文章编号:1004-0609(2014)03-820-06
油酸钠作用下金红石的浮选行为及作用机理
王 军,程宏伟,赵红波,覃文庆,邱冠周
(中南大学 资源加工与生物工程学院,长沙 410083)
摘 要:通过纯矿物试验,研究金红石在油酸钠为捕收剂体系中的浮选行为。当油酸钠用量为20 mg/L、pH值为6~8时,金红石浮选回收率均在80%以上;当pH =7.5时,金红石浮选回收率最高,为85.27%。Zeta电位及红外光谱测试结果表明,油酸钠在金红石表面主要发生了化学吸附,同时也可能存在物理吸附。根据油酸钠溶液化学计算,当pH值为6~8时,油酸钠溶液的优势组分为C17H33COO-和(C17H33COO)22-;而金红石表面Ti4+的羟基化合物主要以[Ti(OH)2]2+和[Ti(OH)3]+形式存在。结合金红石在油酸钠捕收剂体系中的浮选行为,油酸钠在金红石表面的相互作用原理是,金红石表面解离的Ti4+在水溶液中形成羟基化合物[Ti(OH)2]2+和[Ti(OH)3]+络合在金红石表面成为浮选的活性质点,再与C17H33COO-和(C17 H33COO)22-作用形成Ti(C17H33COO)4,从而使金红石疏水上浮;油酸(C17H33COOH(aq))分子和离子 -分子缔合物(C17H33COOH·C17H33COO-)的物理吸附也可能存在。
关键词:金红石;油酸钠;溶液化学;浮选
中图分类号:TD91 文献标志码:A
Flotation behavior and mechanism of rutile in presence of sodium oleate
WANG Jun, CHENG Hong-wei, ZHAO Hong-bo, QIN Wen-qing, QIU Guan-zhou
(School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The flotation behavior of rutile in the system with sodium oleate as collector was studied through pure mineral test. When the dosage of sodium oleate is 20 mg/L, and pH value of the pulp is set from 6 to 8, the flotation recovery of rutile is over 80%. The highest recovery of rutile is 85.27% when pH value of the pulp is 7.5. Zeta potential and IR spectra results show that the chemical adsorption mainly occurs on the surface of rutile for sodium oleate, and physical adsorption may also be present at the same time. Based on the results of solution chemistry calculation, C17H33COO- and (C17H33COO)22- are the main compositions when the pH value of the pulp is 6-8. [Ti(OH)2]2+ and [Ti(OH)3]+ are the major components of the hydroxyl compound of Ti4 +on the surface of rutile. Combined with the results of rutile flotation behavior, the surface interaction mechanism of rutile with sodium oleate is as follows: Ti4+ is exposed on the surface of rutile, and titanium hydroxyl complexes [Ti(OH)2]2 + and [Ti(OH)3] will form because of the dissolution of Ti4+ in water solution of the pulp. These Ti hydroxyl complexes are absorbed on the mineral surface and become the active point of flotation and then react with C17H33COO- and (C17H33COO)22- to form Ti(C17H33COO)4. Besides, physical adsorption of C17H33COOH(aq) and C17H33COOH·C17H33COO- on rutile may also happen.
Key words: rutile; sodium oleate; solution chemistry; flotation
钛被誉为“21 世纪金属”,其主要来源为钛铁矿和金红石矿,我国天然金红石资源中原生金红石矿储量占全国金红石资源总量的86%,而金红石砂矿仅为14%[1-2],因而原生金红石矿的开发和利用对于解决我国钛资源的需求非常关键,而浮选是解决原生金红石矿分离应用的重要手段。
油酸钠作为脂肪酸皂的一种,主要作为氧化矿和盐类矿物的捕收剂。由于脂肪酸具有活泼的羧基官能团,因此几乎可以浮选所有的矿物。通常,在溶液中呈阳离子状态并含有离子键的矿物,如氧化矿、硫酸盐矿物、磷酸盐和萤石,在溶液中呈阳离子状态并含有金属键的矿物,如硫化矿、金和铜等天然金属,具有分子共价键的矿物,如石墨、煤、硫磺、辉钼矿和滑石等,都可以用脂肪酸及其皂类作为捕收剂进行浮选[3]。近年来,油酸钠也广泛应用于金红石的浮选[4-6],并且得到了较好的浮选效果。
许多研究者对油酸钠浮选氧化矿和盐类矿物的机制进行了研究,其中包括赤铁矿、磷灰石[7]、金云母、萤石[8]、白钨矿、铁矿石、石英[9]、方解石[10]、钛辉石[11]、褐铁矿、钛铁矿[12]、红柱石和一水硬铝石等[13]。对于油酸钠浮选此类矿石的机制可归结为3类:1) 矿物晶格中金属离子或者矿物表面的金属离子羟基络合物与油酸根离子发生化学作用形成金属油酸盐;2) 油酸分子、离子缔合物在矿物表面发生物理吸附;3) 化学吸附和物理吸附共存。
目前,有关油酸钠浮选金红石的机制研究较少,LAI等[14]和高利坤[15]认为油酸钠在金红石表面的吸附是由化学因素引起的特性吸附,这种特性吸附的根源可能是化学作用,也可能是氢键作用。本文作者通过研究油酸钠对金红石的捕收性能,并综合考虑矿物表面化学和捕收剂溶液化学,运用动电位测试和红外光谱测试等分析手段,系统讨论油酸钠对金红石的捕收机理。
1 实验
1.1 实验原料
金红石取自湖北枣阳大阜山,原料经弱磁选、强磁选和摇床反复精选后,采用陶瓷球磨机磨细,经过干式筛分取38~74 μm粒级作为浮选试样,化学分析结果显示试样中含有93.49%TiO2(质量分数)。图1所示为样品的XRD谱,由图1可知未检测到杂质峰,说明TiO2纯度较高,符合纯矿物实验的要求。矿浆pH 调整剂为盐酸和氢氧化钠,均为分析纯,油酸钠为化学纯。实验用水为一次蒸馏水。
1.2 浮选实验
浮选实验在容积为40 mL 的XFG 型挂槽式浮选机上进行,浮选温度为25 ℃。每次实验称取矿样2 g,与蒸馏水混合置于浮选槽内,添加pH调整剂后搅拌3 min,加入捕收剂搅拌3 min 后测定pH 值,浮选3 min,浮选过程采取手工刮泡,浮选完成后将刮出的泡沫(精矿)烘干、称量,计算回收率。实验流程如图2所示。
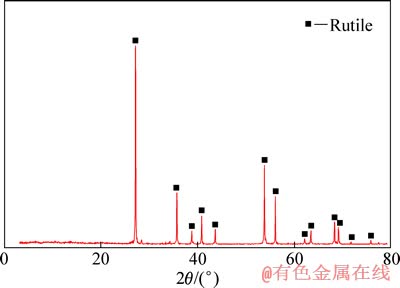
图1 样品的XRD谱
Fig. 1 XRD pattern of sample
图2 金红石浮选实验流程
Fig. 2 Flotation flowchart of rutile
1.3 矿物表面Zeta电位测量
将矿物磨至粒径小于2 μm,每次称样30 mg 置于烧杯中,加入40 mL 蒸馏水并调节溶液pH 值,按照与浮选实验相同的调浆条件加入药剂,采用磁力搅拌器搅拌10 min 后在Zetaplus Zeta 分析仪上测量矿物表面的Zeta电位。
1.4 红外光谱测定
红外光谱测试在Nicolet NEXUS 670 傅里叶变换红外光谱仪上进行测定。采用KBr 压片,测量范围为400~4000 cm-1。红外分析样品制备过程如下:将一定量的矿物放入浮选槽,调节pH 值后加入适量油酸钠溶液,充分搅拌后固液分离,采用相同pH 值的蒸馏水溶液洗涤矿物2 次,所得样品在70 ℃以下烘干,进行红外光谱检测。
2 结果与讨论
2.1 实验结果
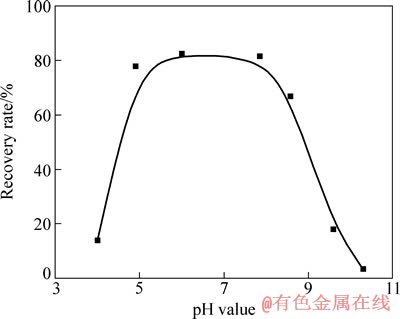
图3 油酸钠用量为20 mg/L时金红石溶液pH 值与矿物回收率的关系
Fig. 3 Relationship between mineral recovery rate and pH value of rutile at dosage of sodium oleate of 20 mg/L
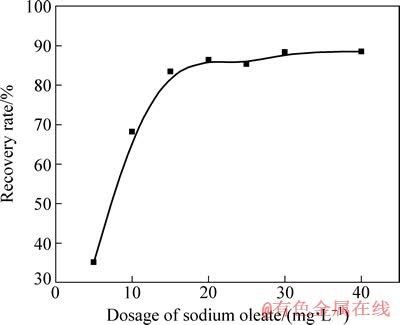
图4 pH值为7.3~7.7时油酸钠用量与矿物回收率的关系
Fig. 4 Relationship between recovery rate of mineral and dosage of sodium oleate at pH value of 7.3-7.7
图3 所示为在不同pH 值下加油酸钠浓度为20 mg/L浮选时金红石的可浮性。由图3 可以看出,当pH 值为5~8 时,金红石都保持了较好的可浮性。图4 所示为当pH值为7.3~7.7 时油酸钠用量与矿物回收率的关系。由图4可知,当油酸钠用量为0~20 mg/L 时,金红石的回收率随其用量的增大而快速提高;进一步增加其用量,回收率增加缓慢;当油酸钠用量达到20 mg/L 时,回收率达到85.27%。由此可见,在适当的浮选环境下,油酸钠对金红石有较强的捕收作用。
2.2 捕收机理
2.2.1 金红石的表面电性
图5 所示为矿物的Zeta电位与pH 值的关系。从图5可以看出,以盐酸和氢氧化钠调节pH 值时,金红石表面在pH值为4.1左右为零电点,这与文献[16]报道结果一致。加入油酸钠使相同pH 值下矿物的Zeta电位发生负移;零电点由4.1降到2.1左右,当pH<2.1时,电位为正且相对于原来发生负移,说明油酸钠在金红石表面发生了吸附,可能为静电吸附,也可能为化学吸附;当pH>2.1时,加入油酸钠使相同pH 值下矿物的Zeta电位发生负移,特别是在金红石可浮性较好的pH 区间内,电位降低的幅度更大,说明油酸根在金红石表面发生吸附。与矿物表面带同种电荷的油酸根可以很好地吸附在矿物表面,表明油酸根与金红石之间的作用力不以静电力为主,而应该与化学作用有关[12]。
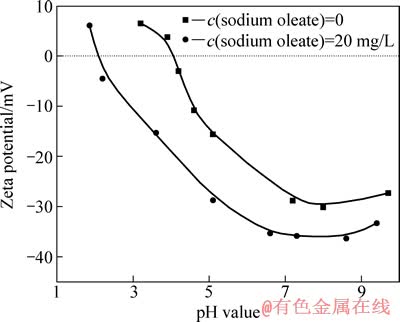
图5 矿物的Zeta电位与pH 值的关系
Fig. 5 Relationship between Zeta potential of minerals and pH value
2.2.2 红外光谱分析
在矿浆pH值为7.5、油酸钠用量为1 g/L条件下,对吸附油酸钠前后的金红石样品和油酸钠进行红外光谱测试,测试结果如图6所示。在金红石的红外光谱 中,只在600 cm-1以内出现数个特征峰[17]。在油酸钠的红外光谱中,在2923和2852 cm-1 处的2个吸收峰是油酸钠中—CH2—和—CH3中C—H 键的对称振动吸收峰,1713、1563、1447 和1426 cm-1 处的峰为R—COOH中—COO—基团的特征吸收峰,724 cm-1处的吸收峰为其面内弯曲振动吸收峰。经油酸钠作用后,金红石的红外光谱发生了明显变化,除金红石的谱带外,还在2924和2854 cm-1 处出现了新的吸收峰,与油酸钠光谱中2923和2852 cm-1 处的亚甲基吸收峰对应,说明药剂油酸钠吸附于金红石表面。另外,在1581和1465 cm-1 处,也出现了新的吸收峰,与油酸钠光谱中1563和1447 cm-1处羧基的特征峰相比,其波数均向高波段移动了约18 cm-1,说明金红石与油酸钠发生了化学吸附。
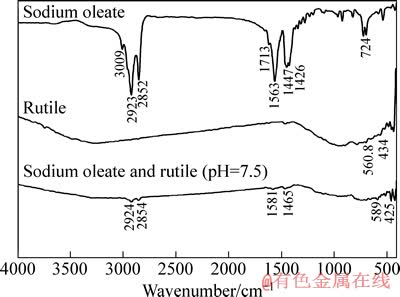
图6 油酸钠与金红石作用前后的红外光谱
Fig. 6 Infrared spectra of sodium oleate and rutile before and after interaction
2.2.3 金红石表面钛质点在水溶液中的形态
由溶液化学理论[15, 18]可知,金红石颗粒表面Ti质点在水溶液中发生溶解和水化反应,生成各种羟基络合物。在水溶液中Ti4+的水解反应为
Ti4++OH-=(TiOH)3+,k1=7.08×10-15 (1)
Ti4++2OH-=[Ti(OH)2]2+,k2=1.32×10-28 (2)
Ti4++3OH-=[Ti(OH)3]+,k3=5.37×10-42 (3)
Ti4++4OH-=[Ti(OH)4],k4=4.68×10-55 (4)
Ti(OH)4(s)=Ti4++4OH-,ksp1=5.01×10-59 (5)
图7所示为Ti4+的水解组分图。当pH<4时,溶液中4种成分都存在;当4<pH<6时,同时有[Ti(OH)2]2+和[Ti(OH)3]+存在,且[Ti(OH)3]+占优势;当pH值为6~11时,仅有[Ti(OH)3]+存在。结合图3可知,在pH为4~10的范围内油酸钠与金红石发生相互作用,使金红石上浮,与油酸钠作用的主要为[Ti(OH)2]2+和[Ti(OH)3]+。而在金红石回收率最大的区域(pH值为6~8),则主要是油酸钠与[Ti(OH)3]+发生作用。
2.2.4 油酸钠溶液的化学性质
油酸在常温常压下的溶解度S=2.51×10-8 mol/L,在通常的浮选用量下,矿浆中油酸的浓度均大于其溶解度,此时水溶液中溶解的油酸RCOOH(aq)与不溶的液态油酸RCOOH (l)形成饱和溶液,平衡如下[18]:
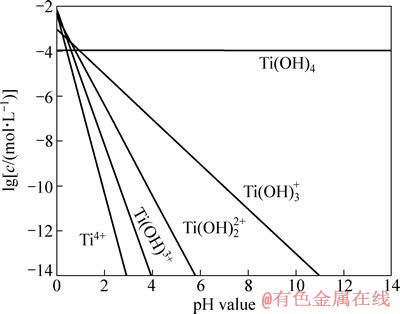
图7 金红石溶液中各组分的lg c和pH值的关系
Fig. 7 Relationship between lg c of rutile solution components and pH value
溶解平衡,
RCOOH(l) RCOOH(aq)
RCOOH(aq)
S=2.51×10-8 (6)
解离平衡,
RCOOH(aq) RCOO+H+
RCOO+H+
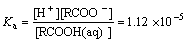 (7)
(7)
离子缔合平衡,
2RCOO- (RCOO)22-
(RCOO)22-
 (8)
(8)
离子-分子缔合平衡,
RCOOH(aq)+RCOO- H(RCOO)2-
H(RCOO)2-
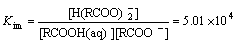 (9)
(9)
质量平衡方程为
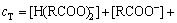
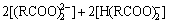 (10)
(10)
将式(6)~(8)代入式(9),并令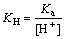 ,得
,得
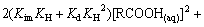
 (11)
(11)
RCOOH (aq)与RCOOH (l)形成平衡的临界pH值由下式确定:
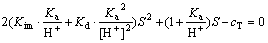 (12)
(12)
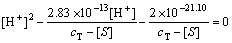 (13)
(13)
式中:cT为油酸钠初始总浓度。由式(12)和(13)可以计算出不同浓度下RCOOH(aq)与RCOOH(l)平衡时的pH 值,并可由式(6)~(12)绘制出不同油酸钠初始浓度时各组分浓度与pH值的关系。图8所示是cT为20 mg/L(6.57×10-5 mol/L)时溶液各组分浓度与pH 值的关系。
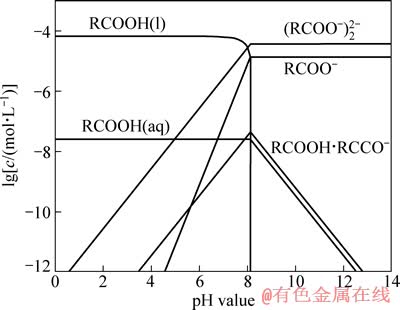
图8 cT为6.75×10-5 mol/L时油酸盐溶液中各组分的lg c 和pH 值的关系
Fig. 8 Relationship between lg c and pH value of sodium oleate solution components at cT of 6.57×10-5 mol/L
在pH值为4~5范围内,随着pH值的增大,油酸钠溶液中RCOO-、(RCOO)22-和RCOOH·RCOO-含量上升,金红石浮选回收率也增大,且在回收率上升较快时,油酸钠的优势组分为RCOOH(aq)和(RCOO)22-。(RCOO)22-组分的变化趋势和回收率的一致,因此,(RCOO)22-应该是决定金红石疏水上浮的活性组分。
在pH=7.5 时,油酸钠溶液优势组分为RCOO-和(RCOO)22-,而红外光谱测试结果表明,金红石表面发生了化学吸附,据此推测矿物表面应该生成了Ti(C17H33 COO)4。此外,溶液中尚存RCOOH(aq)和RCOOH·RCOO-,它们之间也可能存在物理吸附。
3 结论
1) 油酸钠对金红石具有良好的捕收性能,使其在较宽范围内(pH值为5~8)均具有较好可浮性。
2) Zeta电位及红外光谱测试结果表明,油酸钠在金红石表面主要发生了化学吸附,矿物表面生成了Ti(C17H33COO)4,结合油酸钠浮选溶液化学,矿物表面也可能存在C17H33COOH(aq)和C17H33COOH·
C17H33COO-的物理吸附。
3) 综合油酸钠的溶液化学和金红石表面解离的Ti4+在溶液中的溶液组分以及金红石的浮选结果,推测决定金红石浮选回收率的活性组分为RCOO-和(RCOO)22-,而它们主要与金红石表面的[Ti(OH)2]2+和[Ti(OH)3]+作用形成Ti(C17H33COO)4,从而使金红石疏水上浮。
REFERENCES
[1] 王立平, 王 镐, 高 颀, 李献军, 陈战乾, 窦永庆. 我国钛资源分布和生产现状[J]. 稀有金属, 2004, 28(1): 265-267.
WANG Li-ping, WANG Gao, GAO Qi, LI Xian-jun, CHEN Zhan-qian, DOU Yong-qing. Distribution and production status of titanium resources in China[J]. Chinese Journal of Rare Metals, 2004, 28(1): 265-267.
[2] 张 云, 管永诗, 田玉珍. 我国金红石矿资源开发利用现状 [J]. 矿产保护与利用, 2000(5): 27-30.
ZHANG Yun, GUAN Yong-shi, TIAN Yu-zhen. The exploitation and utilization of natural rutile in China[J]. Conservation and Utilization of Mineral Resources, 2000(5): 27-30.
[3] 张泾生, 阙煊兰. 矿用药剂[M]. 北京: 冶金工业出版社, 2008: 69-91.
ZHANG Jing-sheng, QUE Xuan-lan. Reagents for mineral processing[M]. Beijing: Metallurgical Industry Press, 2008: 69-91.
[4] 高利坤, 陈 云. 陕西某金红石矿反浮选试验研究[J]. 金属矿山, 2009(5): 88-91.
GAO Li-kun, CHEN Yun. Research on the pre-concentration of a Shaanxi rutile ore by reverse flotation[J]. Metal Mine, 2009(5): 88-91.
[5] BELARDI G, PIGA L, QUARESIMA S, SHEHU N. Application of physical separation methods for the upgrading of titanium dioxide contained in a fine waste[J]. International Journal of Mineral Processing, 1998, 53(3): 145-156.
[6] 朱建光, 朱玉霜. 浮选药剂的化学原理[M]. 长沙: 中南工业大学出版社, 1996: 64.
ZHU Jian-guan, ZHU Yu-shang. Chemistry fundamentals of flotation reagent[M]. Changsha: Central South University of Technology Press, 1996: 64.
[7] 冯其明, 赵岩森, 张国范. 油酸钠在赤铁矿及磷灰石表面的吸附机理[J]. 中国有色金属学报, 2012, 22(10): 2902-2907.
FENG Qi-ming, ZHAO Yan-sen, ZHANG Guo-fan. Adsorption mechanisms of sodium oleate on surfaces of hematite and apatite[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2902-2907.
[8] 胡岳华, 王淀佐. 脂肪酸钠浮选盐类矿物的作用机理研究[J]. 矿冶工程, 1990, 10(2): 20-24.
HUN Yue-hua, WANG Dian-zuo. Mechanism of fatty acid sodium flotation of salt-type minerals—A study[J]. Mining and Metallurgical Engineering, 1990, 10(2): 20-24.
[9] FUERSTENAU M, CUMMINS W. The role of basic aqueous complexes in anionic flotation of quartz[J]. Trans AIME, 1967, 238: 196-198.
[10] FUERSTENAU M, MILLER J. The role of the hydrocarbon chain in anionic flotation of calcite[J]. Trans AIME, 1967, 238: 153-160.
[11] 张国范, 鄢代翠, 朱阳戈, 冯其明, 王维清. pH 对油酸钠在钛铁矿与钛辉石表面吸附的影响[J]. 中南大学学报: 自然科学版, 2011, 42(10): 2898-2904.
ZHANG Guo-fan, YAN Dai-cui, ZHU Yang-ge, FENG Qi-ming, WANG Wei-qing. Influence of pH on adsorption of sodium oleate on surface of ilmenite and titanaugite[J]. Journal of Central South University: Science and Technology, 2011, 42(10): 2898-2904.
[12] 张国范, 朱阳戈, 冯其明, 卢毅屏, 欧乐明. 油酸钠对微细粒钛铁矿的捕收机理[J]. 中国有色金属学报, 2009, 19(2): 372-377.
ZHANG Guo-fan, ZHU Yang-ge, FENG Qi-ming, LU Yi-ping, OU Le-ming. Flotation of fine ilmenite by sodium oleate[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 372-377.
[13] 张国范, 冯其明, 卢毅屏, 欧乐明. 油酸钠对一水硬铝石和高岭石的捕收机理[J]. 中国有色金属学报, 2001, 11(2): 298-301.
ZHANG Guo-fan, FENG Qi-ming, LU Yi-ping, OU Le-ming. Mechanism on diaspore and kaolinite collected by sodium oleate[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(2): 298-301.
[14] LAI R W, FUERSTENAU D. Model for the surface charge of oxides and flotation response[J]. Trans Soc Min Eng AIME, 1976, 260: 104-107.
[15] 高利坤. 细粒难选金红石矿分步浮选工艺及理论研究[D] . 昆明: 昆明理工大学, 2009.
GAO Li-kun. Distribution floatation theoretic and theoretical research on refractory fine rutile ore[D]. Kunming: Kunming University of Science and Technology, 2009.
[16] SHOWTZU A, POLKIN C. Influence of pulp pH on the floatability of titanium minerals[J]. Russian Journal of Non-ferrous Metals, 1961(2): 27.
[17] 彭文世, 刘高魁. 矿物红外光谱图集[M]. 北京: 科学出版社, 1982: 114.
PENG Wen-shi, LIU Gao-kui. Infrared spectra collection of minerals[M]. Beijing: Science Press, 1982: 114.
[18] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 35.
WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation[M]. Changsha: Hunan Science and Technology Press, 1988: 35.
(编辑 陈卫萍)
基金项目:教育部博士点基金资助项目(2012KJJH02,20120162110008);湖南省科技计划项目(2012SK3233);湖北省博士后创新管理岗位项目(2012年博士后专项);中国有色集团科技计划项目(2012KJJH02,2013KJJH03);国家博士后基金面上项目(2013M531813)
收稿日期:2013-07-24;修订日期:2013-11-20
通信作者:王 军,副教授,博士;电话:0731-88876557;E-mail:wjwq2000@126.com