


Trans. Nonferrous Met. Soc. China 22(2012) 1771-1777
Preparation of UV-visible light responsive photocatalyst from titania-bearing blast furnace slag modified with (NH4)2SO4
LEI Xue-fei1, XUE Xiang-xin2, YANG He2
1. School of Resources and Materials, Northeastern University (Qinhuangdao),Qinhuangdao 066004, China;
2. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 26 July 2011; accepted 13 March 2012
Abstract: Sulfate-modified titanium dioxide-bearing blast furnace slag (STBBFS) photocatalysts were prepared by the high energy ball milling method with (NH4)2SO4 and titanium dioxide-bearing blast furnace slag (TBBFS) as raw materials. X-ray photoelectron spectroscopy(XPS), X-ray diffraction (XRD), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), UV-visible diffuse reflectance absorption spectra (UV-Vis), adsorption experiment and photocatalytic degradation measurement were conducted to characterize the structure, surface status, light absorption capacity, adsorption capacity and photocatalytic activity of the obtained photocatalysts. The adsorption equilibrium was described by the Langmuir isotherm model with a maximum adsorption capacity of 8.25 mg/g of Cr(VI) ions onto the STBBFS photocatalysts. As a result, sulfation of TBBFS improved the photocatalytic activities of STBBFSx photocatalysts. At a low calcination temperature, the photocatalytic activity of STBBFS300 photocatalyst was markedly higher compared with TBBFSx prepared at high calcination temperature, indicating that the photocatalytic activity of STBBFSx photocatalyst was determined by the balanced result between adsorption capacity and perovskite content.
Key words: sulfate modification; titanium dioxide-bearing blast furnace slag; Cr(VI); adsorption capacity; photocatalytic activity
1 Introduction
A new series of photocatalysts containing complex ABO3-type perovskite structure have been studied recently [1,2]. These perovskite compounds as photocatalysts are particularly attractive because of their higher activities which can be easily excited under visible light or UV light irradiation. However, it is an expensive photocatalyst for application in large scale. Consequently, there is a need to search for more economical and effective photocatalyst for application in catalysis and separations.
Currently, the utilization of TBBFS is classified into two parts: partial and overall utilizations. The former uses TBBFS as raw material to refine titanium resources [3]. Although the technique is valid with a clean, low cost and high productivity, the difficulty in purifying products and the low economic value of acquired perovskite material should not be ignored. The latter overall one is indeed to consider TBBFS the whole not only used for constructional materials but also for ecological utilization. In order to efficiently utilize titanium resources and diminish influence of those solid wastes on environment deterioration, ecological utilization methods have been proposed in this respect [4]. As illustrated by our recent works, our groups have reported that the TBBFS as photocatalysts shows UV light photocatalytic activity to some extent [5-9]. XUE et al [6] adopted high-energy ball milling technique and high temperature to get TBBFS700 photocatalysts and concluded that the photocatalytic activity of TBBFS700 photocatalyst reaches 88% that of P25 TiO2 after 6 h. The obvious advantages of TBBFS as photocatalysts are the low costs involved and easy to be isolated. However, in the case of unmodified TBBFS photocatalysts, the photocatalytic activity of TBBFS700 photocatalyst is still lower and photo-reduction process of Cr(VI) is time-consuming.
Here, a simple surface modification was utilized to synthesize a UV-visible light responsive STBBFS photocatalyst. It was well known that the sulfation of TiO2 improved the photocatalytic activity of catalyst [10,11]. In this study, a sulfate-modified TBBFS photocatalyst was synthesized with (NH4)2SO4 and TBBFS as raw materials. The photocatalytic activity of sulfate-modified TBBFS photocatalyst was evaluated by reducing Cr(VI) under UV-Vis light and visible-light irradiation.
2 Experimental
2.1 Preparation of photocatalysts
TBBFS from Panzhihua Iron and Steel Corporation was used in this study. In a typical synthetic procedure, massive TBBFS was broken into pieces; the resulting shatters were then comminuted by disintegrator; subsequently, the powder was mixed with (NH4)2SO4 in the high-energy ball mill for 80 h. One TBBFS without SO42-was also prepared in the same manner in order to use it as a reference. The mass ratio of TBBFS to (NH4)2SO4 was 20:1. After pulverizing, STBBFS and TBBFS powders were calcined in air at a heating rate of 10 ℃/min up to 300, 400, 500 and 600 ℃ and held for 2 h, then cooled down by natural; the resulting catalysts were labeled as STBBFSx and TBBFSx, where x denoted the calcination temperature. The XPS survey spectrum of STBBFS300 catalyst is shown in Fig. 1. Titanium dioxide Degussa P25 was used as a reference without pre-treatment or modification before use in our experiments.

Fig. 1 XPS survey spectrum of STBBFS300 catalyst
2.2 Characterization of photocatalysts
X-ray photoelectron spectroscopy (XPS) measure- ments were done with a Kratos XSAM800 XPS system with Mg Kα source and a charge neutralizer; all the binding energies were referenced to the C 1s peak at 284.8 eV of the surface adventitious carbon. The crystal structure identification of STBBFSx catalyst was obtained by using Philips X’pert X-ray diffractometer with Cu Kα radiation. Morphology of STBBFSx catalyst was recorded with a scanning electron microscope. Average particle diameter of STBBFSx catalyst was got by using a BT-1600 image analyzer. UV-Vis diffuse reflectance spectra were obtained by employing a UV-2550 spectrophotometer. BaSO4 was used as a reflectance standard in a UV-Vis diffuse reflectance experiment. The thermogravimetric analysis (TGA) was carried out using SDT 2960 Simultaneous DSC-TGA instrument, with a heating rate of 10 ℃/min from room temperature to 800 ℃ under air atmosphere.
2.3 Photocatalytic testing
Photocatalytic irradiations were carried out in a multi-tube mixing reaction apparatus equipped with a quartz immersion well, where a 500 W xenon lamp or a 500 W mercury lamp was placed as a radiation source. For all photocatalytic runs, a fresh solution (50 mL) containing 20 mg/L Cr(VI) was simultaneously maintained in suspension by a magnetic stirrer. In each experiment, the catalyst was suspended with a load of 0.5 g/L, solution pH was adjusted to 1.5 with H2SO4 solutions. Prior to irradiation, suspensions were magnetically stirred for 30 min in the dark to ensure the adsorption/desorption equilibrium until the concentration of suspensions was constant. The concentration of suspensions after equilibration was taken as the initial concentration, to discount changes in the dark. At regular intervals in the photocatalytic experiments, 10.0 mL aliquot of the suspensions was withdrawn. Subsequently, the upper transparent solution obtained by centrifugation was collected for UV-Vis absorption spectra measurement. Cr(VI) concentration in the resulting solution was determined colorimetrically at 540 nm using diphenylcarbazide as a color agent. The photocatalytic activity was evaluated by the reduction efficiency (η) of Cr(VI) after experiment:
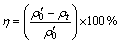 (1)
(1)
where 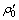 is the initial concentration of Cr(VI) after adsorption equilibrium, and ρt is the concentration of Cr(VI) at any time t, and η is the reduction efficiency of Cr (VI).
is the initial concentration of Cr(VI) after adsorption equilibrium, and ρt is the concentration of Cr(VI) at any time t, and η is the reduction efficiency of Cr (VI).
3 Results and discussion
3.1 Photocatalysts characterization
The XRD patterns of STBBFSx photocatalysts calcined at different temperatures are shown in Fig. 2. It can be observed that the similar patterns were obtained for all STBBFSx photocatalysts, which were identified to be a mixture of perovskite, diopside, akermanite- gehlenite and TiO2 (≥500 ℃). The maximum deviation of the observed peak value corresponding to 33.11° denotes the formation of perovskite. It was obviously observed that the XRD pattern of STBBFSx was different from that of TBBFSx [6], indicating that the surface inorganic modification to TBBFS had some effects on the crystalline phase. Through comparing XRD patterns of TBBFSx and STBBFSx photocatalysts, we find that there is a new crystalline phase (TiO2) in STBBFSx photocatalysts calcined at high temperatures (≥500 ℃). It can be observed from Fig. 2(b) that the calcination temperature influences perovskite content in STBBFSx photocatalysts. As the calcination temperature increases, perovskite content in STBBFSx photocatalysts shows fluctuation. Such a change in phase content may be due to the influence of activation temperature. With increasing the calcination temperature above the activation temperature the periodic circulation of the crystalline phases and surface species of catalysts were promoted, which accounted for the fluctuation of perovskite content in STBBFSx photocatalysts [5].
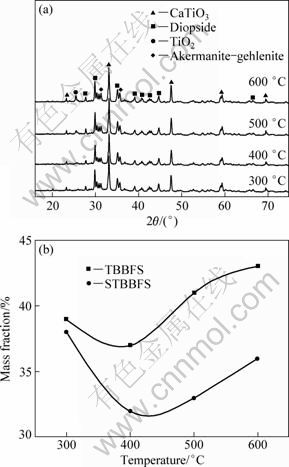
Fig. 2 XRD patterns of STBBFSx photocatalysts calcined at different temperatures (a) and perovskite content of different photocatalysts (b)
SEM images of TBBFSx and STBBFSx photocatalysts are shown in Fig. 3. It is seen from the figures that all the prepared photocatalysts (TBBFSx and STBBFSx) consist of micro-sized particles with concave and convex surface morphology. The particle size is seen to be distributed in the range of 0.10-20 μm. It is also seen that more than 50% of the particles are distributed in the 0.10-0.30 μm range. A certain fraction of photocatalysts is also seen to consist of micro-sized agglomerates. No significant differences were detected in the morphologies of TBBFSx and STBBFSx photocatalysts.

Fig. 3 SEM images of TBBFS600 (a), STBBFS300 (b) and STBBFS600 (c)
Figure 4 shows the TGA curves of TBBFS and STBBFS photocatalysts. From Fig.4, the major mass loss of 1.5% between 35 and 500 ℃ was due to the dehydration of physically absorbed water and crystallization in TBBFS photocatalysts. As seen from Fig. 4, STBBFS sample shows the mass loss mainly in the range of 200-500 ℃, aside from the evaporation of water, an additional mass loss occurred corresponding to the decomposition/desorption of SO42- species on the surface of STBBFSx photocatalysts at high temperature [11]. In addition, the content of undecomposed SO42- was 3.87% for STBBFS300, 3.21% for STBBFS400, 3.16% for STBBFS500 and 3.12% for STBBFS600.

Fig. 4 TGA curves of TBBFS and STBBFS photocatalysts
The light absorbance characteristics of P25 TiO2 and STBBFSx photocatalysts calcined at different temperatures are depicted in Fig. 5. It is demonstrated in the UV-visible diffuse reflectance absorption spectra that STBBFSx photocatalysts have higher absorbance in the UV-visible region. The absorption edge of STBBFSx photocatalysts appeared at about 500 nm. It is evident from the figure that UV-absorption edge of prepared photocatalysts extended to longer wavelength (towards visible region), which is beneficial for improving the photoabsorption and photocatalytic performance of STBBFSx photocatalysts under visible light. Since prepared photocatalysts absorb light over a wider range of wavelength and utilize more light energy, it follows that they have higher photocatalytic activity under sunlight radiation. Generally, the red-shift means the decrease of electron-transfer energy, which is decided mainly by chemical structure [12]. Additionally, the fact that visible absorption capacity (>470 nm) of STBBFSx photocatalysts strengthens with the decrease of calcination temperature indicates that there exist moderate SO42- species on the surface of STBBFSx photocatalysts calcined at lower temperature (see Fig. 3), which can slightly improve the visible light absorption of STBBFSx photocatalysts.

Fig. 5 UV-Vis diffuse reflectance absorption spectra of P25 TiO2 and STBBFSx photocatalysts calcined at different temperatures
3.2 Cr(VI) adsorption isotherms
Analysis of the information about the adsorption of reactants on the surface of catalyst is very important for understanding the photocatalytic activity of catalyst. The two most popular isotherm theories are the Langmuir and the Freundlich theories.
The parameters of Cr(VI) adsorption on the catalysts, obtained by fitting the experimental data using the Langmuir and Freundlich adsorption models, are listed in Table 1. The Langmuir adsorption model fitted better the experimental results than the Freundlich model for all the materials, which suggests that Cr(VI) adsorption by calcined TBBFSx or STBBFSx photocatalysts was apparently with monolayer coverage of adsorbed molecules. In addition, it can be seen from Table 1, the maximum adsorption capacity of STBBFSx photocatalysts was slightly higher than that of TBBFSx photocatalysts, suggesting that the presence of SO42- on catalyst surface increases the adsorption capacity of STBBFSx photocatalysts. Moreover, it was generally accepted that the surface acid sites of the sulfated catalysts are involved in the process of Cr(VI) reduction and the acid environment of the catalyst surface may markedly facilitate the Cr(VI) adsorption [13]. Considering that marked desorption/decomposition of surface SO42- was observed at calcination temperature higher than 200 ℃ (see Fig. 4), the lower adsorption capacity of STBBFSx calcined at higher temperature may also be ascribed to the decrease of surface acidity. Therefore, sulfation of TBBFS improved surface acidity of catalysts and therefore increased the adsorption capacity of STBBFSx.
3.3 Comparison of photocatalytic activities of TBBFSx and STBBFSx photocatalysts
The photocatalytic performance of photocatalysts (TBBFSx, STBBFSx) were evaluated by the photo-reduction efficiency of Cr(VI) after 2 h irradiation under UV-visible light. To clearly compare the photocatalytic performance of various photocatalysts, the photo- reduction efficiency of Cr(VI) in different photocatalysts is shown in Fig. 6. It is found that TBBFSx showed very poor photocatalytic activity, but STBBFSx photocatalysts (calcination temperature<500 ℃) exhibited notably high reactivity.
Table 1 Langmuir and Freundlich constants for adsorption of Cr(VI) onto TBBFSx and STBBFSx photocatalysts


Fig. 6 Dependence of reduction efficiency of photocatalysts on calcinations temperature
It is generally thought that the photocatalytic performance depended on the absorbance, the phase structure, and the adsorption capacity of photocatalyst [14,15]. In our experiments, the absorbance of all photocatalysts was almost the same in UV-visible region, so the different photocatalytic activities should depend on the latter two factors. In addition, another important founding from Fig. 6 is that with the increase of calcination temperature from 300 to 600 ℃, the photocatalytic performance of STBBFSx photocatalysts firstly decreased and then increased. On the whole, the change trend of photocatalytic performance of STBBFSx photocatalysts was in agreement with that of perovskite content (see Fig. 2(b)), indicating that the photocatalytic activities of STBBFSx photocatalysts were affected by their perovskite content. This result agrees with that of TBBFSx photocatalysts [6]. However, the decreasing trend shown in Fig. 6 was different with that of Fig. 2(b) at calcination temperature below 500 ℃. The perovskite content from 300 to 500 ℃ had a slow decline, and the photocatalytic activity decreased rapidly. This difference should be attributed to the adsorption capacity. When calcination temperature increased from 300 to 500 ℃, the adsorption capacity quickly reduced (as listed in Table 1). So, the photocatalytic performance of STBBFSx photocatalysts exhibited a rapid decline with calcination temperature (<500 ℃). As a result, sulfation of TBBFS improved the photocatalytic activities of STBBFSx photocatalysts. At a low calcination temperature, the photocatalytic activity of STBBFS300 photocatalyst was markedly higher than that of TBBFS prepared at high calcination temperature, indicating that the photocatalytic activities of STBBFSx photocatalysts were determined by the balanced result between adsorption capacity and perovskite content.
Figure 7 shows photocatalytic reduction results of Cr(VI) conducted with P25 TiO2 and STBBFS300 photocatalysts under visible-light and UV-Vis light irradiation, respectively. Overall, it is obvious that the UV-Vis and visible-light photocatalytic activities of STBBFS300 photocatalyst are higher than those of P25 TiO2. We also find that the visible-light photocatalytic activities of both STBBFS300 and P25 TiO2 are lower than under UV-Vis light irradiation, which means that the irradiation light is a key factor for photocatalysis. The different activities between P25 TiO2 and STBBFS300 under UV-Vis light and visible-light irradiation may have multiple origins. Firstly, bare TiO2 had no visible absorption capability (absorbing mainly in the range of 240-300 nm), but STBBFS300 photocatalyst had a broad light absorption capability (240-800 nm). The broad light absorption capability of STBBFS300 photocatalyst might be favorable to photocatalysis under UV-visible light irradiation. Secondly, the surface sulfation on STBBFSx photocatalysts could strongly enhance surface acidity, which was also able to improve the adsorption efficiency of Cr(VI) (see Fig.7(b)) on STBBFS300 and hence the photocatalytic performance of STBBFS300 photocatalyst. Moreover, as shown in Fig. 2(b), the phase content of perovskite is less than 40% in STBBFS300, suggesting that the photocatalytic activity of the perovskite phase formed in STBBFS300 is higher than that of P25 TiO2. Consequently, STBBFS300 photocatalyst is promising candidate for Cr(VI) photoreduction with high photocatalytic activity.

Fig. 7 Photocatalytic reduction efficiency (a) and adsorption efficiency of Cr(VI) with STBBFS300 and P25 TiO2 (b)
4 Conclusions
1) Perovskite types STBBFSx photocatalysts were prepared by a simple but powerful approach through the high energy ball milling method at different calcination temperatures.
2) The adsorption equilibrium was described by the Langmuir isotherm model with a maximum adsorption capacity of 8.25 mg/g of Cr(VI) ions onto the STBBFS300 photocatalyst.
3) The photocatalytic activity of STBBFS300 photocatalyst was markedly higher than that of others, indicating that the photocatalytic activities of STBBFSx photocatalysts were determined by the balanced result between adsorption capacity and perovskite content.
References
[1] AKHGAR B N, PAZOUKI M, RANJBAR M, HOSSEINNIA A, KEYANPOUR-RAD M. Preparation of nanosized synthetic rutile from ilmenite concentrate [J]. Miner Eng, 2010, 23(7): 587-589.
[2] YANG Y, SUN Y B, JIANG Y S. Structure and photocatalytic property of perovskite and perovskite-related compounds [J]. Mater Chem Phys, 2006, 96(2-3): 234-239.
[3] ZHANG L, ZHAN L N, WANG M Y, LI G.Q, SUI Z T. Precipitation selectivity of perovskite phase from Ti-bearing blast furnace slag under dynamic oxidation conditions [J]. J Non-Cryst Solids, 2007, 353(22-23): 2214-2220.
[4] YANG He, XUE Xiang-xin, ZUO Liang, YANG Zhong-dong. Photocatalytic degradation of methylene blue with blast furnace slag containing Titanium [J]. The Chinese Journal of Process Engineering, 2004, 4(3): 265-268. (in Chinese)
[5] LEI Xue-fei, XUE Xiang-xin. Preparation, characterization and photocatalytic activity of sulfuric acid-modified titanium-bearing blast furnace slag [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(12): 2294-2298.
[6] XUE Xiang-xin, LEI Xue-fei, YANG He, DUAN Pei-ning. Photocatalytic reduction of Cr(VI) by sulfate modified titanium-bearing blast furnace slag [J]. Journal of Northeastern University: Natural Science, 2009, 30(2): 221-225. (in Chinese)
[7] LEI Xue-fei, XUE Xiang-xin. Photocatalytic reduction of Cr(VI) in Cr(VI)-citric acid-ferric nitrate compound system [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 383-388. (in Chinese)
[8] LEI X F, XUE X X. Preparation and characterization of perovskite type titania-bearing blast furnace slag photocatalyst [J]. Mater Sci Semicond Process, 2008, 11(4): 117-121.
[9] LEI Xue-fei, XUE Xiang-xin. Effect of the different compound systems on photocatalytic reduction of Cr(VI) by titanium-bearing blast furnace slag [J]. Acta Chimica Sinica, 2008, 66(22): 2539-2546. (in Chinese)
[10] MOHAPATRA P, SAMANTARAY S K, PARIDA K. Photocatalytic reduction of hexavalent chromium in aqueous solution over sulphate modified titania [J]. J Photochem Photobiol A, 2005, 170(2): 189-194.
[11] SRINIVASAN R, KEOGH R A, MILBURN D R, DAVIS B H. Sulfated zirconia catalysts: Characterization by TGA/DTA mass spectrometry [J]. J Catal, 1995, 153(1): 123-130.
[12] JIANG D, XU Y, WU D, SUN Y H. Visible-light responsive dye-modified TiO2 photocatalyst [J]. J Solid State Chem, 2008, 181(3): 593-602.
[13] PAPADAM T, XEKOUKOULOTAKIS N P, POULISO I, MANTZAVINOS D. Photocatalytic transformation of acid orange 20 and Cr(VI) in aqueous TiO2 suspensions [J]. J Photochem Photobiol A, 2007, 186(2-3): 308-315.
[14] JIANG F, ZHENG Z, XU Z Y, ZHENG S R, GUO Z B, CHEN L Q. Aqueous Cr(VI) photo-reduction catalyzed by TiO2 and sulfated TiO2 [J]. J Hazard Mater B, 2006, 134(1-3): 94-103.
[15] YU J G, WANG W G, CHENG B, SU B L. Enhancement of photocatalytic activity of mesporous TiO2 powders by hydrothermal surface fluorination treatment [J]. J Phys Chem C, 2009, 113(16): 6743-6750.
紫外-可见光催化活性的硫酸铵改性
含钛高炉渣光催化剂的制备
雷雪飞1,薛向欣2,杨 合2
1. 东北大学(秦皇岛分校) 资源与材料学院,秦皇岛 066004;
2. 东北大学 材料与冶金学院,沈阳 110819
摘 要:以含钛高炉渣和硫酸铵为原料,利用高能球磨法制备硫酸盐掺杂的含钛高炉渣(STBBFS)光催化剂。利用X射线光电子能谱(XPS)、X射线衍射(XRD)、扫描电镜(SEM)、紫外-可见吸收光谱(UV-Vis)、热重(TGA)分析以及暗态吸附Cr(VI)废水、光催化还原Cr(VI)废水实验对STBBFS催化剂的物相、表面结构、光吸收能力、吸附容量以及光催化活性进行表征。结果表明:Cr(VI)在STBBFS催化剂表面上的吸附遵循Langmuir吸附等温线模型;掺杂硫酸盐后,STBBFS催化剂的吸附容量增大为8.25 mg/g;在300 ℃煅烧后,STBBFS催化剂由于存在较高的钙钛矿含量、吸附容量及表面酸性,从而具有较高的光催化活性。
关键词:硫酸盐改性;含钛高炉渣;六价铬;吸附容量;光催化活性
(Edited by YANG Hua)
Foundation item: Project (2007CB613504) supported by the National Basic Research Program of China; Project (307009) supported by the Foundation for Key Program of Ministry of Education, China; Project (N110423003) supported by the Fundamental Research Funds for the Central Universities, China; Project (E2012501012) supported by Natural Science Foundation-Steel and Iron Foundation of Hebei Province, China
Corresponding author: LEI Xue-fei; Tel: +86-335-8047521; Email: leixuefei69@163.com
DOI: 10.1016/S1003-6326(11)61386-1