal arrays design and statistical analysis method. Porous titanium implants with porosity of 60% were prepared by metal injection molding. Gelatin microspheres were prepared by improved emulsified cold condensation method and loaded with TGF-β1 by swelling in aqueous TGF-β1 solution. The morphology of the microspheres were observed by scanning electron microscopic (SEM). The encapsulation rate and drug content were tested with TGF-β1 ELISA kit. The porous titanium implants were coated with TGF-β1 loaded gelatin microspheres and characterized by drug release kinetics. The results show that the porosity and pore size of porous titanium implants are (62.02±1.82)% and 50-300 ?m, respectively. The compression yield strength is (49.21±10.81) MPa, and elastic modulus is (5.81±1.32) GPa. The diameter of gelatin microspheres decreases with the decrease of gelatin concentration and the increase of stirring speed and cross-linking time. However, cross-linking agent has no distinguished influence on the diameter. The diameter of gelatin microspheres is (21.42±3.67) ?m in average. The drug content is (0.91±0.02) μg/g, and encapsulation rate is (91.41±1.82)%. In vitro, (94.2±3.4)% of TGF-β1 were released after 14 d. The optimized preparation parameters of gelatin microspheres are as follows: gelatin concentration 10% (mass fraction), stirring speed 800 r/min, cross linking agent 0.1 mL, and crosslinking time 2 h. The porous titanium implants can be coated with 5% (mass fraction) gelatin and 20 g/L TGF-β1 loaded gelatin microspheres, and the structure of pores are kept completely. In vitro, TGF-β1 can be released for 14 d.
Key words: porous titanium; transforming growth factor-β1; gelatin; microspheres; orthogonal arrays design
钛和钛合金因具有良好力学性能和生物相容性,已被广泛应用于骨科的人工关节假体及牙植入体。现用的钛植入体多为全致密型,存在如下不足:其力学性能与周围骨质不匹配(致密钛弹性模量为110 GPa,骨质弹性模量为2~18 GPa),易在植入体的骨界面发生应力集中和应力遮挡效应,使骨界面产生慢性疲劳破坏,不利于植入体的长期稳定[1];钛缺乏生物活性,无骨诱导和骨传导能力,植入后骨整合时间需3~6月。多孔结构的植入体能实现植入体的力学性能与骨质的力学性质相匹配,避免骨质界面应力屏蔽效应,且为提供骨组织长入植入体的结构条件,实现生物固 定[2-3];多孔结构还能为生物活性涂层提供支架,避免植入剪切力发生破坏,有利于提高钛植入体生物活性。
对植入材料表面进行改性或涂层处理是提高植入材料生物活性的主要方法,已有研究证实在骨替代材料表面吸附多种生长因子可以促进成骨细胞的分化、基质分泌及钙化,包括增加有丝分裂因子(如IGF-1),增加骨细胞活性的因子(如TGF-β1)和诱导成骨的因子(如BMPS等)[4-5]。TGF-β1能增大成骨细胞活性,有较强的成骨和成软骨作用,改变骨膜的细胞构成和成骨细胞的结构,诱导矿化的骨形成[6-7]。但由于外源性生长因子稳定性弱,生物膜透过性差,半衰期短,在体内很快被稀释和分解代谢,直接应用效果不理想。缓释微球载体作为新型药物缓释控释系统之一,具有缓释药物,靶向输送,在保证药物治疗作用的前提下减少给药剂量,降低药物毒性等优点[8],目前,适用于制备微球的生物可降解材料包括聚乳酸、明胶、壳聚糖等。聚乳酸体内降解产物为乳酸,可致局部发生炎症反应[9-10]。明胶作为生物降解材料,有如下优点:组织相容性好,在体内可自然降解,降解产物不引起组织炎症反应[11],是由氨基酸与肽类交联形成的直链聚合物,能进行多种表面修饰及反应,且含有RGD生物活性短肽(即精氨酸-甘氨酸-天冬氨酸的三肽结构,可与细胞表面受体结合,有利于细胞表面粘附分子识别[12];在冻干过程中,明胶还可以取代水与多肽形成氢键,以稳定多肽的天然构象,从而保持生长因子活性;明胶微球为实体微粒,被包裹药物释放速度相对缓慢,足以达到缓释的要求[13-14]。在此,本文作者采用MIM技术制备60%孔隙率多孔钛植入体,以明胶作为缓释微球及涂层材料,用正交实验法研究明胶浓度、搅拌速度、交联剂用量和交联时间对明胶微球粒径的影响,优化工艺参数,并制备一定粒径的复合TGF-β1微球;利用渗涂法使其嵌入多孔钛植入体表层孔隙内形成涂层,采用体外释药试验法检测缓释微球涂层的载药、释药特性。
1 材料与方法
1.1 多孔钛植入体的制备
将氢化脱氢钛粉(粒径<77 μm)、氯化钠粉(粒径<290 μm),混合均匀后与粘结剂混炼制粒,采用注射成形技术制备生坯,经正庚烷溶剂浸泡4 h溶剂脱脂和300 ℃热脱脂,于80 ℃水浴脱盐8 h,在10-3 Pa真空下于1 150 ℃烧结3 h,随炉冷却,制备多孔钛植入体的直径为8 mm,厚度为3 mm。用扫描电镜(SEM)检测试样的表面微结构特点,用阿基米德法检测试样的孔隙度,用压汞法检测平均孔径和孔径分布,通过抗压强度试验检测试样的抗压缩强度和弹性模量。
1.2 载TGF-β1明胶微球的制备
采用L9(34)正交实验设计优化乳化交联法工艺参数(表1),考查明胶的浓度、搅拌速度、交联剂用量及交联时间对微球粒径的影响,在每种实验条件下重复2次。将含有10 g/L Span-80的液体石蜡35 mL预热至60 ℃,在快速搅拌下滴加同样温度的明胶水溶液5 mL,调节搅拌速度,继续搅拌10 min,形成油包水乳剂后,立即冰浴。乳剂固化后,加入适量25%(质量分数)戊二醛溶液行交联反应,控制搅拌速度和时间,再用适量丙酮、异丙醇洗涤3次后经真空干燥,用双蒸水清洗残留的有机溶剂后冻干。参照文献[15],在每1 mg干燥微球中加入200 μg/L的TGF-β1溶液5 ?L,在温度为4 ℃,pH值为7.4的条件下保存24 h,微球充分溶胀后,离心(1 200~1 500 r/min) 15 min,用双蒸水洗涤微球后冻干,钴-60照射灭菌后封存。
表1 明胶微球正交试验因素水平表
Table 1 Factors and levels for preparation of gelatin microspheres

1.3 载TGF-β1明胶微球形态及粒径测定
用激光衍射粒度分析仪测定载TGF-β1明胶微球平均粒径和粒度分布。对干燥TGF-β1明胶微球喷金后,采用扫描电镜观察其形貌。
1.4 载 TGF-β1明胶微球的包封率及载药量测定
取干燥空白微球样品分成5组,每组微球10 mg,加入200 μg/L的TGF-β1溶液50 μL,在4 ℃,pH=7.4条件下充分浸润、膨胀24 h后离心转速为(1 200~1 500 r/min)15 min,离心后取上清液,并将微球重悬于PBS中洗涤后离心,收集离心液和洗涤液用PBS定容至10 mL,取100 μL作为待测样品,依据TGF-β1 ELISA试剂盒操作说明,用酶联免疫仪于波长450 nm时测定标准品及待测样品OD值并绘制标准曲线,根据结果计算包封率及载药量。

1.5 载 TGF-β1明胶微球涂层多孔钛植入体的制备
在50 ℃,将明胶溶于双蒸水中,配制5%(质量分数)的明胶溶液。将多孔钛植入体浸泡于明胶溶液中,在负压条件下处理10 min,使明胶溶液充分渗入多孔钛内部形成薄膜涂层,取出后,将样品在50 ℃干燥24 h。用无水乙醇配制10,20和30 g/L TGF-β1明胶微球悬液,在超声条件下分散后,取100 μL TGF-β1明胶微球悬液滴至上述多孔钛植入体表面,使微球悬液渗入植入体孔隙内,于4 ℃时干燥;再用2.5%(质量分数)的戊二醛溶液浸泡上述样品,使明胶微球与明胶涂层进行交联反应,浸泡30 min后,用无水乙醇清洗样品3次,每次10 min,去除样品中残留的戊二醛,冷冻干燥,经钴-60照射灭菌后封存。采用扫描电镜检测载 TGF-β1明胶微球涂层多孔钛样品的表面微结构。
1.6 体外释药实验
随机取TGF-β1明胶微球涂层多孔钛植入体5个,置于无菌24孔细胞培养板,加入2 mL PBS缓冲液,置于37 ℃恒温培养箱中浸泡24 h,暂时取出试样,摇匀浸出液后,再吸取至EP管中于4 ℃的环境中保存,即为释放24 h的待测浸泡液。再将已取出试样放入无菌24孔细胞培养板内,加入PBS液2 mL,以同样方法留取实验开始后2,4,6,8,10,12,14 d的浸出液,于4 ℃的环境中保存。依据TGF-β1 ELISA试剂盒操作说明,用酶联免疫仪于450 nm波长下测定浸出液OD值,与标准曲线对照,计算各时间点样本TGF-β1浓度。将各个时间点的释放液TGF-β1含量分别与前一段时间的TGF-β1释放总量累加,即为该时间的TGF-β1释放总量,绘制TGF-β1释放曲线。
1.7 统计分析
以SPSS13.0对实验结果进行统计学分析,计量数据用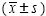 表示,采用单因素方差分析,显著性差异P<0.05,表明有统计学差异。
表示,采用单因素方差分析,显著性差异P<0.05,表明有统计学差异。
2 结果与讨论
2.1 多孔钛植入体表面结构及力学性能检测
采用注射成形技术制备的多孔钛植入体试样如图1所示。可见,试样具有均匀分布的连通孔隙结构,孔隙率为(62.02±1.82)%,孔径范围为50~300 μm,抗压缩强度为(63.23±12.81) MPa,弹性模量为(0.95±0.61) GPa,其力学性能与松质骨力学性能相匹配,弹性模量与松质骨弹性模量(0.9~1.7 GPa)[16]接近,能避免植入骨质界面应力集中和应力屏蔽效应,有利于骨界面应力传递。多孔植入体的孔隙度、孔径及连通孔结构是影响骨质长入多孔结构的主要因素,Holy等[17]发现孔径为100~150 μm比较适合新生骨的生长;Itala等[18]的研究结果表明,当孔径为50~125 μm时,骨组织也能生长良好,孔径小于50 μm 的孔隙结构有纤维组织长入,但基质不能矿化成骨;Otsukia等[19-20]的研究结果表明,连通孔结构有利于成骨细胞的迁移和新生血管组织长入,而血管组织的长入为孔隙内成骨细胞的生长和骨基质矿化创造条件;孔隙率的增加提高了种植体的渗透性,有利于各孔隙之间营养物质的交换、氧气的进入及代谢产物的排出,有利于细胞的增殖分化[21-22]。本研究制备的多孔钛植入体已满足上述结构要求。

图1 扫描电镜观察多孔钛孔隙结构
Fig.1 Scanning electron microscopic image of porous titanium
2.2 工艺条件对TGF-β1明胶微球制备工艺的影响
微球制备采用乳化冷凝聚合交联法,其机制是等电点为5.0的酸性明胶在水中溶解后,分子伸展为纤维状,在冷冻脱水的条件下卷曲,并由多个分子凝聚成球状微粒,在交联剂(戊二醛)的作用下,明胶分子形成稳定性良好的三维网状结构。
交联反应中明胶浓度、搅拌速度、交联剂用量、交联时间的等因素对微球粒径形态有显著影响[23]。按平均粒径评定制备工艺的影响因素,实验设计与结果如表2所示,方差分析结果如表3所示。
从表2可知,各因素水平从优至劣的顺序是:A1,A2,A3;B3,B2,B1;C2,C1,C3;D3,D1,D2。从表3的方差分析结果可见,各因素对明胶微球制备工艺的影响重要次序从大至小依次为A,B,D,C,其中明胶浓度、搅拌速度、交联时间对明胶微球的粒径影响有显著性差异(P<0.05),交联剂用量对微球粒径影响无显著性差异(P>0.05)。降低明胶溶液浓度能增大乳液分散均匀性,降低微球粒径。当搅拌速度小时,粒径大;随着搅拌速度增加,粒径减小。但当速度大到一定程度时,粒径变小不明显。增加交联时间,微球交联度增加,有利于微球粒径的稳定。
表2 明胶微球正交设计实验安排及结果
Table 2 Design and results of orthogonal experiment
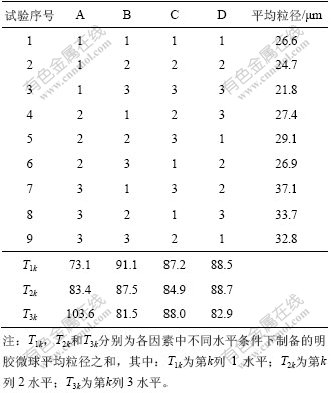
表3 方差分析
Table 3 Tests of between-subjects effects

最佳明胶微球制备工艺条件如下:A1B3C1D3,即明胶浓度为10%,搅拌速度为800 r/min,交联剂用量为0.1 mL,交联时间为2 h。制备的明胶微球粒径符合多孔钛孔隙内涂层的要求。
2.3 TGF-β1明胶微球表面形貌及粒径分布
本研究制备的多孔钛种植体孔隙的孔径为50~300 μm,TGF-β1明胶微球需比多孔钛表层孔隙的直径小1~2个数量级,有利于微球进入孔隙内,沉积形成涂层,避免因微球粒径过大而阻塞多孔钛孔隙。通过正交设计优化最佳制备工艺所得的载TGF-β1明胶缓释微球为淡黄色疏松粉末,加双蒸水后为淡黄色乳状溶液,分散性良好。在扫描电镜下微球呈圆球型,粒径较均匀,表面光滑,未见微孔和裂纹(见图2)。在激光衍射粒度分析仪下测得明胶微球粒径为10~40 μm,占80.25%,平均粒径为(21.42±3.67) μm(见图3)。

图2 TGF-β1明胶微球的SEM像
Fig.2 SEM image of TGF-β1 loaded gelatin microspheres

图3 TGF-β1明胶微球粒径分布
Fig.3 Size distribution (in diameter) of TGF-β1 loaded gelatin microspheres
2.4 TGF-β1明胶微球的包封率和载药量
根据TGF-β1标准品所绘制的标准曲线方程为:Y=-0.000 6X+0.917 1(X为TGF-β1浓度,Y为OD值),检测TGF-β1浓度线性范围为1~1 000 ng/L。由此测得最佳制备工艺所得TGF-β1明胶微球的包封率为(91.41±1.82)%,载药量为(0.91±0.02) μg/g。表明空白明胶微球有很强的吸水性能,水溶性生长因子很容易被吸附进入并包裹于微球内部。
2.5 载TGF-β1缓释微球涂层多孔钛植入体表面形态
明胶溶液与多孔钛之间有较好的润湿性而吸附并渗入孔隙结构内,形成明胶涂层并紧密结合,在交联过程中,明胶涂层与TGF-β1明胶微球通过戊二醛交联作用形成网络结构,使分子链间作用力加强,在多孔钛表面及孔隙内形成载TGF-β1明胶微球涂层,避免了缓释微球从多孔钛表面孔隙内脱落。经5%明胶溶液涂层处理后分别渗涂10,20,30 g/LTGF-β1明胶微球液的多孔钛植入体微结构如图4所示。从图4(a) 与(b)可见,渗涂20 g/L TGF-β1明胶微球后,多孔钛植入体孔隙内微球沉积量明显多于渗涂10 g/L TGF-β1明胶微球组的沉降量,且分布较均匀,孔隙结构未阻塞,只是在多孔钛的孔壁上和小间隙内沉积了大量明胶微球;渗涂30 g/L TGF-β1明胶微球液,由于微球的分散度降低,导致孔隙被阻塞(图4(c))。

ρ(TGF-β1)/(g?L-1): (a) 10; (b) 20; (c) 30
图4 不同浓度的TGF-β1明胶微球渗涂后多孔钛孔隙内SEM像
Fig.4 SEM images of porous titaniums coated with different concentrations TGF-β1 loaded gelatin microspheres
2.6 载TGF-β1明胶微球涂层多孔钛植入体体外释药特性
TGF-β1缓释微球的释药速度主要取决于生长因子的扩散速度和载体材料的降解速度。在明胶微球碱性条件下完全降解约需2月,人体体液pH值为7.35~7.45,符合明胶微球降解条件。载TGF-β1明胶缓释微球涂层多孔钛种植体体外释放曲线如图5所示。从图5可见,明胶微球在释放介质中溶胀后,生长因子逐渐释放,在释药初期速度较快,在24 h内达到20%左右,随着时间的增加,速度逐渐减缓并平稳释放,14 d时累计释放(94.2±3.4)%。Reddi等[24]的研究结果表明,种植体植入10~12 d后,成骨细胞开始分化矿化成骨,12~18 d成骨细胞活性增强,种植体骨质界面开始骨改建。TGF-β1能增大成骨细胞活性,有较强的成骨和成软骨作用,能改变成骨细胞的结构,诱导矿化骨形成。本研究在多孔钛种植体表层孔隙内沉积载TGF-β1缓释微球,持续释放TGF-β1可达14 d,因而,种植体植入骨质后,其骨界面的TGF-β1浓度能维持较高,有利于成骨细胞对植入体的早期反应,但最佳载药量还需通过体外细胞试验及动物实验进一步研究。

图5 载TGF-β1明胶缓释微球涂层多孔钛种植体体外释放曲线
Fig.5 Release profile of porous titanium coated with TGF-β1 loaded gelatin microspheres
3 结 论
a. 采用正交设计优化了乳化冷凝聚合交联法制备明胶缓释微球的最佳工艺参数如下:明胶浓度为10%、搅拌速度为800 r/min,交联剂用量为0.1 mL,交联时间为2 h。该条件下制得的明胶微球平均粒径为(21.42±3.67) μm,载TGF-β1明胶微球的载药量为(0.91±0.02) μg/g,包封率为(91.41±1.82)%。
b. 5%明胶溶液处理多孔钛表面再渗涂20 g/L TGF-β1明胶缓释微球后,在多孔钛表层孔隙内可形成较均匀的TGF-β1缓释微球涂层,且不阻塞多孔钛表面孔隙结构。
c. 载TGF-β1明胶缓释微球涂层多孔钛植入体体外释药时间达14 d,具有持续释放TGF-β1的作用。
参考文献:
[1] Ryan G, Pandit A, Apatsidis D P. Fabrication methods of porous metals for use in orthopaedic applications[J]. Biomaterials, 2006, 27(13): 2651-2670.
[2] Lee B H, Lee C, Kim D G, et al. Effect of surface structure on biomechanical properties and osseointegration[J]. Materials Science and Engineering C, 2008, 28(8): 1448-1461.
[3] Le Guehennec L, Lopez-Heredia M A, Enkel B, et al. Osteoblastic cell behaviour on different titanium implant surfaces[J]. Acta Biomaterialia, 2008, 4(3): 535-543.
[4] Michel B, Francesca B, Massimilliano L, et al. Effect of different growth factors on human osteoblasts activities: A possible application in bone regeneration for tissue engineering[J]. Biomolecular Engineering, 2007, 24(6): 613-618.
[5] Liu Y L, Enggista L, Kuffer F A, et al. The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration[J]. Biomaterials, 2007, 28(16): 2677-2686.
[6] Liu Q, Rauth A M, Wu X Y, et al. Immobilization and bioactivity of glucose oxidase in hydrogel microspheres formulated by an emulsification-internal gelation-adsorption- polyelectrolyte coating method[J]. International Journal of Pharmaceutics, 2007, 339(1/2): 148-156.
[7] GUO Chang-an, LIU Xue-guang. Novel gene-modified-tissue engineering of cartilage using stable transforming growth factor-β1-transfected mesenchymal stem cells grown on chitosan scaffolds[J]. Journal of Bioscience and Bioengineering, 2007, 103(6): 547-556.
[8] Kawai K, Suzuki S, Tabata Y, et al. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis[J]. Biomaterials, 2000, 21(5): 489-499.
[9] Shim W S, Kim J H, Park H, et al. Biodegradability and biocompatibility of a pH and thermo-sensitive hydrogel formed from a sulfonamide-modified poly (ε-caprolactone-co-lactide)- poly(ethyleneglycol)-poly(ε-caprolactone-co-lactide)blockcopolymer[J]. Biomaterials, 2006, 27(30): 5178-5185.
[10] Zhou Z H, Ruan J M, Zhou Z C, et al. Bioactivity of bioresorbable composite based on bioactive glass and poly-L-lactide[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(2): 394-399.
[11] Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on bio-degradation of gelatin hydrogel[J]. J Biomer Sci Polym Ed, 2001, 12: 77-88.
[12] Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond[J]. Biomaterials, 2003, 24(10): 4385-4415.
[13] Cortesi R, Esposito E, Osti M, et al. Dextran cross-linked gelatin microspheres as a drug delivery system[J]. European Journal of Pharmaceutical Sciences, 1999, 47(12): 153-160.
[14] Chen F M, Zhao Y M, Wu H, et al. Enhancement of periodontal tissue regeneration by local decontrolled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres[J]. Journal of Controlled Release, 2006, 114: 209-222.
[15] Holland T A, Tabata Y, Mikos A G, et al. In vitro release of transforming growth factor-β1 from gelatin microparticles encapsulated in biodegrada-ble, injectable oligo (poly- ethyleneglycol-fumarate)hydrogels[J]. Journal of Controlled Release, 2003, 91(3): 299-313.
[16] Mahony A M, Williams J L, Katz J O, et al. Anisotropic elastic properties of cancellous bone from a human edentulous mandible[J]. Clin Oral Implants Res, 2000, 11(5): 415-421.
[17] Holy C E, Fialkov J A, Davies J E, et al. Use of a biomimetic strategy to engineer bone[J]. Biomed Mater Res, 2003, 65(4): 447-453.
[18] Itala A I, Ylanen H O, Ekholm C, et al. Pore diameter of more than 100 micron is not requisite for bone ingrowth in rabbits[J]. J Biomed Mater Res, 2001, 58(6): 679-683.
[19] Otsukia B, Takemotoa M, Fujibayashia S, et al. Pore throat size and connectivity determine bone and tissue in growth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants[J]. Biomaterials, 2006, 27(35): 5892-5900.
[20] Mastrogiacomo M, Scaglione S, Martinetti R, et al. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics[J]. Biomaterials, 2006, 27(17): 3230-3237.
[21] Oh I H, Nomura N, Masahashi N, et al. Mechanical properties of porous titanium compacts prepared by powder sintering[J]. Scripta Mater, 2003, 49(12): 1197-1202.
[22] Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis[J]. Biomaterials, 2005, 26(27): 5474-5491.
[23] Rao J, Ramesh D, Rao K. Controlled-release systems for proteins based on gelatin microspheres[J]. J Biomat Sci Polym E, 1994, 6(5): 391-398.
[24] Reddi A H, Wientroub S, Muthukumaran N. Biologic principles of bone induction[J]. Orthop Clin North Am, 1987, 18(2): 207-212.
收稿日期:2009-04-07;修回日期:2009-06-22
基金项目:国家自然科学基金资助项目(35770576);国家“863”计划项目(2007AA03Z114);湖南省自然科学基金资助项目(2007JJ5109)
通信作者:陈良建(1967-),男,湖南攸县人,博士,副教授,从事新型钛种植体开发与临床研究;电话:0731-88618554;E-mail: chen0313@xy3yy.com