文章编号:1004-0609(2015)04-1063-09
基于正交试验的电置换提镉工艺优化及电化学研究
何 静,戚春萍,段良洪,叶龙刚,张家玮,王夏阳
(中南大学 冶金与环境学院,长沙 410083)
摘 要:提出一种基于微电流作用下的提镉新工艺,采取正交试验考察各工艺参数对海绵镉提取过程的影响,并对ZnSO4+CdSO4混合溶液中Zn2+、Cd2+的阴极行为进行电化学研究。结果表明:各因素对提镉率的影响由大到小的顺序依次为温度、pH、极距、电流密度、阴阳极面积比;最佳工艺条件为pH=1、极距3 cm、电流密度400 A/m2、温度90 ℃、阴阳极面积比1:2,在此条件下电置换1 h提镉率达到79.31%;镉先于锌在阴极析出,当锌开始析出时,溶液中镉离子浓度已降到很低,最低可降至0.528 mg/L。混合溶液阴极沉积的表观活化能为6.379 kJ/mol,在60~90 ℃时属于扩散步骤控制。
关键词:镉;微电流;锌板置换;电化学
中图分类号:TF8 文献标志码:A
Process optimization and electrochemistry study of electrical replacement for cadmium extraction based on orthogonal experiments
HE Jing, QI Chun-ping, DUAN Liang-hong, YE Long-gang, ZHANG Jia-wei, WANG Xia-yang
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: A new process of cadmium extraction under micro-current was proposed. In order to determine the optimum process parameters, five main effect factors were investigated by an orthogonal experiment. Electrochemical experiments of the cathodic behavior of Zn2+, Cd2+ in mixed solution of CdSO4+ZnSO4 were conducted. The results show that the influence factors on extraction rate decrease in such an order: temperature, pH, polar plate distance, current density, plate area ratio. The optimal conditions are obtained at pH of 1, polar plate distance of 3 cm, current density of 400 A/m2, temperature of 90 ℃ and plate area ratio of 1:2. The maximum extraction rate is 79.31%. Cadmium is prior to precipitate than zinc. The concentration of Cd2+ in solution decreases to the minimum of 0.528 mg/L while zinc begins to deposit. The apparent activation energy of deposition is 6.379 kJ/mol. The diffusion-controlled is the dominant process at temperature ranging in 60-90 ℃.
Key words: cadmium; micro-current; zinc plate cementation; electrochemistry
镉主要用于制造合金、电镀、电池和颜料等,同时还是原子反应堆控制棒的重要成分[1-2]。镉的毒性 较大[3],其提取和使用过程都需引起特别注意,国内外都曾发生严重的镉中毒事件。由于镉多伴生在铅锌矿中,因此主要作为铅锌冶炼的副产物回收[4]。
目前,工业上镉主要在湿法炼锌过程生产,硫酸锌浸出液净化产生的铜镉渣经造液后再经两段锌粉转换生产纯度80%左右的粗镉,该工艺存在锌粉用量大、生产成本高、置换率低和海绵镉纯度低等缺点[5]。特别是残液的镉浓度高,后续处理难度大,如处理不妥将对人类健康及环境产生致命危害[3]。其他从铜镉渣中回收镉的方法有:邓小华[6]提出用锌片代替锌粉置换除镉,所得的海绵镉品位较高,但反应过程较平缓,反应时间长;梁龙伟[7]开发出一种湿法炼锌三段净化新工艺,主要过程为低温锌粉除反溶镉,净化深度高,Cd含量基本稳定在l mg/L以下,但仍需消耗大量锌粉。
针对以上问题,国内外对提镉新工艺进行了大量研究,提出的提镉方法主要有离子交换法、溶剂萃取法、液膜法和吸附法等。离子交换法采用N503(N,N-二仲辛基乙酰胺)萃淋树脂[8]或高分子配位体亚胺基二乙酸树脂(D401)[9]吸附。REDDY等[10]研究了基于有机磷的萃取剂-TOPS99、PC88A、Cyanex272及其混合物对硫酸盐溶液中的Cd(Ⅱ)进行萃取回收;JHA等[11]则综合回收电子废弃物中的铜、锌、镉和镍,在采用LIX84萃铜后分别用10%H2SO4和10%HCl(质量分数)反萃回收锌和镉。黄炳辉等[12]提出利用液膜技术从电镀废水中回收低浓度镉,汤兵等[13]在此基础上利用液膜内相结晶技术,建立萃取-反萃-结晶同时进行的分离过程,在高浓度干扰离子体系中实现炼锌渣浸出液的净化并提取镉。此外,吸附法提镉的研究也广受重视,新型吸附剂包括金红石型纳米TiO2[14]、锰矿[15]和赤泥[16]等。但这些方法都存在一定的缺陷,离子交换法处理效果好,但树脂易受污染或氧化失效,再生频繁,操作费用高;膜分离法污染物去除率高,能回收废水中的镉盐,工艺简单,但其投资较高,膜孔易堵塞。相对来说,生物法因能耗少、成本低、效率高、易操作、不产生二次污染而逐渐发展起来。
为解决现有镉提取的问题,本文作者提出一种含镉液在微电流作用下提取镉的新工艺,即在非均匀电场微电流作用下,用锌板做置换板(阳极),钛网做导电板(阴极)进行电场强化置换,使锌板极缓慢溶解,可消除传统工艺中因包裹而使反应减慢的问题,促进转换反应的不断进行。并综合考察了pH、极距、电流密度、温度和阴阳极面积比这5个因素对提镉率的影响,进行正交试验,优化工艺参数,以确定适合微电流作用下提取海绵镉的最佳工艺参数组合。并在低于90 ℃的温度下,对33.82 g/L CdSO4+93.6 g/L ZnSO4混合溶液进行了循环伏安、线性扫描和Tafel测试。
1 实验
1.1 试验原料
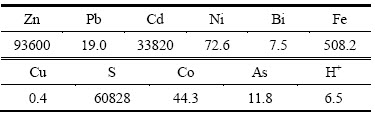
本试验中原料为湖南水口山炼锌厂中铜镉渣的一次浸出液,其主要成分经ICP-AES分析结果如表1所列。
表1 一次浸出液的主要化学成分
Table 1 Chemical compositions of primary leaching solution (mg/L)
1.2 试验原理及方法
本实验的置换原理是基于Zn的标准电位(-0.7628 V)较Cd的标准电极电位(-0.4026 V)更负,故电解过程中镉离子可在锌板表面被置换出,同时锌板表面在微电流作用及酸性条件下均发生溶解反应,使表面附着的镉不断脱落,从而使置换反应得以正常进行[17]。电极反应如下:
置换镉绵,
Zn+Cd2+=Zn2++Cd (1)
阳极溶解,
Zn-2e=Zn2+ (2)
Zn+H+=Zn2++H2 (3)
正交试验采用直接电解的方法,电解时采用可溶性阳极,即含Zn含量>99%的锌片,阴极为长方形钛网,不加添加剂。主要考察pH值、极距、电流密度、温度和阴阳极面积比等工艺条件对提镉率的影响。选取5因素4水平的L16(45)正交试验表,共进行16组试验:具体的实验安排如表2所列。
表2 电置换提镉正交试验因素水平表
Table 2 Factor levels of orthogonal test of electrical replacement for Cd extraction

根据表2安排进行了16组实验:量取一定体积的一次浸出液于烧杯中,置于恒温加热器中加热到指定温度,通电电解1 h后刮下阴阳极板上的沉积物,洗净、真空干燥,用ICP(型号IRIS IntrepidⅡXSP,美国热电公司生产)分析其中的Zn和Cd含量;再取置换后液过滤,同样用ICP分析其中的Zn和Cd含量。根据分析结果按下式计算提镉率(E Cd):
 (4)
(4)
式中:c1和c2分别为电解初始和结束时溶液中的镉浓度;V1和V2分别为电解初始和结束时溶液的体积。
配制与表1中Cd2+、Zn2+浓度相同的ZnSO4+ CdSO4混合溶液;在低于90℃的温度下利用CHI660C型电化学工作站(上海辰华仪器有限公司生产)进行循环伏安曲线、线性扫描伏安曲线和塔菲尔曲线测试。
2 结果与分析
2.1 正交试验
2.1.1 正交试验结果
根据表2的试验安排进行了电置换提镉正交试验,并计算提镉率,得出正交试验结果,如表3所列。根据正交试验结果进行极差分析,以找出最优条件和各影响因素对指标影响的主次顺序。试验极差分析结果如表3所列,表中Ⅰ、Ⅱ、Ⅲ、Ⅳ对应数值为各因子水平综合值,K1、K2、K3、K4对应数值为综合平均值,R为极差。
表3 电置换镉正交试验计算结果
Table 3 Orthogonal test results of electrical replacement of Cd extraction

由表3可知,仅考虑提镉率这一指标,则本次正交试验的最优条件为A1B1C4D4E3,即pH值为1,极距3 cm,电流密度400 A/m2,温度90 ℃,阴阳极面积比1:2;各因素对提镉率影响程度从大到小的顺序为:温度、pH、极距、电流密度、阴阳极面积比。
2.1.2 正交试验结果分析
为了更直观地表示各因素水平与指标的关系,以因素水平为横坐标,指标的和值(即同一水平提镉率和的平均值)为纵坐标,绘出因素与指标关系趋势,其结果如图1所示。
从图1(a)中可以看出,随着pH值的降低,提镉率逐渐增大。酸度大加快了锌板表面的锌板溶解,锌板溶解后可露出新的反应表面,从而加快置换反应的进行,使得镉的置换率提高。然而酸度太大时,析出的镉容易“复溶”,且pH越低,镉“复溶”现象越严重。
极距对提镉率的影响如图1(b)所示,在一定范围内,提镉率随着极距的增大而减小,在极距为7 cm时,提镉率降到最低值,由于增大极距会增长离子迁移距离,降低提取率。
如图1(c)所示,随着电流密度的增大,提镉率先减小而后逐渐增大。这是因为电流密度越高,单位面积单位时间通过的电量越多,从而促使了电解液中的镉离子得到电子而析出。
5个因素中温度对镉置换的影响最显著,且随着温度的升高,提镉率逐渐增大。这是由于温度升高,电解液黏度下降且离子扩散系数增大,加快了镉离子的运动,从而使溶液中镉离子成分均匀。此外,温度升高也加速了锌板表面的溶解,同时加快化学反应速率。但温度继续升高时,提镉率增加不明显,海绵镉纯度却急剧下降,同时温度升高,溶液蒸发量大,锌板消耗多,生产成本高。此外,由于温度升高,氢的超电压降低,在置换的同时会使析出的氢增多,影响锌置换镉的反应,因此,温度不宜过高。
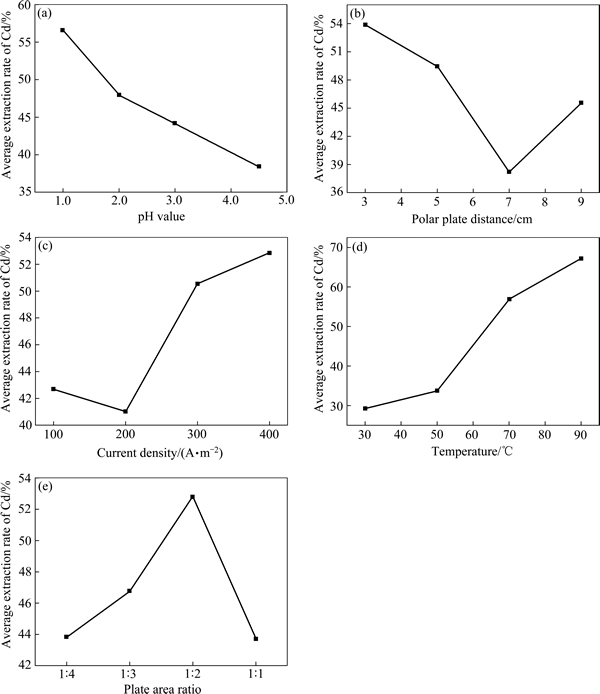
图1 电置换过程各因素与提镉率的关系
Fig. 1 Relationship among effect factors and extraction rate of Cd for electrical replacement
由图1(e)可知,随阴阳极面积比的增大,提镉率先缓慢增加后急剧下降。一方面阳极面积增大促进了锌板置换镉;另一方面,又由于试验过程中电流密度均以最初的阳极面积所计算,故电积过程随着阳极面积的增加,实际电流密度会降低,从而又抑制了镉的电沉积。实际过程中同时存在镉的置换和镉的电积,因此,随着阴阳极面积比的增大,提镉率出现一个峰值。
2.1.3 验证试验
根据正交试验结果,在预测的最优提镉条件下进行了验证试验。即在pH=1、极距3 cm、电流密度400 A/m2、温度90 ℃、阴阳极面积比1:2下进行了3次重复试验,其结果见表4。
表4 电置换提镉验证试验结果
Table 4 Confirmation test results of electrical replacement for cadmium extraction
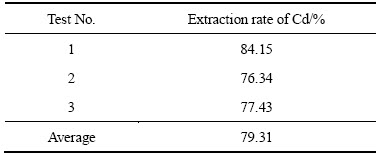
由表4可知,最优条件下所得总提镉率的平均值为79.31%,高于上述正交试验所得的提镉率,进一步证明了正交试验的正确性。正交试验所得海绵镉品位均在80%以上,直收率约78.2%,海绵镉品位与传统置换工艺(两段锌粉置换生产80%左右的粗镉)相比稍高。取验证试验所得的产品海绵镉,在真空干燥箱中烘24 h后,研磨,用孔径74 μm的筛子筛分得到的粉末进行X射线衍射分析,XRD谱如图2所示。
由图2可知,主要物相为单质镉,同时还伴有少量的Cd(OH)2和CdSO4,可能是由于冲洗不完全和在冲洗过程中酸度降低水解所引起的。说明产品纯度较高,没有其他杂峰出现。

图2 海绵镉的XRD谱
Fig. 2 XRD pattern of sponge cadmium
2.2 电化学实验
通过正交试验得到温度对提镉率的影响最显著,故将温度作为电化学实验的变量,并调节电解液pH值为最佳pH=1。
2.2.1 循环伏安测试
图3所示为93.6 g/L ZnSO4+33.82 g/L CdSO4混合溶液在温度为(70±2) ℃、扫描速率100 mV/s、扫描区间-1.19~1.80 V时的循环伏安曲线。
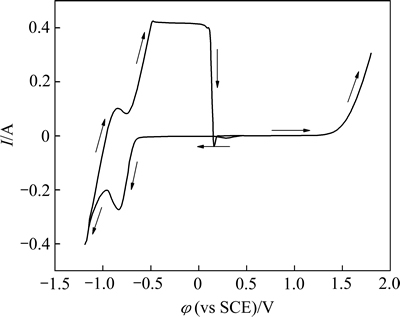
图3 93.6 g/L ZnSO4+33.82 g/L CdSO4混合溶液的循环伏安曲线
Fig. 3 CV curve of mixed solution of 33.82 g/L CdSO4+93.60 g/L ZnSO4
由图3可知,在扫描速度为100 mV/s时,由开路电位负扫至电压约-0.421 V时,电流开始增大,推断为溶液中镉离子的析出反应(Cd2++2e→Cd),同时可观察到逐渐有金属沉积在研究电极表面。当往更负电位扫至约-0.620 V时,电流快速增加,于-0.833 V处形成一阴极还原波峰。根据析出电位推断为锌离子的析出反应,并观察到持续有金属沉积在研究电极表面。当往更负电位继续扫至约-0.958 V时,观察到研究电极表面产生大量气泡,推断为氢气生成(2H++2e→H2)的反应电流波形。正向回扫过程于φp,a=-0.841 V处有一阳极氧化峰,与锌的析出峰近似对称,同时可观察到铂片上的沉积物开始逐渐溶解,推断此反应为沉积的锌发生返溶(Zn→Zn2++2e)。继续正扫则形成一与镉的析出峰近似对称的阳极峰,同时也可观察到铂片表面的沉积物继续溶解进入溶液,推断此处反应为沉积的镉也发生了溶解(Cd→Cd2++2e)。随后,当电位扫至约1.256 V时,观察到铂电极表面产生大量气泡出现,推断为氧气生成反应(2H2O→O2+4H++4e)的电流波形。
图4所示为混合溶液在不同温度下扫描速率为30 mV/s、扫描区间-1~1.80 V时的循环伏安曲线。
由图4可知,不同温度下混合溶液中镉、锌的析出电位略有变化,具体数值见表5。

图4 不同温度下CdSO4+ZnSO4混合溶液的循环伏安曲线
Fig. 4 CV curves of mixed solution of CdSO4+ZnSO4 at different temperatures
表5 镉、锌的析出电位与温度的关系
Table 5 Relationship between deposition potential of Cd, Zn and temperature

根据表5数据按下式计算:
 (5)
(5)
其中:E1和E2分别为镉和锌的析出电位,V;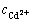 为锌析出时溶液中的镉浓度,mol/L。计算结果如表6所列。
为锌析出时溶液中的镉浓度,mol/L。计算结果如表6所列。
表6 不同温度下锌开始析出时溶液中的Cd2+浓度
Table 6 Concentration of Cd2+ in solution when zinc depositing at different temperature
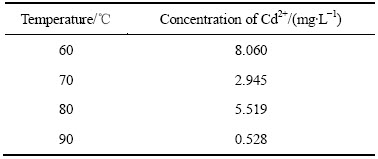
由表6可知,在锌析出前溶液中镉离子浓度已经降到很低,且随着温度的升高,Cd2+的终点浓度呈降低的趋势,说明温度越高,镉在阴极沉积越完全。
2.2.2 线性扫描伏安测试
图5所示为混合溶液在不同温度下扫描速率为30 mV/s时测得的线性扫描伏安曲线。
由图5(a)可知,随温度升高,阳极析氧起始电位逐渐负移,同一电位下阳极反应的电流增加,说明阳极腐蚀速度加快。由图5(b)可以看到,随温度升高,阴极析出起始电位正移,且波峰越来越不明显,温度90 ℃时,锌离子的还原波峰与氢气生成的反应重叠,导致两者不宜区分。随温度的升高,同一电位下阴极反应的电流值也呈增加的趋势。
温度对镉电化学行为的影响,主要体现在电化学反应过程。电化学反应控制步骤是极化过程时,有如下关系式[18]:
 (6)
(6)
式中:J为电流密度,A/m2;E为表观活化能,J/mol;T为温度,K。对阴极反应不同温度下的lgJ与T -1作图并拟合,其曲线如图6所示。
图6经线性回归(R=0.9861)得到:
 (7)
(7)
结合式(6)和式(7)可以计算表观活化能E=6.379kJ/mol。从表观活化能来看,阴极反应在该扫描电位范围内属于浓度极化控制。
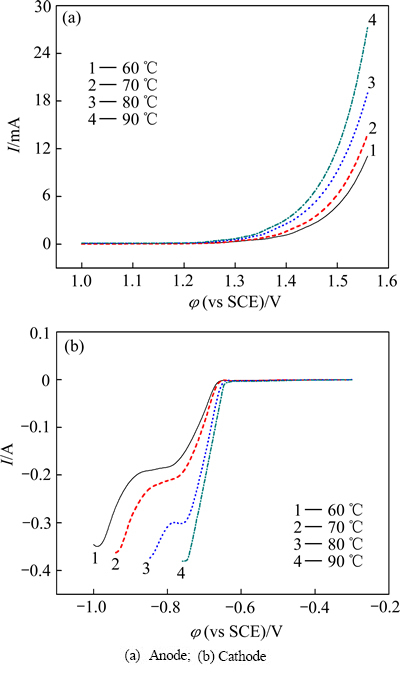
图5 不同温度下33.82 g/L CdSO4+93.60 g/L ZnSO4混合溶液的线性扫描伏安曲线
Fig. 5 LSV curves of mixed solution of 33.82 g/L CdSO4+ 93.60 g/L ZnSO4 under different temperatures

图6 阴极反应的lgJ-T -1曲线
Fig. 6 Curves of lgJ-T -1 during cathodic reaction
2.2.3 Tafel测试
Tafel曲线由开路电位往两边依次为线性区、弱极化区和塔菲尔区。塔菲尔区又称为强极化区,当极化足够大,即η≥120/n=60 mV(n为转移电子数)时,符合Tafel公式[19]:
 (8)
(8)
图7所示为CdSO4+ZnSO4混合溶液在不同温度下扫描速率为5 mV/s时测得的Tafel曲线。

图7 不同温度下33.82 g/L CdSO4+93.60 g/L ZnSO4混合溶液的Tafel曲线
Fig. 7 Tafel curves of mixed solution of 33.82 g/L CdSO4+93.60 g/L ZnSO4 under different temperatures
从图7可以看出,电位在-0.11~-0.01 V之间时,曲线呈较好的直线关系,直线经线性回归(Rl=0.99)并结合式(7)求得各温度下的传递系数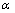 和交换电流密度J0,其结果如表7所列。
和交换电流密度J0,其结果如表7所列。
表7 各温度下阴极反应的传递系数 和交换电流密度J0
和交换电流密度J0
Table 7 Transfer coefficient and exchange current density J0 of cathodic reaction under different temperatures
and exchange current density J0 of cathodic reaction under different temperatures

同理,电位在0.17~0.27 V之间时求得各温度下的传递系数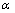 和交换电流密度J0,其结果如表8所列。
和交换电流密度J0,其结果如表8所列。
由表7和8可知,随温度的升高,阴阳极交换电流密度逐渐增大;而传递系数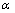 也随之发生波动。
也随之发生波动。
表8 各温度下阳极反应的传递系数 和交换电流密度J0
和交换电流密度J0
Table 8 Transfer coefficient and exchange current density J0 of anodic reaction under different temperatures
and exchange current density J0 of anodic reaction under different temperatures

3 结论
1) 正交试验及其验证试验,得出了微电流作用下提取海绵镉的最佳工艺条件为pH值为1、极距3 cm、电流密度400 A/m2、温度90 ℃、阴阳极面积比1:2,最优条件下电置换1 h总提镉率达到79.31%。各因素对提镉率影响的大小顺序依次为温度、pH、极距、电流密度、阴阳极面积比。本工艺中得到的海绵镉品位在80%以上,与传统置换工艺相比稍高。海绵镉的X射线衍射分析也表明产品纯度较高,得到海绵镉主要物相为镉单质。
2) 镉、锌在阴极的沉积均先于氢气的析出,且金属镉先于锌在阴极沉积。对CdSO4+ZnSO4混合溶液循环伏安测试表明,锌开始析出时,溶液中镉离子浓度已经降到很低,最低可降到0.528 mg/L,且随温度的升高,呈降低的趋势。
3) 混合溶液阴极沉积的表观活化能为6.379 kJ/mol,在60~90 ℃之间属于扩散步骤控制。镉和锌在阴极沉积的交换电流密度随温度升高逐渐增大,传递系数也随温度发生一些波动。
REFERENCES
[1] JU Shao-hua, LU Shuai-dan, PENG Jin-hui, ZHANG Li-bo, SRINIVASAKANNAN C, GUO Sheng-hui, LI Wei. Removal of cadmium from aqueous solutions using red mud granulated with cement[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(12): 3140-3146.
[2] 邱运仁, 郜国英, 刘 敏. 络合-超滤技术处理含镉废水[J]. 中国有色金属学报, 2011, 21(8): 2012-2016.
QIU Yun-ren, GAO Guo-ying, LIU Min. The treatment of wastewater containing cadmium by complexation-ultrafiltration [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(8): 2012-2016.
[3] ZHANG Xiao-xi, CHAI Li-yuan, TANG Jian-xin, LIU Xue-duan, YANG Zhi-hui. Taxonomy characterization and cadmium biosorption of fungus strain[J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 2759-2765.
[4] 刘 远, 郑雅杰, 孙召明. 锌冶炼含镉烟尘制备高纯镉粉的新工艺[J]. 中国有色金属学报, 2014, 24(4): 1070-1075.
LIU Yuan, ZHENG Ya-jie, SUN Zhao-ming. The new process of preparing high purity cadmium powder by dust containing cadmium produced in zinc smelter[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(4): 1070-1075.
[5] 梅光贵. 湿法炼锌学[M]. 长沙: 中南大学出版社, 2001.
MEI Guang-gui. The zinc hydrometallurgy[M]. Changsha: Central South University Press, 2001.
[6] 邓小华. 铜镉渣中回收金属锌、铜、镉的研究[D]. 上海: 同济大学, 2005.
DENG Xiao-hua. Recovery of zinc, copper, cadmium from copper and cadmium sediment[D]. Shanghai: Tongji University, 2005.
[7] 梁龙伟. 湿法炼锌新三段净化工艺研究[D]. 昆明: 昆明理工大学, 2012.
LIANG Long-wei. Process research of new three-segments purifying in the zinc hydrometallurgy[D]. Kunming: Kunming University of Technology, 2012.
[8] 陈建荣, 林建军, 吴小华, 袁欣星, 周静芬. N503萃淋树脂吸附镉的研究[J]. 中国有色金属学报, 1998, 8(3): 507-510.
CHEN Jian-rong, LIN Jian-jun, WU Xiao-hua, YUAN Xin-xing, ZHOU Jing-fen. The research of cadmium adsorption of N503 extraction leaching resin[J]. The Chinese Journal of Nonferrous Metals, 1998, 8(3): 507-510.
[9] 莫建军, 熊春华. 亚胺基二乙酸树脂对镉的吸附性能及其机理[J]. 中国有色金属学报, 2006, 16(5): 924-928.
MO Jian-jun, XIONG Chun-hua. The cadmium adsorption properties and mechanism of iminodiacetic acid resin[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(5): 924-928.
[10] REDDY B R, PRIYA D N, KUMAR J R. Solvent extraction of cadmium (Ⅱ) from sulphate solutions using TOPS 99, PC 88A, Cyanex 272 and their mixtures[J]. Hydrometallurgy, 2004, 74: 277-283.
[11] JHA M K, GUPTA D, CHOUBEY P K, KUMAR V, JEONG J, LEE J. Solvent extraction of copper, zinc, cadmium and nickel from sulfate solution in mixer settler unit (MSU)[J]. Separation and Purification Technology, 2014, 122(10): 119-127.
[12] 黄炳辉, 张仲甫, 汪德先. 用液膜技术提取镉的研究[J]. 膜科学与技术, 1989, 9(2): 56-63.
HUANG Bin-hui, ZHANG Zhong-fu, WANG De-xian. Study on handling of wastewater containing cadmium using liquid membrane[J]. Membrane Science and Technology, 1989, 9(2): 56-63.
[13] 汤 兵, 简弃非, 万印华, 王向德, 张秀娟. 液膜结晶法提取高锌低镉体系中镉的研究[J]. 膜科学与技术, 1999, 19(1): 24-30.
TANG Bing, JIAN Qi-fei, WAN Yin-hua, WANG Xiang-de, ZHANG Xiu-juan. Recovering cadmium in the system of high concentration zinc and low concentration cadmium by liquid membrane crystallizing technique[J]. Membrane Science and Technology, 1999, 19(1): 24-30.
[14] 刘雪岩, 杨丽君, 金燕利, 张 蕾, 徐天赐, 李 娜. 纳米TiO2对镉(Ⅱ)的吸附性能[J]. 中国有色金属学报, 2011, 21(11): 2971-2977.
LIU Xue-yan, YANG Li-jun, JIN Yan-li, ZHANG Lei, XU Tian-ci, LI Na. The cadmium(Ⅱ) adsorption properties of nanometer TiO2[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(11): 2971-2977.
[15]  . Adsorption of lead and cadmium ions from aqueous solutions using manganoxide minerals[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(12): 3131-3139.
. Adsorption of lead and cadmium ions from aqueous solutions using manganoxide minerals[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(12): 3131-3139.
[16] MA Y Q, LIN C X, JIANG Y H, LU W Z, SI C H, LIU Y. Competitive removal of water-borne copper, zinc and cadmium by a CaCO3-dominated red mud[J]. Journal of Hazardous Materials, 2009, 172(2/3): 1288-1296.
[17] SAFARZADEH M S, MORADKHANI D. The electrowinning of cadmium in the presence of zinc[J]. Hydrometallurgy, 2010, 105(1/2): 168-171.
[18] 杨声海. Zn(Ⅱ)-NH3-NH4Cl-H2O体系制备高纯锌理论及应用[D]. 长沙: 中南大学, 2003.
YANG Sheng-hai. Theory and application studies on preparing high purity zinc in the system of Zn(Ⅱ)-NH3-NH4Cl-H2O[D]. Changsha: Central South University, 2003.
[19] 田昭武. 电化学研究方法[M]. 北京: 科学出版社, 1984.
TIAN Zhao-wu. Electrochemical research techniques[M]. Beijing: Science Press, 1984.
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51174240);湖南省科技重大专项(2012FJ1010)
收稿日期:2014-08-11;修订日期:2014-11-07
通信作者:何 静,教授;电话:0731-88830470;E-mail: he6213@163.com