
Electrical conductivity of Na3AlF6-AlF3-Al2O3-CaF2-LiF(NaCl)system electrolyte
KAN Hong-min(阚洪敏), WANG Zhao-wen(王兆文), BAN Yun-gang(班允刚),
SHI Zhong-ning(石忠宁), QIU Zhu-xian(邱竹贤)
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 22 July 2006; accepted 26 November 2006
Abstract: A PGSTAT 30 and a BOOSTER 20A were used to measure cell impedance. Electrical conductivity was gained by the Continuously Varying Cell Constant Technique. Electrical conductivity of KCl was measured for comparison. The results prove that the method is reliable and accurate. The electrical conductivity of Na3AlF6-AlF3-Al2O3-CaF2-LiF(NaCl) system was studied by this method. Activation energy of conductance was obtained based on the experiment results. The experiments show that electrical conductivity is increased greatly with NaCl and LiF added. Increasing 1%LiF(mass fraction) results in corresponding increase of 0.0276 S/cm for superheat condition of 15 ℃. For NaCl, it is 0.024 S/cm. Electrical conductivity is increased by 0.003 S/cm with 1℃ temperature increase. The electrical conductivity is lower than that predicted by the WANG Model and higher than that predicted by the Choudhary Model.
Key words: electrical conductivity; aluminium electrolyte; additive; superheat; activation energy of conductance
1 Introduction
The electrical conductivity of cryolitic melts has been an area of active research in recent years[1]. The electrical conductivity is of particular importance, since it affects the ohmic voltage drop in the electrolytic cell. In the industrial production of aluminum, it is desirable to have an electrolyte with a high electrical conductivity to increase the current efficiency and reduce energy consumption. On the other hand, research on electrical conductivity can help in studies of structure of melts.
The major difficulty in determining electrical conductivity of fused fluorides is to select a suitable material for the conductance cell. It should not be attacked by the melt; and it should have dimensional stability at these temperatures. Pyrolytic boron nitride is impervious to melts. It has a very high electrical resistivity and acts as an insulator. It seems that pyrolytic boron nitride is the most suitable material for the purpose[2-11].
There are many measurement methods of electrical conductivity[2-8,12-14], such as 4-electrode method, Wheatstone bridge and Kelvi bridge method. Bridge methods can eliminate errors from lead resistance. But the reproducibility of the measurements is bad for the need of continuity for the conductance cell. 4-electrode method does not require determination of the cell constant to measure conductivity of melts. While this method works well in many electrolytes, and it does not seem to give satisfactory result in cryolite melts[4]. A novel experimental technique with moving electrodes (Continuously Varying Cell Constant) for tube-type cell made of pyrolytic boron nitride was developed by WANG et al[9-10] and by KIM and SADOWAY[11], to increase the reproducibility of the measurements.
In this study, AC-techniques are used to measure impedance with a sine wave signal and small amplitude in high frequency range. The CVCC experiment technique, together with a pyrolytic boron nitride conductance cell, was used to determine the electrical conductivity. The objective of the present work is to provide conductivity data for cryolite-based melts. The electrical conductivity results are compared with those predicted by WANG et al[10] and the CHOUDHARY equation[15]. The effects of AlF3, LiF, NaCl and temperature on the electrical conductivity are discussed in theory. And the conductance activation energy is given.
2 Theory
In the measurement of electrical conductivity, the real component of the impedance, named Rm, may be expressed as follows:
Rm=R0+Rf+ΔR (1)
where R0 is the ohmic resistance of the electrolyte; Rf is the polarization resistance of the electrolyte due to frequency effects; and ΔR is the contact resistance between wires and electrodes.
AC-techniques are used to measure impedance with a sine wave signal and small amplitude in high frequency range. Lowering of the sine wave amplitude to the smallest possible value reduces effects associated with any electrode reaction. Rf is decreased to an infinitely small value and can therefore be omitted.
The electrical conductivity of molten cryolitic bath is measured using CVCC technique[9]. Because of the linearity of Rm versus the cell constant, the electrical conductivity of electrolyte, k, can be obtained by using
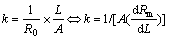 (2)
(2)
where k is the conductivity of molten salts, in S/cm; A is the inner cross-sectional area of conductivity cell, in cm2, which is known through calibration; and dRm/dL is the slope of the resistance of the measured circuit versus the programmed variation of the conductivity cell length.
CVCC is accomplished by linearly varying the length of the conductivity cell, L, by moving the Pt-disc electrode while keeping the cell cross area, A, unchanged. The slope is therefore derived through a series of circuit resistance measurements versus the programmed lengths of the conductivity cell. The electrical conductivity derived from Eqn.(2) is free of the extraneous conductivity effects such as wire contact resistance.
3 Experimental
A PGSTAT30 and a BOOSTER 20A were used for the measurements of the cell impedance. The amplitude was 10 mV, the frequency was varied from 100 Hz to 100 kHz, and 31 readings are taken within this range. A personal computer was used for controlling PGSTAT30 and BOOSTER 20A for collection of the data by Frequency Response Analysis software.
The CVCC theory and technique was firstly tested in 1 mol/L KCl aqueous solutions and then verified in molten KCl. The cross-sectional area, A, of the tube-type cell was firstly known in 1 mol/L KCl aqueous solutions, that is A=19.24 cm2. Secondly, dRm/dL=0.229 in molten KCl at 800 ℃ is presented in Fig.1. Electrical conductivity, k, of molten KCl is 2.265 S/cm according to Eqn.(2). Molten KCl has a reported electrical conductivity value of 2.25 S/cm at 800 ℃. The average error, compared with the literature values, is only 0.67%. This showed that the CVCC technique was a very successful method to obtain very high accuracy in measuring electrical conductivity of molten salts. Finally, electrical conductivity of cryolite-based melts was measured. Cryolite-based electrolyte composition and temperature range are presented in Table 1.
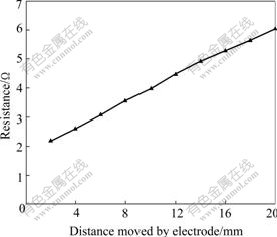
Fig.1 Total resistance of circuit vs distance moved by electrode at 100 kHz for KCl at 800 ℃
Table 1 Cryolite-based electrolyte composition and tempera- ture range

4 Results and discussion
4.1 Effect of LiF
Figs.2-4 show the electrical conductivity values of cryolitic melts as a function of LiF content. Increase in LiF content results in increase of the electrical conductivity. Firstly, the effect is probably due to the reaction of LiF with the ‘free’ AlF3 under formation of lithium cryolite after LiF addition into the acidic melt. This reaction decreases the acidity of the electrolyte, making it more ionic and increasing thereby the electrical conductivity. Secondly, because the radius (volume) of Li+ is small, there is less obstruction. It moves easily in electric field. Thirdly, it gains less polarization and binding force from contrary electric change because it has relative few changes and outer electrons. These factors are useful to increasing the electrical conductivity.
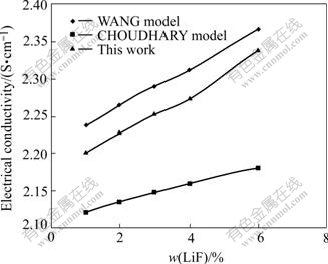
Fig.2 Electrical conductivity as function of LiF content at 15 ℃ superheat for r=2.2, 3%Al2O3, 4%CaF2
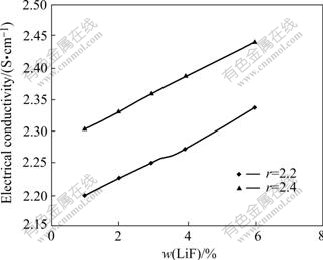
Fig.3 Electrical conductivity as function of LiF content at 15 ℃ superheat with 3%Al2O3 and 4%CaF2 for r=2.2 and r=2.4
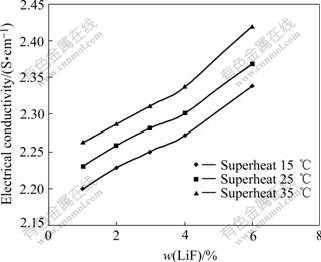
Fig.4 Electrical conductivity as function of LiF content at 15, 25, 35 ℃ superheat for r=2.2, 3%Al2O3, 4% CaF2
4.1.1 Comparison of measured conductivity results in this work with two models
Fig.2 shows the comparison of measured conductivity results in this work with the CHOUDHARY model and the WANG model.
Increase in LiF content results in increases of the electrical conductivity for the three methods. But the electrical conductivity value and the increasing rate of the conductivity for the three models are different under the same condition. All the data in this work are higher than those predicted by the CHOUDHARY model[15], lower than those predicted by the WANG model. An increase of every 1% LiF in the bath results in about 0.027 6 S/cm conductivity increase for this work, 0.026 S/cm for the WANG model and only 0.012 2 S/cm for the CHOUDHARY model[15].
4.1.2 Effect of LiF for different r
Fig.3 shows the electrical conductivity values of cryolitic melts as a function of LiF content for r=2.2 and r=2.4. Increase in r results in increase of the electrical conductivity. The electrical conductivity for r=2.2 is lower by 0.107 S/cm than the electrical conductivity for r=2.4 under the same condition.
Ions in Na3AlF6-Al2O3 system include Na+, 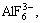
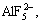
 F- and Al-O-F complex. The formation of Al-O-F ion complex is shown as follows:
F- and Al-O-F complex. The formation of Al-O-F ion complex is shown as follows:
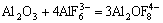 (3)
(3)
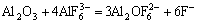 (4)
(4)
 (5)
(5)
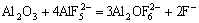 (6)
(6)
With the increase of AlF3, ion complex will increase according to the reactions. Formation of the complex reduces the ion mobility and, therefore, reduces the electrical conductivity of molten electrolyte[9-10].
4.1.3 Effect of LiF for different superheat
Fig.4 shows electrical conductivity values of cryolitic melts as a function of LiF content at superheat of 15, 25 and 35 ℃, for r=2.2, 3%Al2O3, 4%CaF2. Increasing superheat results in increase of the electrical conductivity. For 1%LiF content, the electrical conductivity increases to 2.26 S/cm from 2.2 S/cm when the superheat is increased to 35 ℃ from 15 ℃. For 6% LiF content, the electrical conductivity increases to 2.42 S/cm from 2.34 S/cm when the superheat is increased to 35 ℃ from 15 ℃.
It is beneficial for industrial production. But use of LiF is restricted because of its expensive price. According to the need of industrial production, proper LiF content and superheat can be chosen to reduce cost and improve economic benefits.
4.2 Effect of NaCl
Fig.5 shows the electrical conductivity values of cryolitic melts as function of NaCl content. Increasing NaCl content results in increase of the electrical conductivity. As for Li+, the radius (volume) of Na+ is small, which gives less obstruction. It moves easily in electric field. Furthermore, it gains less polarization and binding force from contrary electric change because it has relative few changes and outer electron. According to Ref.[1], all electric current is transmitted by Na+ in neutral melt and 90% current is transmitted by Na+ in acid melt. After NaCl addition into the melt, the number of Na+ increases greatly. So the electrical conductivity increases. Increasing 1%NaCl content results in a corresponding conductivity increase of 0.024 S/cm for the 15 ℃ superheat, r=2.2, 3%Al2O3 and 4%CaF2 condition.
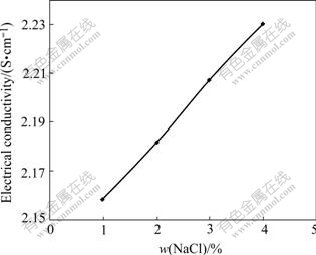
Fig.5 Electrical conductivity as function of NaCl content at 15 ℃ superheat, r=2.2, 3%Al2O3 and 4%CaF2
4.3 Effect of temperature
4.3.1 Comparison of isothermal electrical conductivity with that under superheat condition
Fig.6 shows that LiF addition results in a greater increase of the isothermal electrical conductivity than that under constant superheat conditions. The explanation of this behavior is that the LiF greatly decreases the freezing point of the cryolitic baths and, as a result, a very high superheat results from the high content of the LiF under the condition of isothermal temperatures. An increase of every 1%LiF in the bath results in about 0.027 6 S/cm increase in the electrical conductivity under 15 ℃ superheat, r=2.2, 3%Al2O3 and 4%CaF2 condition, and the increase in electrical conductivity is about 0.05 S/cm at 955 ℃.
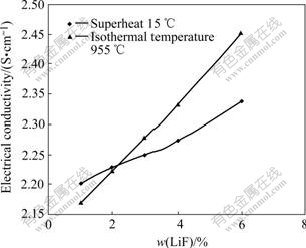
Fig.6 Comparison of LiF content influence on electrical conductivity at isothermal 955 ℃ and constant 15 ℃ superheat for r=2.2, 3%Al2O3, 4%CaF2
4.3.2 Effect of bath temperature
The influence of bath temperature on the electrical conductivity is shown in Fig.7 for r=2.2, 3%Al2O3, 4%CaF2 containing respectively 1%LiF, 3%LiF, and 6%LiF. The electrical conductivity of cryolitic melts increases with increasing bath temperature. The increased conductivity rate is about 0.003 S/(cm?℃). The explanation of this effect is that the heat movement of particles speeds up when temperature increases. Bonding energy between ion and ion with contrary charge is weakened because of acceleration of heat movement. Directional movement of ion is easier. The number of free ion increases because of decomposition of more ion complexes with increasing temperature. Decomposition of the ion complexes increases the ion mobility and thereby, increases the electrical conductivity.
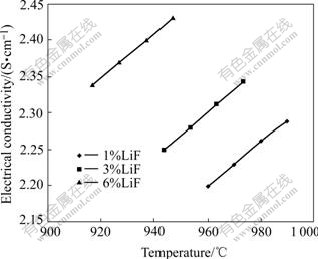
Fig.7 Electrical conductivity as function of bath temperature for r=2.2, 3%Al2O3, 4%CaF2
4.4 Activation energy of conductance
The experimental data obtained in this work for the same composition and different temperatures can be described by linear relationships with respect to the temperature:
k=A+B/T (7)
The parameters were obtained by a least square method.
The temperature dependence of the electrical conductivity of molten salts of predominantly ionic characteristic is given in the form of a basic Arrhenius equation:
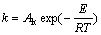 (8)
(8)
where k is the specific conductivity, in S/cm; Ak is the coefficient related to chemical composition of melt; E is the activation energy of conductance, in J/mol; T is the temperature of the melt, in K; R is the universal gas constant, 8.314 J/(mol?K).
The logarithm form of Eqn.(8) is
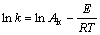 (9)
(9)
When lnAk=A, 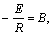
Then 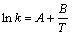 (10)
(10)
The activation energy of conductance can be obtained by the equations. The activation energy of conductance is given in Table 2.
Table 2 Electrical conductivity and activation energy of conductance of different electrolyte systems containing 3%Al2O3, 4%CaF2 and r=2.2
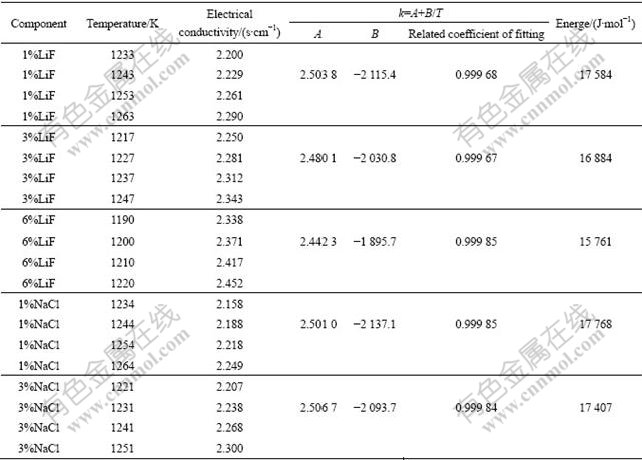
Table 2 shows that when the activation energy of conductance of the system is great under certain condition, the capacity of the electric conduction of ion is bad and the electrical conductivity is low. On the contrary, when the activation energy of conductance of the system is little under certain condition, the electrical conductivity of the system is high.
5 Conclusions
1) The electrical conductivity is increased greatly by NaCl and LiF addition. Increasing 1%LiF content results in a corresponding conductivity increase of 0.027 6 S/cm for superheat of 15 ℃. For NaCl, it is 0.024 S/cm.
2) Electrical conductivity is increased by 0.003 S/cm with 1 ℃ temperature increase.
3) The electrical conductivity is lower than that predicted by the WANG model and higher than that predicted by the CHOUDHARY model.
4) The activation energy of conductance is obtained based on the experiment results.
References
[1] QIU Zhu-xian. Principle and Application of Aluminum Electrolysis [M]. Xuzhou: China University of Mining and Technology Press, 1998: 93-112. (in Chinese)
[2] H?VE? J, THONSTAD J. Electrical conductivity of low-melting electrolytes for aluminium smelting [J]. Electrochem Soc, 2004, 49: 5111-5114.
[3] H?VE? J, THONSTAD J, STERTEN A. Electrical conductivity of cryolite-based ternary mixtures Na3AlF6-Al2O3-CaF2 and Na3AlF6-Al2O3-MgF2 [J]. Electrochimica Acta, 1993, 38(15): 2165-2169.
[4] FELLNER P, KOBBELT O, STERTEN A. Electrical conductivity of molten cryolite-based binary mixtures obtained with a tube-type cell made of pyrolytic boron nitride [J]. Electrochimica Acta, 1993, 38(4): 589-592.
[5] H?VE? J, THONSTAD J, STERTEN A. Electrical conductivity of molten cryolite-based mixtures obtained with a tube-type cell made of pyrolytic boron nitride [J]. Metallurgical and Materials Transactions B, 1996, 27(4): 255-261.
[6] FELLNER P, MIDTLYNG S, STERTEN A. Electrical conductivity of low melting baths for aluminium electrolysis: the system Na3AlF6-Li3AlF6-AlF3 and the influence of additions of Al2O3, CaF2 and MgF2 [J]. Journal of Applied Electrochemistry, 1993, 23: 78-81.
[7] HAARBERG G M, THONSTAD J, EGAN J J. Electrical conductivity measurements in cryolite alumina melts in the presence of aluminium[C]//Light Metals 1996. Warrendale: TMS, 1996: 221-225.
[8] CHRENKOV? M, DANEK V, SILN? A. Density, electrical conductivity and viscosity of low melting baths for aluminium electrolysis[C]//Light Metals 1996. Warrendale: TMS, 1996: 227-232.
[9] WANG X W, RAY D P, ALTON T T. Electrical conductivity of cryolitic melts [C]//Light Metals 1992. Warrendale: TMS, 1992. 481-488.
[10] WANG X W, RAY D P, ALTON T T. A multiple regression equation for the electrical conductivity of cryolitic melts [C]//Light Metals 1993. Warrendale: TMS, 1993. 247-255.
[11] KIM K B, SADOWAY D R. Electrical conductivity measurements of molten alkaline-earth fluoride [J]. Electrochem Soc, 1992, 139: 1027-1033.
[12] LI Guo-hua, LI De-xiang. Physicochemical properties of NaF-AlF3-BaCl2-NaCl system and electrolyte composition choice [J]. The Chinese Journal of Nonferrous Metals, 1994, 4(1): 28-32. (in Chinese)
[13] SUN Ben-liang, ZHAI Yu-chun, TIAN Yan-wen. Measurement of conductance and melting point temperature of NaCl-KCl(1:1)-ScCl3 system [J]. Chinese Rare Earths, 1999, 20(2): 26-28. (in Chinese)
[14] WANG L, ALTON T T, NOLAN E R. The electrical conductivity of cryolitic melts containing aluminum carbide [C]//Light Metals 1994. Warrendale: TMS, 1994: 177-185.
[15] CHOUDHARY G. Electrical conductivity for aluminum cell electrolyte between 950-1 025 ℃ by regression equation [J]. Electrochem Soc, 1973, 120(3): 381-383.
Foundation item: Project(50334030) supported by the National Natural Science Foundation of China
Corresponding author: KAN Hong-min; Tel: +86-24-83680245; E-mail: kanhongmin2002@163.com
(Edited by YANG Bing)