DOI: 10.11817/j.ysxb.1004.0609.2020-39520
闪锌矿加压浸出体系下Mn2+催化机理与动力学
陈丽杰1, 2,龚 傲1, 2,吴选高1, 2,刘 燕1, 2,张廷安1, 2,田 磊1, 2
(1. 江西理工大学 绿色冶金与过程强化研究所,赣州 341000;
2. 东北大学 多金属共生矿生态化冶金教育部重点实验室,沈阳 110819)
摘 要:研究了闪锌矿加压搅拌浸出体系下Mn2+催化机理与动力学。将ZnS和FeS烧结制备人造闪锌矿,并用于加压酸浸实验。通过使用自行设计的电位高压釜研究压力浸出系统的电位变化。结果表明:随着浸出时间的推移直到浸出结束,体系电位持续升高,故Mn2+在多大程度上能够实现催化作用,取决于Mn2+氧化为MnO2的反应速度和控制条件。随着温度、硫酸浓度、氧分压的增大,相应的氧压浸出速率及浸出率均有较大的提高,最终,通过闪锌矿氧压酸浸动力学模型的分析得出,在Mn2+催化浸出体系下,反应活化能为27.34 kJ/mol,硫酸浓度、氧分压及Mn2+用量的反应级数分别为1.23、1.64和0.36,表明其应遵循化学反应及表面扩散混合控制的收缩核模型,并建立了相应的动力学方程。
关键词:Mn2+催化机理;人造闪锌矿;浸出动力学;加压浸出;体系电位
文章编号:1004-0609(2020)-09-2151-11 中图分类号:TF813 文献标志码:A
闪锌矿具有共价键晶格结构,其溶解度非常低,因此,闪锌矿的加压浸出动力学非常缓慢[1-4]。然而,在合适的催化剂存在下,浸出速率可以显着提高[5-7]。硫化物通常是半导体,浸出过程本质上是电化学反 应[8-9],其中,闪锌矿中的晶格缺陷和阳离子杂质对电化学反应过程中的浸出动力学显著的影响[10],因此,固溶体中的杂质包括矿相和离子,对加压浸出过程是非常重要的。在正常情况下,闪锌矿中的Fe2+、Cu2+和Mn2+不仅可以起到载O2的作用,还可以参与原电池的形成,促进价金属的浸出。在浸出过程中,Fe2+、Cu2+和Mn2+的浓度和扩散速率决定了Zn的浸出率。在足够的O2和硫酸浓度条件下,Fe2+、Cu2+、Mn2+和其他阳离子在反应体系中达到动态平衡,起到催化剂和氧化剂的作用[11-14]。
许多研究人员研究了不同金属离子对硫化物矿物溶解的催化作用[15-17],GHOSH等[18]使用Cu2+作为氧化催化剂研究了闪锌矿在氨中的浸出动力学。结果表明,Cu2+具有良好的催化作用,反应受化学表面反应过程控制,表观活化能为48.3 kJ/mol,O2分压、氨浓度和Cu2+浓度的表观反应级数分别为0.2、0.3和0.3。Cu2+的催化作用归因于氧化还原电子对Cu2+/Cu+,其中铜胺[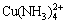 ]氧化闪锌矿得到Zn2+和还原态亚铜胺[Cu(NH3)2+],接着还原态亚铜胺[Cu(NH3)2+]被溶解O ([O]S)氧化。SCOTT等[19]研究了Cu、Bi、Ru、Mo和Fe对ZnS加压浸出条件下的催化作用。结果表明,Ag、Hg、Pb和Sn等金属虽然能从矿相中置换出Zn,但对提高ZnS加压浸出的浸出率和浸出动力学上并无帮助,因此,Cu2+和Bi2+等离子作为催化剂应满足两个条件:1) 催化剂离子必须掺入金属硫化物晶格的表面层中;2) 催化剂离子必须是能够形成氧化还原电子,并能够很容易催化转移硫化物上的电子到氧化剂上去。BALLESTER等[20]研究了Cu2+对闪锌矿浸出的催化作用。结果表明,Cu2+对酸溶液闪锌矿浸出的催化作用可以用浸出反应模型来解释。在不存在Cu2+的情况下,闪锌矿的氧化浸出是缓慢的,因为在矿物表面上形成致密的元素S层阻滞了反应的进行。然而,在Cu2+存在下,浸出溶液具有更高的导电性,促进电子通过闪锌矿表面的传输,这加速了有价值金属的溶解。郑国渠等[21]研究了在高锰硫酸锌(30 g/L Mn2+)溶液中电解提取Zn的影响因素。研究发现,在酸性溶液中,Mn可以以三价离子形式稳定存在,因此推断在闪锌矿加压浸出过程中离子Mn2+/Mn3+催化效果可能更直接有效。MULAK等[22]研究了在Cu2+和Fe3+存在的条件下Ni3S2溶解的机理。结果表明,由于中间产物(金属铜和硫化铜)的不溶性,在60 ℃以下的2.0 mol/L HNO3溶液中没有Cu2+的催化作用。在80 ℃时,Cu2+或Fe3+参与硫化物离子的氧化,形成比H2S气体更快被HNO3氧化的高活性中间产物(铜铁的硫化物)。Cu2+和Fe3+的催化作用主要表现在相应的高活性中间产物的生成,它们可以迅速被HNO3氧化浸出,从而再生继续进行催化反应。
]氧化闪锌矿得到Zn2+和还原态亚铜胺[Cu(NH3)2+],接着还原态亚铜胺[Cu(NH3)2+]被溶解O ([O]S)氧化。SCOTT等[19]研究了Cu、Bi、Ru、Mo和Fe对ZnS加压浸出条件下的催化作用。结果表明,Ag、Hg、Pb和Sn等金属虽然能从矿相中置换出Zn,但对提高ZnS加压浸出的浸出率和浸出动力学上并无帮助,因此,Cu2+和Bi2+等离子作为催化剂应满足两个条件:1) 催化剂离子必须掺入金属硫化物晶格的表面层中;2) 催化剂离子必须是能够形成氧化还原电子,并能够很容易催化转移硫化物上的电子到氧化剂上去。BALLESTER等[20]研究了Cu2+对闪锌矿浸出的催化作用。结果表明,Cu2+对酸溶液闪锌矿浸出的催化作用可以用浸出反应模型来解释。在不存在Cu2+的情况下,闪锌矿的氧化浸出是缓慢的,因为在矿物表面上形成致密的元素S层阻滞了反应的进行。然而,在Cu2+存在下,浸出溶液具有更高的导电性,促进电子通过闪锌矿表面的传输,这加速了有价值金属的溶解。郑国渠等[21]研究了在高锰硫酸锌(30 g/L Mn2+)溶液中电解提取Zn的影响因素。研究发现,在酸性溶液中,Mn可以以三价离子形式稳定存在,因此推断在闪锌矿加压浸出过程中离子Mn2+/Mn3+催化效果可能更直接有效。MULAK等[22]研究了在Cu2+和Fe3+存在的条件下Ni3S2溶解的机理。结果表明,由于中间产物(金属铜和硫化铜)的不溶性,在60 ℃以下的2.0 mol/L HNO3溶液中没有Cu2+的催化作用。在80 ℃时,Cu2+或Fe3+参与硫化物离子的氧化,形成比H2S气体更快被HNO3氧化的高活性中间产物(铜铁的硫化物)。Cu2+和Fe3+的催化作用主要表现在相应的高活性中间产物的生成,它们可以迅速被HNO3氧化浸出,从而再生继续进行催化反应。
上述已经提出了几种机制来解释闪锌矿加压浸出过程中催化机理,包括可以作为“氧载体”的氧化还原电子对和生成高活性硫化中间产物的金属阳离子,其将电子从S2-转移到氧化剂上去。但在上述文献中,氧化还原电子对的催化理论与Mn2+催化剂体系中闪锌矿压力浸出的机理研究的还不够深入。因此,不能排除加压浸出过程闪锌矿中众多杂质阳离子的干扰,而且不能获得氧化还原电子对在加压浸出过程中的电位变化规律。鉴于此,在本研究中,使用人造闪锌矿排除杂质离子的干扰,并使用自制的电位高压釜进行体系电位的测定,通过实验来阐明浸出系统中Mn2+的催化机理和电位变化规律,并考虑了几个变量对加压浸出过程的动力学影响。研究结果有助于提高闪锌矿中Zn及其他有价金属的回收率,并为实现复杂伴生矿的综合利用提供基础数据。
1 实验
1.1 实验原料
闪锌矿中的硫化锌为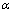 型;因此,为了获得人造闪锌矿(
型;因此,为了获得人造闪锌矿( -ZnS),将
-ZnS),将 -ZnS(分析试剂)置于高温管式炉中,并通入惰性气体后升温,固态烧结的温度和时间分别为1223 K和120 min,为了得到均匀的物相,烧结结束后继续在1123 K温度下保温60 min。最终得到的人造闪锌矿的XRD谱如图1所示。
-ZnS(分析试剂)置于高温管式炉中,并通入惰性气体后升温,固态烧结的温度和时间分别为1223 K和120 min,为了得到均匀的物相,烧结结束后继续在1123 K温度下保温60 min。最终得到的人造闪锌矿的XRD谱如图1所示。
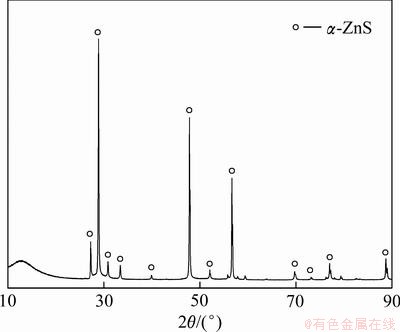
图1 人造闪锌矿的XRD谱
Fig. 1 XRD pattern of artificial sphalerite
1.2 实验设备
氧压浸出实验在FCFD 2-1.0型电位高压釜中进行(一种可在线测量加压湿法体系电位的高压反应釜,专利申请号2015106179797)。釜体是锆材制造,容积为2000 mL,最大压力可达6.0 MPa,最高温度可以达到573 K。FCFD 2-1.0型电位高压釜的示意图和实物图分别见图2和3。
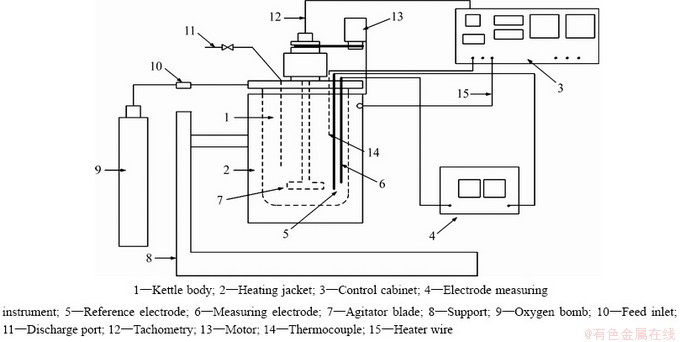
图2 电位高压釜示意图
Fig. 2 Device attachment of potential autoclave
1.3 实验步骤
首先,将800 mL水溶液、20.0 g人造闪锌矿和0.3 g木质素磺酸钙加入到电位高压釜中并加热至设定温度。接着用氧气瓶中的氧气将200 mL硫酸溶液和MnSO4溶液压入电位高压釜中; 当温度达到设定点并且在反应期间以500 r/min搅拌搅拌器叶片时,通过电位计记录电位变化。
然后,以适当的时间间隔取出5 mL浸出溶液,并使用电感耦合等离子体(ICP)发射光谱仪(Leeman,USA)分析Zn2+的浓度。 实验流程图如图4所示。
有价元素的浸出率计算如下:
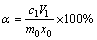 (1)
(1)
式中:c1是滤液中有价元素的浓度,g/L;V1是滤液的体积,L;m0是实验中使用的样品质量,g;x0是样品中有价元素的质量分数,%。
2 结果与讨论
2.1 Mn2+氧化还原催化浸出机理预测
Mn2+以氧化还原形式参与到闪锌矿氧压浸出过程的化学方程式见式(2)和(3):
MnO2+2H++H2S→Mn2++2H2O+S (2)
Mn2++H2O+1/2O2→MnO2+2H+ (3)
图3 FCFD 2-1.0型电位反应釜
Fig. 3 FCFD 2-1.0 potential autoclave
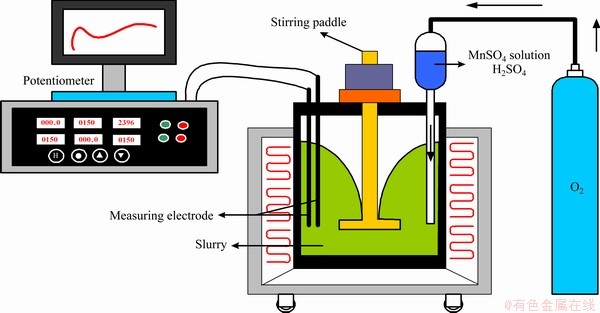
图4 人造闪锌矿在加压浸出过程中Mn2+催化的流程图
Fig. 4 Flowchart of Mn2+ catalyzed in O2-pressure acid leaching of artificial sphalerite
Mn-Zn-S-H2O系的φ-pH图如图5所示。Mn元素也能实现类似的氧化还原过程及Mn4+与Mn2+之间的相互转化,从而实现阳离子氧化还原催化,但相较而言,浸出体系中Mn4+是以MnO2形式存在的,在溶解氧作用下Mn4+与Mn2+的相互转化较为困难。
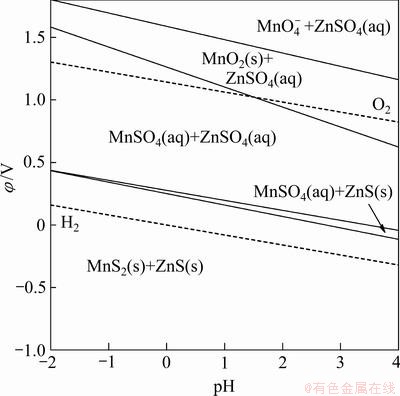
图5 Mn-Zn-S-H2O系φ-pH图
Fig. 5 φ-pH figure of Mn-Zn-S-H2O
Mn2+催化体系下人造闪锌矿颗粒氧压浸出的示意图如图6所示。在纯ZnS颗粒的浸出过程中,H2SO4先与ZnS发生反应生成H2S和Zn2+,然后H2S中的S2-被硫酸溶液中的溶解氧[O]S氧化为单质硫,H+被释放后继续与ZnS反应,但在此浸出过程中,氧气溶解进入浸出液中的速度非常缓慢,大幅降低了反应的浸出速率。
在Mn2+催化体系中,H2SO4先与ZnS发生反应生成H2S和Zn2+,H2S中的S2-被Mn4+氧化为单质硫,H+被释放后继续与ZnS反应,Mn4+则被还原为Mn2+。而硫酸溶液中Mn2+被溶解氧[O]S氧化为Mn4+,重新回到体系中继续参加氧化还原反应。在此浸出过程中,Mn2+被硫酸溶液中溶解氧[O]S的氧化速度与S2-被硫酸溶液中Mn4+的氧化速度都非常快,这就大幅地提高了反应的总浸出速率。氧化还原电子对Mn2+/Mn4+的形成实现催化剂的循环,即所谓的变价离子原电池催化过程。
2.2 Mn2+氧化还原催化体系相对电位变化分析
为更好地研究浸出过程中Mn2+的催化行为,本研究在温度403 K、硫酸浓度110 g/L、氧分压0.8 MPa、Mn2+的添加量0.05 mol、搅拌转速500 r/min的条件下,考察了Mn2+对人造闪锌矿富氧酸浸过程的相对电位变化。描绘了Mn2+对人造闪锌矿富氧酸浸体系的电位变化过程。
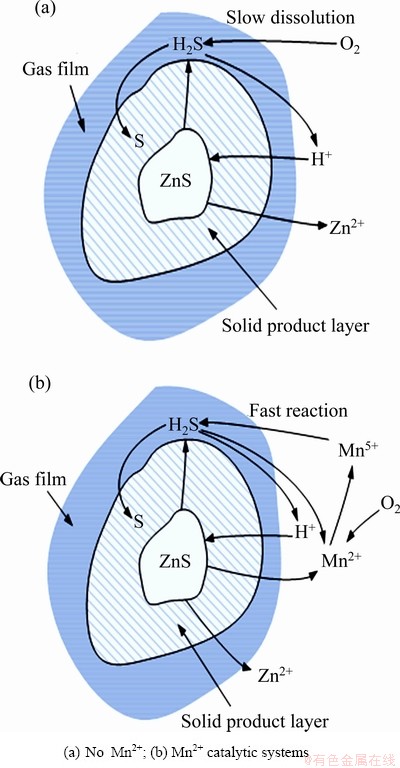
图6 Mn2+催化体系下人造闪锌矿颗粒氧压浸出的示意图
Fig. 6 Schematic diagram of oxygen pressure leaching artificial sphalerite particles in Mn2+ catalytic systems
由图7(a)可以看出:1) 加入硫酸、通入氧气后,电势急剧升高,虽然有一部分H+被消耗生成H2S,但初始酸溶过程较为困难,更为重要的是,因为没有如Fe3+/Fe2+、Mn2+/MnO2电子对的存在,溶解氧无法被大量消耗,因为在矿物表面生成H2S气膜的破坏是浸出过程持续进行的动力学基础,生成单质硫的过程必然很难进行,这也解释了电势不会下降,是因为H2S气膜氧化破坏反应的困难;2) 浸出开始阶段,H2S被溶解氧缓慢氧化,体系电势逐渐升高;3) 浸出后期,基本达到浸出平衡状态。
由图7(b)可以看出,Mn2+氧化还原催化体系的电位变化趋势反而与图7(a)人造闪锌矿的氧压浸出过程相似。表明溶解氧氧化Mn2+为MnO2的化学反应过程较为缓慢,但随着浸出时间的推移直到浸出结束,体系电位持续升高,这是由于溶解氧氧化Mn2+的反应在持续进行,而生成的MnO2能够较为快速地破坏矿物表面生成的H2S气膜层。从整体上来看,由于氧化反应的持续进行(溶解氧能够得到不断补充),体系电位是逐渐升高的,但在局部区域也存在着MnO2氧化H2S,造成体系电位的降低,使得在整个浸出周期均会出现表征氧化还原反应的振荡波形的出现。综合以上分析,在Mn2+氧化还原催化体系中,浸出过程的控制步骤为Mn2+氧化为MnO2的化学反应,实现了控制步骤的转移,因此,Mn2+在多大程度上能够实现催化作用,取决于Mn2+氧化为MnO2的反应条件。
2.3 闪锌矿氧压酸浸动力学方程
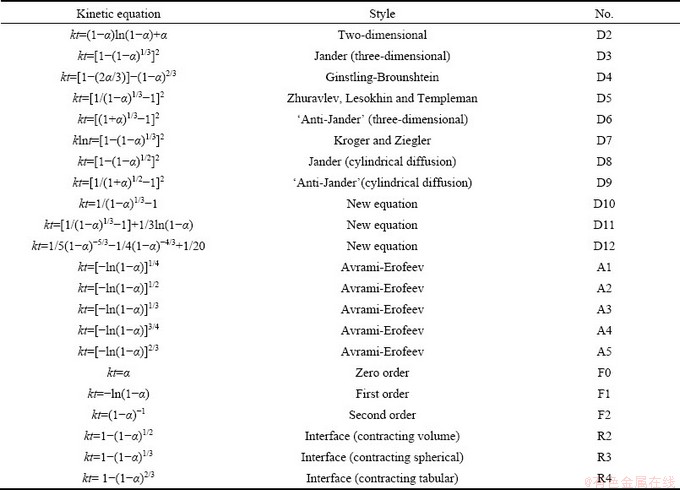
不同的液固反应的动力学方程见表1。其中,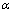 为浸出率,k为反应速率常数,t为反应时间。
为浸出率,k为反应速率常数,t为反应时间。
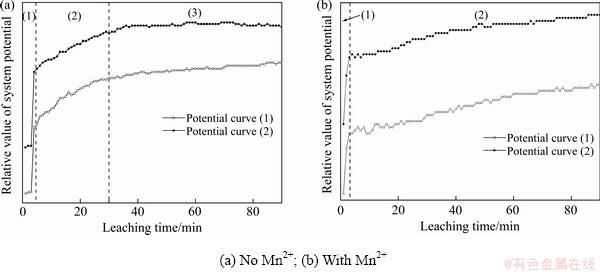
图7 闪锌矿氧压浸出体系过程电势变化
Fig. 7 Potential change of catalytic systems in oxygen pressure leaching process
表1 不同液固反应的动力学方程
Table 1 Kinetic equations of different dynamic reaction
2.3.1 温度影响及活化能的求解
以人造闪锌矿为原料,将MnSO4(N=0.05 mol)以硫酸盐溶液的形式通过后加料阀加入到浸出体系,在硫酸浓度(A)110 g/L、氧分压(p)0.8 MPa条件下,研究了不同温度下(温度为363、383、403、423 K)浸出90 min内Zn的浸出率,如图8所示。
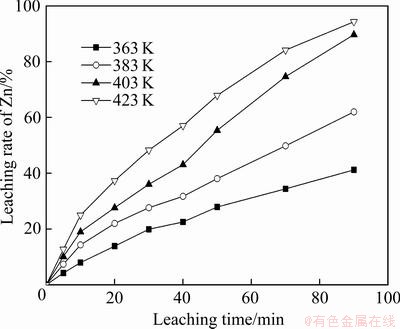
图8 反应温度对Zn的浸出率的影响
Fig. 8 Effect of reaction temperature on leaching rate of zinc
为了确定动力学方程,将不同温度下Zn的浸出率代入表2中,其拟合的相关系数见表2。
表2 不同温度下动力学方程拟合的相关系数
Table 2 Result of linear correlation of each kinetic equation at different temperatures
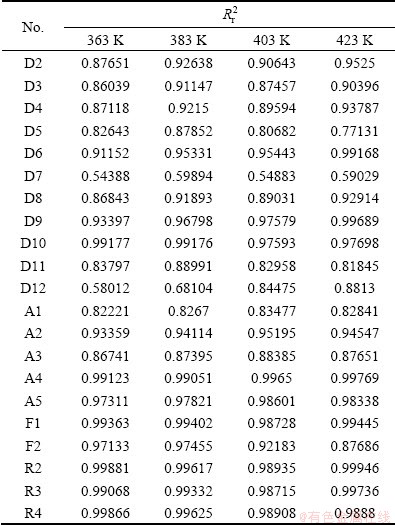
从表2中各方程拟合的相关系数可以看出,R2、R3、R4的拟合相关系数的线性关系非常好,但是根据人造闪锌矿的电镜图谱可以看出,人造闪锌矿的颗粒呈类球状 (见图9),故取R3用来分析人造闪锌矿氧压酸浸的行为。

图9 人造闪锌矿的SEM像
Fig. 9 SEM image of artificial sphalerite
因此,对于Mn2+氧化还原催化体系,可以设定动力学模型方程为
 (4)
(4)
式中: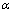 为富氧浸出过程中金属的浸出率,%;k0为浸出反应的动力学速率前指因子;Ea为浸出体系反应活化能,kJ/mol;R为摩尔气体常数,8.314 J/(mol·K);T、A、p、N分别为浸出温度、硫酸浓度、氧分压、Mn2+的物质的量;rn、ra、rp分别为催化剂用量、硫酸浓度、氧分压的反应级数;t为浸出反应时间,min。
为富氧浸出过程中金属的浸出率,%;k0为浸出反应的动力学速率前指因子;Ea为浸出体系反应活化能,kJ/mol;R为摩尔气体常数,8.314 J/(mol·K);T、A、p、N分别为浸出温度、硫酸浓度、氧分压、Mn2+的物质的量;rn、ra、rp分别为催化剂用量、硫酸浓度、氧分压的反应级数;t为浸出反应时间,min。
图10(a)给出了经过浸出模型拟合后的拟合直线并得到不同温度下4条直线的斜率kT,图10(b)给出了ln(kT)与1/T的拟合直线,线性拟合的相关系数在图中标出。根据阿伦尼乌斯公式(见式(5))和图10(b)中拟合直线的斜率,可求出此条件下的活化能:
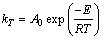 (5)
(5)
式中:kT是化学反应速率常数;A0为指前因子;E为活化能,kJ/mol;T为反应温度,K;R摩尔气体常数,8.314 J/(mol·K)。
代入k=-3288.29,求得E=27.34 kJ/mol,根据CHRISTOPHER等[23]的研究结果,针对于闪锌矿氧压浸出动力学,活化能在24~27 kJ/mol之间处于扩散与化学反应混合控制,大于27 kJ/mol则一般可认为化学反应是限速步骤。所以Mn2+催化条件下人造闪锌矿氧压浸出的限速步骤为矿物界面H2S气膜氧化化学反应。
2.3.2 硫酸浓度影响及硫酸反应级数的求解
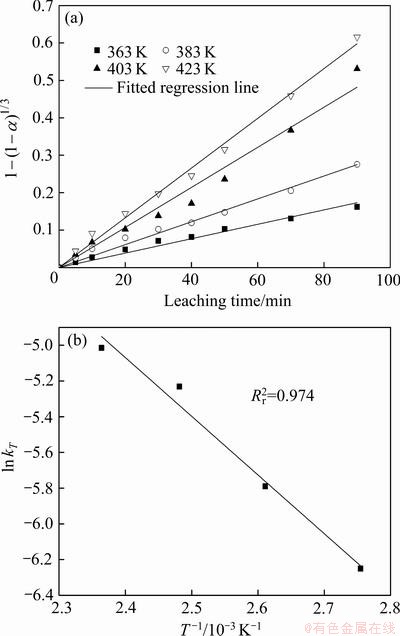
图10 在Mn2+催化体系中不同温度下闪锌矿中Zn浸出率和ln kT-1/T
Fig. 10 Leaching rate of zinc(a) and ln kT-1/T(b) of sphalerite at different temperatures in Mn2+ catalytic systems
以人造闪锌矿为原料,将MnSO4(N=0.05 mol)以硫酸盐溶液的形式通过后加料阀加入到浸出体系,在温度(T)403 K、氧分压(p)0.8 MPa条件下,研究在不同硫酸浓度下(A为50、80、110、140 g/L),浸出90 min内Zn的浸出率及动力学分析,如图11所示。
图11(a)所示为不同硫酸浓度下Zn浸出率随时间的变化曲线,图11(b)所示为经过浸出模型拟合后的拟合直线并得到不同硫酸浓度下4条直线的斜率kA,图11(c)所示为ln(kA)与ln[H2SO4]的拟合直线,并得到其斜率求出活化能,线性拟合的相关系数在图中标出。基于动力学模型进行拟合给出硫酸浓度的反应级数为1.23,这与自析出催化体系中等Fe含量体系一致,这也部分佐证了Mn氧化还原催化浸出闪锌矿的动力学模型不应该只归因于化学反应控制,在一定程度上也应该考虑扩散控制。
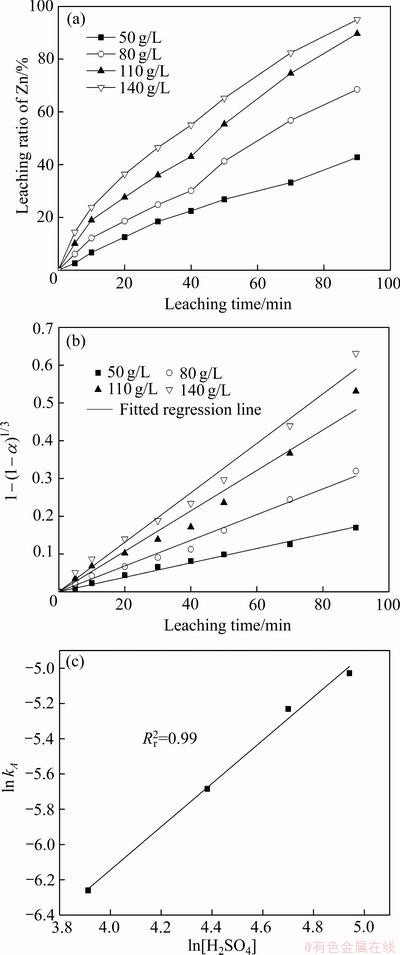
图11 不同硫酸浓度A下Mn阳离子氧化还原催化闪锌矿时Zn浸出率、浸出率线性拟合及ln kA-ln [H2SO4]
Fig. 11 Zn leaching rate(a), linear fitting of leaching rate(b) and ln kA-ln [H2SO4](c) of sphalerite at different H2SO4 concentrations in Mn2+ catalytic systems
2.3.3 氧分压影响及氧压反应级数的求解
以人造闪锌矿为原料,将MnSO4(N=0.05 mol)以硫酸盐溶液的形式通过后加料阀加入到浸出体系,在温度(T)403 K、硫酸浓度(A)110 g/L时,探究了在不同硫酸浓度和氧分压(p为0.4、0.6、0.8、1.0 MPa)浸出90 min内Zn的浸出率及动力学分析,如图12所示。
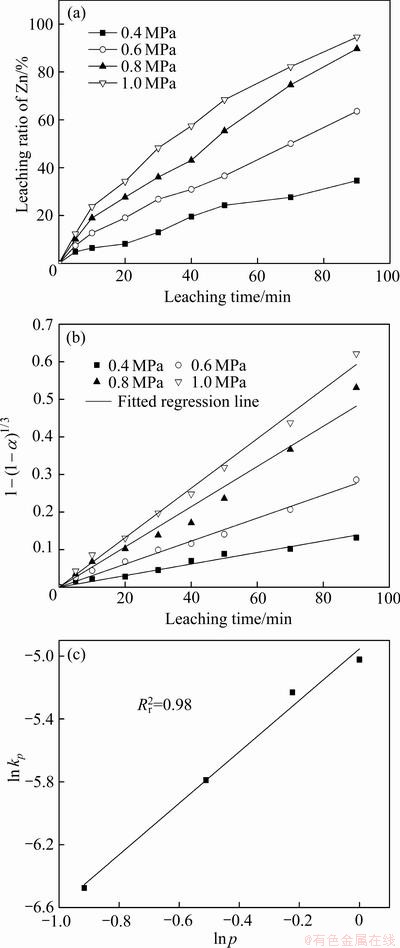
图12 不同氧分压p下Mn2+氧化还原催化闪锌矿时Zn的浸出率、浸出率线性拟合及ln kp-ln p
Fig. 12 Zn leaching rate(a), linear fitting of leaching rate(b) and (c) ln kp-ln p of sphalerite at different oxygen partial pressure in Mn2+ catalytic systems
图12(a)所示为不同氧分压下Zn浸出率随时间的变化曲线,图12(b)所示为经过浸出模型拟合后的拟合直线并得到不同氧分压下4条直线的斜率kp,图12(c)所示为ln(kp)与ln(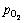 )的拟合直线并得到其斜率求出活化能,线性拟合的相关系数在图中标出。基于动力学模型进行拟合给出氧分压的反应级数为1.64。
)的拟合直线并得到其斜率求出活化能,线性拟合的相关系数在图中标出。基于动力学模型进行拟合给出氧分压的反应级数为1.64。
2.3.4 Mn2+用量影响及催化剂用量级数的求解
以人造闪锌矿为原料,在温度(T)403 K、硫酸浓度(A)110 g/L、氧分压(p)0.8 MPa的条件下,分别将MnSO4(N为0.01、0.03、0.05、0.07 mol)以硫酸盐溶液的形式通过后加料阀加入到浸出体系,考察了不同Mn2+用量在浸出90 min内锌的浸出率及动力学分析,如图13所示。
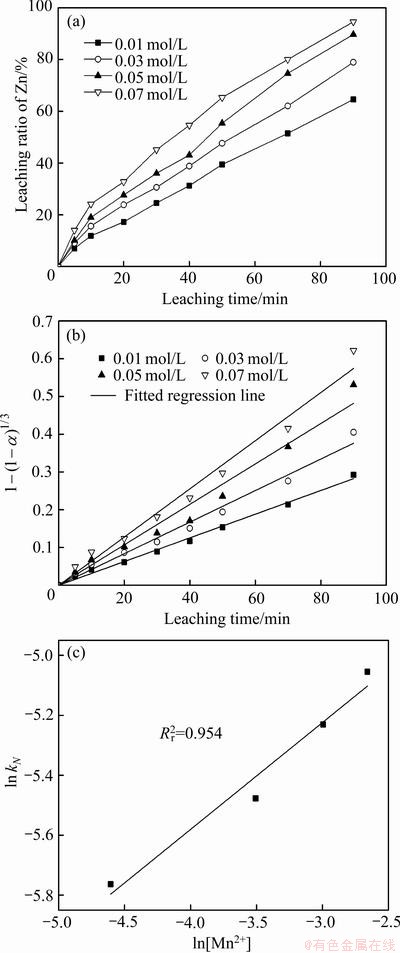
图13 不同催化剂用量N下Mn2+氧化还原催化闪锌矿时Zn的浸出率、浸出率线性拟合及ln kN-ln[Mn2+]
Fig. 13 Zn leaching rate(a), linear fitting of leaching rate(b) and ln kN-ln[Mn2+](c) of sphaleriteat amount of Mn2+ in Mn2+ catalytic systems
图13(a)所示为不同氧分压下锌浸出率随时间的变化曲线,图13(b)所示为经过浸出模型拟合后的拟合直线并得到不同氧分压下4条直线的斜率kN,图13(c)所示为ln(kN)与ln[Mn2+]的拟合直线并得到其斜率求出活化能,线性拟合的相关系数在图中标出。与之前的动力学浸出要素不一样,在不同的催化剂量浸出时,浸出率之间并没有出现明显的跳跃(尤其是不同温度之间),表明催化剂量对浸出体系的最终影响并不是特别大,即在本研究的实验条件下,催化剂量在一定程度上已经过饱和了。依据线性拟合的结果给出的反应级数为0.36,进一步证明催化剂量的的影响效果较小。
2.3.5 动力学方程的建立
综合上述不同条件下的Mn2+催化体系人造闪锌矿氧压浸出数据可以发现,随着温度、硫酸浓度、氧分压的增大,相应的氧压浸出速率及浸出率均有较大的提高,这是由于增强相应的反应条件,矿物的酸浸速率及颗粒表面H2S气膜氧化速率均有较大提高。最终,通过闪锌矿氧压酸浸动力学模型的分析,该体系下的动力学方程可以表示为
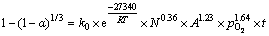 (6)
(6)
然后作出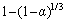 与
与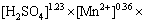
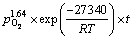 的拟合关系,结果见图14。虽然在图中的点有些分散,但是拟合直线的相关系数已超过0.98。因此,从图14可以得出,K0为3.85×10-3。
的拟合关系,结果见图14。虽然在图中的点有些分散,但是拟合直线的相关系数已超过0.98。因此,从图14可以得出,K0为3.85×10-3。
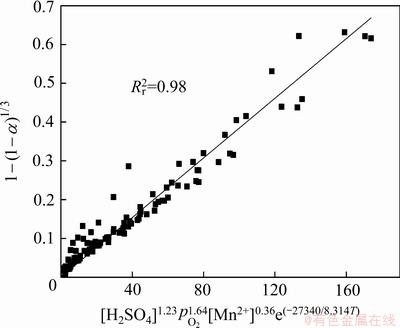
图14 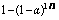 和
和
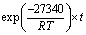 之间的关系
之间的关系
Fig. 14 Relationships between 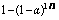 and
and 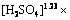
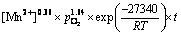
最终,可得到Mn2+催化体系人造闪锌矿氧压浸出的动力学方程为
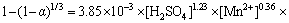
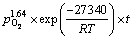 (7)
(7)
3 结论
1) 随着浸出时间的推移直到浸出结束,体系电位持续升高,这是由于溶解氧氧化Mn2+的反应在持续进行,而生成的MnO2能够较为快速地破坏矿物表面生成的H2S气膜层。在Mn2+氧化还原催化体系,浸出过程的控制步骤为Mn2+氧化为MnO2的化学反应,实现了控制步骤的转移,因此,Mn2+在多大程度上能够实现催化作用,取决于Mn2+氧化为MnO2的反应速度与控制条件。
2) 随着温度、硫酸浓度、氧分压的增大,相应的氧压浸出速率及浸出率均有较大的提高,这是由于增强相应的反应条件、矿物的酸浸速率及颗粒表面H2S气膜氧化速率均有较大提高。最终,通过闪锌矿氧压酸浸动力学模型的分析,在Mn2+催化浸出体系下,反应活化能为27.34 kJ/mol,硫酸浓度、氧分压及Mn2+用量的反应级数分别为1.23、1.64和0.36,表明其应遵循化学反应及表面扩散混合控制的收缩核模型,给出了其在本研究条件下的氧压浸出动力学模型方程为
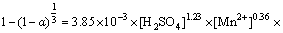
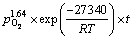
REFERENCES
[1] HARVEY, T J, YEN W T, PATERSON J G. A kinetic investigation into the pressure oxidation of sphalerite from a complex concentrate[J]. Minerals Engineering, 1993, 6(8/10): 949-967.
[2] JAN R J, HEPWORTH M T, FOX V G. A kinetic study on the pressure leaching of sphalerite[J]. Metallurgical and Materials Transactions B, 2007, 7(3): 353-361.
[3] TIAN Lei, LIU Yan, ZHANG Ting-an, Lü Guo-zhi, ZHOU Shuang, ZHANG Guo-quan. Kinetics of indium dissolution from marmatite with high indium content in pressure acid leaching[J]. Rare Metals, 2017, 36(1): 69-76.
[4] NEVEUX L, CHICHE D, BAZER-BACHI D, FAVERGEON L, PIJOLAT M. New insight on the ZnO sulfidation reaction: Evidences for an outward growth process of the ZnS phase[J]. Chemical Engineering Journal, 2012, 181: 508-515.
[5] 陈建华, 曾小钦, 陈 晔, 张辉鹏. 含空位和杂质缺陷的闪锌矿电子结构的第一性原理计算[J]. 中国有色金属学报, 2010, 20(4): 765-771.
CHEN Jian-hua, ZENG Xiao-qin, CHEN Ye, ZHANG Hui-peng. First-principle theory calculations of electronic strucfure of sphalerite with vacancy and impurity[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(4): 765-771.
[6] NIEDERKORN J S. Kinetic-study on catalytic leaching of sphalerite[J]. JOM, 1985, 37(7): 53-56.
[7] LIU Zhi-xiong, YIN Zhou-lan, HU Hui-ping, CHEN Qi-yuan. Catalytic-oxidative leaching of low-grade complex zinc ore by Cu(Ⅱ) ions produced from copper ore in ammonia-ammonium sulfate solution[J]. Metallurgical and Materials Transactions B-process, 2012, 43(5): 1019-1026.
[8] 杨洪英, 杨 立, 魏绪钧. 氧化亚铁硫杆菌(SH-T)氧化毒砂的机理[J]. 中国有色金属学报, 2001, 11(2): 323-327.
YANG Hong-ying, YANG Li, WEI Xu-jun. Mechanism of oxidizing arsenopyrite by Thiobacillus ferrooxidans (SH-T)[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(2): 323-327.
[9] HABASHI F. Dissolution of minerals and hydrometallurgical processes[J]. Naturwissenschaften, 1983, 70(8): 403-411.
[10] LI Y, KAWASHIMA N, LI J, CHANDRA A P, GERSON A R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite[J]. Advances in Colloid and Interface Science, 2013, 197: 1-32.
[11] 陈建华, 陈 晔, 曾小钦, 李玉琼. 铁杂质对闪锌矿表面电子结构及活化影响的第一性原理研究[J]. 中国有色金属学报, 2009, 19(8): 1517-1523.
CHEN Jian-hua, CHEN Ye, ZENG Xiao-qin, LI Yu-qiong. First principle study of effect of Fe impurity on electronic structure and activation of sphalerite surface[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(8): 1517-1523.
[12] 付中梦, 邓志敢, 魏 昶, 李兴彬, 李存兄, 樊 刚. 锌精矿与锌浸渣协同浸出及氧化转化行为[J]. 中国有色金属学报, 2018, 28(10): 2086-2093.
FU Zhong-meng, DENG Zhi-gan, WEI Chang, LI Xing-bin, LI Cun-xiong, FAN Gang. Simultaneous leaching of zinc residue and zinc concentrate and oxidative conversion behavior[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(10): 2086-2093.
[13] VLADIMIR V Z, ARTO L, MATTI L, TUOMAS K. A mechanistic kinetic model for direct pressure leaching of iron containing sphalerite concentrate[J]. Chemical Engineering Research & Design, 2017, 118: 131-141.
[14] 王少芬, 方 正, 龙 姝, 陈阳国. 二氧化锰和硫化矿同时发电浸出的初步探索[J]. 有色金属, 2004, 56(1): 56-60.
WANG Shao-feng, FANG Zheng, LONG Shu, CHEN Yang-guo. Electrogenerative simultaneously leaching of sulfide minerals and MnO2[J]. Non-ferrous Metal, 2004, 56(1): 56-60.
[15] ESCUDERO M E, GONZALEZ F. The catalytic effect of some cations on the biological leaching of a Spanish complex sulphide[J]. Hydrometallurgy, 1993, 34: 151-169.
[16] BALLESTER A, GONZALEZ F. The use of catalytic ions in bioleaching[J]. Hydrometallurgy, 1992, 29: 145-160.
[17] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part Ⅲ: Effect of redox potential on the silver-catalyzed process[J]. Hydrometallurgy, 2008, 93: 97-105.
[18] GHOSH M K, DAS R P, BISWAS A K. Oxidative ammonia leaching of sphalerite Part Ⅱ: Cu(Ⅱ)-catalyzed kinetics[J]. International Journal of Mineral Processing, 2003, 70(1/4): 221-234.
[19] SCOTT T R, DYSON N F. The catalyzed oxidation of zinc sulfide under acid pressure leaching conditions[J]. Transactions of the Metallurgical Society of AIME, 1968, 242: 1815-1821.
[20] BALLESTER A, GONZALEZ F. The influence of various ions in the bioleaching of metal sulphides[J]. Hydrometallurgy, 1990, 23: 221-235.
[21] 郑国渠, 梅光贵, 钟竹前. 高锰硫酸锌溶液电解金属锌研究[J]. 有色金属(冶炼部分), 1993(5): 31-34.
ZHENG Guo-qu, MEI Guang-gui, ZHONG Zhu-qian. Study on electrolytic metallic zinc in high manganese sulfate solution[J]. Nonferrous Metals (Extractive Metallurgy), 1993(5): 31-34.
[22] MULAK W. The catalytic action of cupric and ferric ions in nitric acid leaching of Ni3S2[J]. Hydrometallurgy, 1987, 17: 201-214.
[23] SOKIC M D, MARKOVIC B, ZIVKOVIC D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid[J]. Hydrometallurgy, 2009, 95(3/4): 273-279.
Catalytic mechanism and kinetics of Mn2+ under sphalerite pressure leaching system
CHEN Li-jie1, 2, GONG Ao1, 2, WU Xuan-gao1, 2, LIU Yan1, 2, ZHANG Ting-an1, 2, TIAN Lei1, 2
(1. Institute of Green Metallurgy and Process Intensification, Jiangxi University of Science and Technology, Ganzhou 341000, China;
2. Key Laboratory for Ecological Metallurgy of Multimetallic Mineral, Ministry of Education, Northeastern University, Shenyang 110819, China)
Abstract: The catalytic mechanism and kinetics of Mn2+ under the sphalerite pressurized agitation leaching system were studied. Artificial sphalerite was prepared by sintering ZnS and FeS and used in pressure acid leaching experiment. The potential changes of pressure leaching system were studied by using the autoclave designed by ourselves. The results show that as the leaching time goes on until the end of leaching, the system potential continues to increase, so the extent to which Mn2+ can achieve catalytic action depended on the reaction conditions of oxidation of Mn2+ into MnO2. With the increases of temperature, acidity and oxygen partial pressure, the corresponding oxygen pressure leaching speed and leaching rate all have large increase at the end, through the analysis of sphalerite oxygen pressure acid leaching kinetics model, in manganese ion catalyzed leaching system, the activation energy is 27.34 kJ/mol, the dosage of sulfuric acid, oxygen partial pressure and Mn2+ reaction series are 1.23, 1.64 and 0.36, respectively, showing that it should follow and surface chemical reaction diffusion retract model of hybrid control, and the corresponding dynamic equation is established.
Key words: Mn2+ catalytic mechanism; artificial sphalerite; leaching kinetics; pressure leaching; system potential
Foundation item: Projects(51804136, 51764016) supported by the National Natural Science Foundation of China; Project(U1402271) supported by the National Natural Science Foundation of Yunnan Joint Key Fund; Project(20181BAB216017) supported by the Natural Science Foundation of Jiangxi Province, China
Received date: 2019-05-30; Accepted date: 2019-09-26
Corresponding author: TIAN Lei; Tel: +86-797-8312047; E-mail: tianleijx@163.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51804136,51764016);国家自然科学基金云南联合重点基金资助项目(U1402271);江西省自然科学基金资助项目(20181BAB216017)
收稿日期:2019-05-30;修订日期:2019-09-26
通信作者:田 磊,副教授,博士;电话:0797-8312047;E-mail:tianleijx@163.com