β-环糊精衍生物对萘普生对映体的包合作用
陈圆圆1,刘佳佳1,唐课文1, 2,张文灵1
(1. 中南大学 化学化工学院,湖南 长沙 410083;
2. 湖南理工学院 化学化工系,湖南 岳阳 414000)
摘 要:采用紫外分光光度法和液相色谱法研究β-环糊精衍生物与萘普生的包合作用,考察羟丙基β-环糊精与萘普生包合反应的热力学常数;加入乙醇和改变溶剂的离子强度以进一步对其包合机理进行探讨;用红外光谱法 对固体包合物进行表征。研究结果表明:羟丙基β-环糊精和萘普生的包合比为1?1,整个包合反应的热力学常数?G,?H和?S都小于0,说明该过程为自发放热过程;在水溶液中,主体的包合能力较弱;加入乙醇以后表观包结稳定常数降低;溶剂离子强度增加有利于包合反应的发生;萘普生溶解度随pH值增大及羟丙基β-环糊精浓度的增大而增大,但表观稳定常数却随pH值增大而减小;环糊精衍生物对R, S-萘普生外消旋体具有不同的识别能力。
关键词:萘普生;β-环糊精衍生物;包合作用;紫外分光光度法;液相色谱法
中图分类号:O065.8 文献标识码:A 文章编号:1672-7207(2008)03-0474-06
Inclusion between β-cyclodextrin derivatives and naproxen
CHEN Yuan-yuan1, LIU Jia-jia1, TANG Ke-wen1, 2, ZHANG Wen-ling1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology,
Yueyang 414000, China)
Abstract: The inclusion stability constants of naproxen(NAP) with β-cyclodextrin derivatives (HPCD, HECD, MECD) in aqueous solution were studied by UV(ultraviolet rays) and HPLC(high performance liquid chromatography). Thermodynamic parameters for the binding of NAP and HPCD were investigated. The effects of alcohol and ionic strength on inclusion behavior were investigated. Stable inclusion complex in solid state was characterized by IR spectra. The results show that host-guest complex with molar ratio of 1?1 is formed. Three hosts all have weak binding ability with NAP in aqueous solution. The solubility of naproxen increases with the increase of pH value and the concentration of HPCD, but the apparent stability constants decrease with the increase of pH value. ?G, ?H and ?S are all less than 0, so the inclusion process is a spontaneous and exothermic process. Binding ability of HPCD increases with the increase of ionic strength; β-cyclodextrin derivatives have chiral recognition ability for R- and S-naproxen.
Key words: NAP; β-cyclodextrin derivatives; inclusion; ultraviolet rays; high performance liquid chromatography
近年来,β-环糊精(CD)及环糊精衍生物的分子识别应用备受关注[1-2]。CD的外亲水、内疏水特殊结构,使它能对一些客体分子有很强的包合能力。研究结果表明,进入CD筒形空腔内部的客体分子得到保护,从而表现为抗氧化、抗光照、热稳定性增加、缓释、增溶等特点。人们对β-CD的包合作用机理进行了研究,一般认为包合反应的发生主要与主客体分子间三维空间排列的相互匹配性、范德华力、色散力、偶 极-偶极相互作用、电荷转移作用、静电作用力、氢键、疏水作用等分子间相互作用有关[3-4]。羟丙基β-环糊精、羟乙基β-环糊精和甲基β-环糊精是CD 分子空腔端口的部分羟基被取代得到的衍生物,其水溶性 较CD大大提高,又由于空腔立体结构没有改变,所以,β-CD仍具有很强的包合能力[5]。
萘普生(naproxen,简称NAP) 即α-甲基-6-甲氧基-2-萘乙酸,是一种重要的非甾体消炎、解热、镇痛 药,主要用于治疗类风湿关节炎、风湿性脊椎炎和产后、术后止痛等疾病。其α-位有1个手性碳原子,存在1对光学异构体,其中S-萘普生在体内的抗炎活性是R-型的35倍,为了减少给药量和对人体产生的毒副作用,必须对其进行手性拆分。目前,采用β-环糊精对萘普生外消体进行拆分的研究未见报道。在此,本文作者采用紫外光谱和液相色谱法研究水溶性β-CD衍生物对萘普生外消旋体的包合作用,以期为萘普生新剂型的制备和消旋体的拆分提供理论依据。
1 试剂与实验仪器
a. 试剂:萘普生,浙江仙居药业有限公司生产,质量分数大于98%,熔点为163~164 ℃;羟丙基β-环糊精(HPCD)、羟乙基β-环糊精(HECD)和甲基β-环糊精(MECD),山东新大精细化工有限公司生产。
b. 实验仪器:TU-1901双光束紫外可见光分光光度计,北京谱析通用仪器有限公司制造;10 mm石英比色皿;高效液相色谱HPLC1200,德国安捷伦科技有限公司制造;Nicolet 560傅里叶变换红外光谱 仪,美国Nicolet公司制造。
2 实验内容与方法
2.1 检测波长的确定
取一定量的萘普生对照品,用适量乙醇溶解,再用蒸馏水配制成澄清溶液。HPCD用蒸馏水配制成澄清溶液,在200~400 nm内扫描。结果表明,萘普生在230 nm处的吸光度最大,HPCD 在此处无吸收,故选230 nm为实验检测波长。
2.2 标准曲线的绘制
称取萘普生10 mg,置于100 mL量瓶中,加无水乙醇溶解,并稀释至刻度,摇匀,分别量取0.1,0.2,0.3,0.4,0.5和0.6 mL 置于10 mL容量瓶中,加水至刻度,在最大吸收波长(230 nm)处测吸光度,以萘普生浓度(X)为横坐标,吸光度(Y)为纵坐标,绘制标准曲线,经回归处理得线性回归方程:Y = 0.362 49X+ 0.137 47 (相关系数为0.999 9)。由此可见,萘普生浓度在1.0~6.0 mg/ L 范围内基本呈线性关系。
2.3 包合常数的测定
移取1 mL已配制好的1.5×10-4 mol/L NAP溶液于10 mL容量瓶中,分别加入不同量的5×10-2 mol/L HPCD水溶液,使其浓度为0,0.010,0.015,0.020,0.025,0.030和0.035 mol/L。依据实验要求改变温度(288,298,308 K),乙醇浓度(体积分数为1%,3%,7%,10%,15%,20%,25%)和Na2SO4 浓度(0.05,0.1,0.15,0.2,0.25 mol/L)。定容后经常温超声振荡,静置一段时间后,进行紫外分析,最大吸收波长选择在230 nm处。以相应浓度的HPCD水溶液作参比溶液,扣除溶剂水和HPCD的吸收量。
2.4 主体与客体形成超分子体系的化学计量
假定环糊精(H)与萘普生(G)间形成化学计量1?1的超分子包合物,则体系中存在如下平衡:
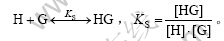
式中:“[ ]”表示浓度,mol/L。在实验中,保持萘普生的浓度远远小于环糊精的浓度,即[H]>>[G]。因此,形成超分子包合物的稳定常数可根据修饰的Benesi-Hildebrand方程进行计算[6-7]:

2.5 HPCD与NAP的增溶作用
称取HPCD 适量,配制成浓度分别为0,5,10,15,20 和25 mmol/ L 的HPCD 磷酸缓冲溶液(pH值为2.0,5.0,6.5),取上述溶液各25 mL,加30 mg萘普生,超声30 min,置于特定温度的水浴振荡器中振荡3 d。过滤,弃去初滤液,取上清液经0.45 μm 微孔滤膜过滤,滤液用水适当稀释后用紫外分光光度计测定其在最大波长处(230 nm)的吸光度,计算药物含量。以HPCD含量为横坐标,药物含量为纵坐标,绘制溶解度曲线。
2.6 R,S-NAP 的HPLC分析方法
取NAP 0.01 g用乙醇溶解至100 mL,用水稀释配制成10~200 mg/L系列溶液,色谱条件参照文献[8]中方法:色谱柱为C18ODS柱(长×宽为4.6 mm×250 nm);流动相为乙醇和0.5%的三乙胺水溶液(pH=3.5),两者体积比为15?85;pH值用乙酸调节,其中,含25 mmol/L β-CD;柱温为25 ℃,流速为1.0 mL/min;检测波长为254 nm。以峰面积(A)与R,S-NAP浓度(c)进行回归,线性回归方程分别为AS-NAP=1.816 87×106c- 21.885 46 (r = 0.999 1);AR-NAP=1.762 9×106c-29.310 54 (r =0.999 1);R,S-NAP浓度在10~200 mg/L范围内呈良好线性关系。
2.7 不同β-CD衍生物与R,S-NAP选择性的增溶 作用
称取β-CD衍生物(甲基β-CD,羟乙基β-CD,羟丙基β-CD)适量,配制成浓度分别为0,5,10,15,20 和25 mmol/L溶液,取上述溶液各10 mL,加30 mg萘普生,超声30 min,置于特定温度的水浴振荡器中振荡3 d。过滤,弃去初滤液,取上清液经0.45 μm 微孔滤膜过滤,滤液用水适当稀释后用HPLC测定峰面积,计算药物含量。以β-CD衍生物含量为横坐标,药物含量为纵坐标,绘制溶解度曲线。
2.8 NAP-HPCD 包合物的制备
称取NAP和HPCD适量,两者摩尔比为1?1,用少量蒸馏水将HPCD磨匀,分次加入NAP,置研钵中充分磨成糊状,于40 ℃干燥,过0.18 mm筛即得。
3 结果与讨论
3.1 NAP在羟丙基β-环糊精介质中的紫外光谱分析
图1所示为R,S-萘普生与β-环糊精体系的紫外光谱图。可见,加入环糊精前后,在200~400 nm范围内,最大吸收波长基本上无位移,但吸光度随环糊精浓度增加而上升,这表明NAP分子与CD之间存在某种相互作用。该现象可能与CD空腔内高电子流密度诱导客体分子电子发生移动有关,也可能由于β-CD 与萘普生发生了主客体包合反应,而包合程度受分子匹配性、电子效应和空间效应等的影响。
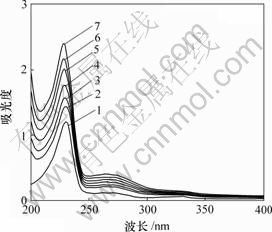
c(HPCD)/(mol?L-1): 1—0; 2—0.010; 3—0.015; 4—0.020;
5—0.025; 6—0.030; 7—0.035
图1 HPCD浓度对NAP 吸收光谱的影响
Fig.1 Influence of HPCD concentration on ultraviolet spectrum of NAP
3.2 乙醇对包合作用的影响
为了进一步考察NAP与HPCD 包合过程中的主要驱动力,研究了乙醇对包合平衡常数的影响。图2所示为NAP与HPCD的lg K随乙醇体积分数的变化关系。可以看出,随着水溶液中有机溶剂含量的增加,NAP和环糊精的稳定常数降低。这可能是因为疏水相互作用是环糊精在水体系中进行分子键合的一个重要驱动力,这样,水溶液中有机组分含量的增加必将导致疏水驱动力下降。
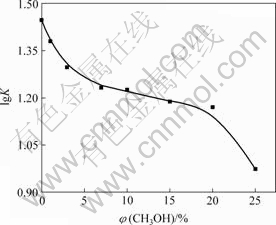
图2 乙醇浓度对NAP与HPCD lg K的影响
Fig.2 Influence of ethanol concentrates on lg K of NAP and HPCD
3.3 离子强度对包合平衡常数的影响
Na2SO4 的加入是为了改变体系的离子强度,因为离子强度的增大将使体系的疏水作用增强[9]。图3所示为NAP与HPCD的lgK随Na2SO4浓度的变化情况。从图3可以看到,随着体系疏水作用的增强,NAP与HPCD的平衡常数急剧增大,这从另一个方面证明了上述结论的正确性,即对于NAP与HPCD的包合过程,疏水作用是一个主要驱动力。
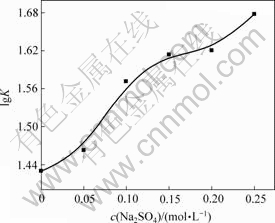
图3 离子强度对NAP与HPCD lg K的影响
Fig.3 Influence of Na2SO4 concentrations on lg K of
NAP and HPCD
3.4 HPCD与NAP包合作用的热力学常数
根据HPCD与NAP的分子结构推断其包合反应的推动力主要为疏水作用、范德华力、氢键以及空腔内高能水的释放。这里对该包合反应的包合稳定常数及相关热力学常数进行研究。根据反应温度及该温度下的稳定常数K,可由范特霍夫方程计算各热力学参数[10]。

以ln K 对1/T进行线性回归,得到:Y=3 404.9X-8.5,r=0.998 0。由直线斜率和截距可分别求出包合反应的焓变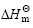 和熵变
和熵变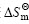 ,各热力学参数见表1。
,各热力学参数见表1。
表1 不同温度下NAP和HPCD的包合稳定常数和热力学常数
Table 1 Stability constants and thermodynamic parameters for binding of NAP and HPCD at different temperatures
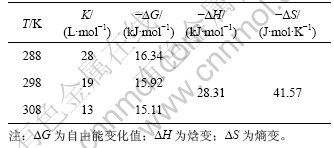
从表1可知:
a. NAP和HPCD的包合反应适宜在低温下进行;
b. ?G<0,说明该包合过程是一个自发过程;
c. ?H<0,?S<0,说明该反应是放热反应,负的焓变来源于主客体分子的范德华力及释放HPCD空腔内的高能水;分子的几何形状引起的空间障碍和空腔对客体分子平移和旋转自由度的限制引起负的熵 变[11]。
3.5 HPCD与NAP的增溶作用
不同羟丙基-β-环糊精浓度,不同pH条件下羟丙基-β-环糊精包合萘普生的结果如图4所示。由图4可知,萘普生的浓度随HPCD浓度的增大而增大,该体系相溶解度图属于AL型,呈线性增加,表明HPCD 对萘普生以摩尔比1?1进行包合。此时,正反应速率和逆反应速率相等,溶液溶解度达到饱和。
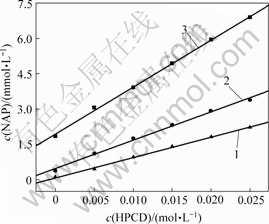
pH: 1—2.0;2—5.0;3—6.5
图4 不同pH值下HPCD与NAP的相溶解度
Fig.4 Phase solubility of naproxen and HPCD concentrations at different pH values
表观稳定常数(K)[12]是衡量包合物稳定性的重要参数,包合物的表观稳定常数由回归方程求得:
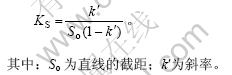
对图4进行线性回归,得出不同pH值条件下的回归方程,据此又可得出不同pH 值时的表观稳定常数,结果见表2。
表2 不同pH值下的回归方程和表观稳定常数
Table 2 Regression equation and stability constants at different pH values

由表2可见,萘普生溶解度随pH值增大及HPCD 浓度的增大而增大,但表观稳定常数却随pH值的增大而减小,表观稳定常数越小,说明HPCD 对药物稳定作用越弱,解离度升高,使HPCD包合作用减弱。pH值为2.0时,NAP(pKa=4.2)完全处于非电离状态,此时,包合物呈现较高的稳定常数[13],因为环糊精衍生物的疏水性空腔对不带电状态下的药物具有更强的亲和力。
3.6 不同β-CD衍生物对R, S-NAP选择性的增溶作用
萘普生外消旋体在给定的HPLC条件下达到了所需要的分离效果,其色谱图如图5所示。采用该方法可检测不同β-CD衍生物对R,S-NAP选择性增溶作用。
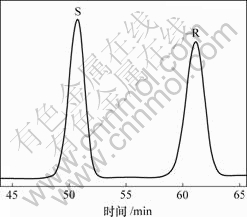
图5 NAP外消旋体的色谱图
Fig.5 Chromatogram of NAP
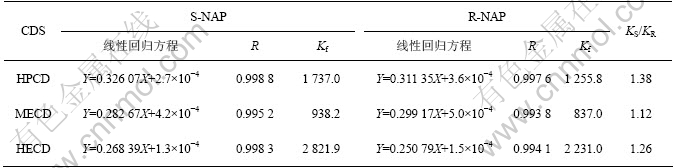
β- CD 衍生物甲基-β-环糊精、羟乙基-β-环糊精、羟丙基-β-环糊精对R,S-NAP选择性增溶作用的结果如表3所示。
从表3可以看出,S-萘普生与环糊精衍生物有较强的包合作用;各体系的KS/KR都不大。这些结果表明,环糊精衍生物对萘普生分子的包合作用与手性识别能力既可能与主、客体之间尺寸适合关系、萘普生分子中各基团的空间排布等有关,也可能与主体腔径、大环构造、修饰基团的结构与性质等因素有关。组成相同、空间结构稍有差别的R-,S-萘普生外消旋体与同一主体具有不同程度的键合作用,表明可以感知客体结构上的细微差异,KS/KR最大达到1.38,使异构体较好地分离,意味着利用这类环状主体可以实现对手性萘普生的鉴定、分离以及对外消旋体的化学拆分。
表3 不同主体对萘普生的增溶作用
Table 3 Solublization of naproxen with HECD, HPCD and MECD
3.7 红外光谱研究
比较HPCD,NAP,HPCD 和NAP混合物及NAP-HPCD 包合物的红外光谱(如图6所示),发现NAP的羰基的振动吸收峰在1 710 cm-1处,HPCD 和NAP混合物中羰基的振动吸收峰没有移动,而在NAP-HPCD包合物中羰基的振动吸收峰基本上都消失,论证了HPCD与NAP 包合物的形成和存在。
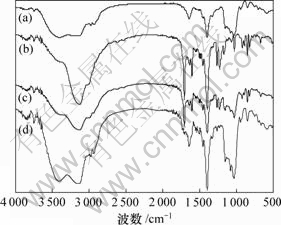
(a) HPCD;(b) NAP;(c) HPCD与NAP混合物;(d) HPCD与NAP包合物
图6 HPCD,NAP 及其混合物、包合物的红外光谱图
Fig.6 IR spectra of HPCD, NAP, mixture of HPCD and NAP and inclusion complex
4 结 论
a. 羟丙基β-环糊精和萘普生的包合比为1?1;加入乙醇以后表观包合稳定常数降低。溶剂离子强度的增加有利于包合反应的发生。整个包合反应的热力学常数?G,?H和?S都小于0,该过程为自发放热过程。
b. 萘普生溶解度随pH值增大及HPCD 浓度的增大而增大,但表观稳定常数却随pH值的增大而减小,红外光谱的研究结果也论证了HPCD与NAP 包合物的形成和存在。
c. 环糊精衍生物对R,S-萘普生外消旋体具有不同的识别能力,具有一定的手性识别效果。
参考文献:
[1] 宋乐新, 柯晓康, 郭子建. 荧光标识的环糊精二聚体与小肽衍生物之间的包合行为研究[J]. 化学学报, 2001, 60(8): 1419-1427.
SONG Le-xin, KE Xiao-kang, GUO Zi-jian. Study on inclusion behavior of labeling CD dimers with a fluorescence tracer to small peptide derivatives[J]. Acta Chim Sinica, 2001, 60(8): 1419-1427.
[2] Giordano F, Novak C, Moyano J R. Thermal analysis of cyclodextrin and their inclusion compounds[J]. Thermochim Acta, 2001, 380: 123-151.
[3] Sbai M, Lyazidi S, Ait Lerner D A, et al. Modified β-cylclodextrins as enhancers of fluorescence emission of carbazole alkaloid derivatives[J]. Analytica Chimica Acta, 1995, 303: 47-55.
[4] 杨 郁, 双少敏, 钞建宾, 等. β-环糊精对氨基苯甲酸同分异构体的分子识别作用研究[J]. 化学学报, 2004, 62(2): 176-182.
YANG Yu, SHUANG Shao-min, CHAO Jian-bin, et al. Study on the molecular recognition interaction of β-cyclodextrin with aminobenzoic acid isomer[J]. Acta Chim Sinica, 2004, 62(2): 176-182.
[5] Agnes B B, Lajos B. Influence of the guests, the type and degree of substitution on inclusion complex formation of substituted β-cyclodextrins[J]. Talanta, 1999, 49(3): 577-585.
[6] Jurgen K, Charles A H. Modelling multiple chemical equilibria in chiral partition systems[J]. Chemical Engineering Science, 2001, 56: 5853-5864.
[7] Pickering P J, Chaudhuri J B. Equilibrium and kinetic studies of the enantios-elective complexation of (D/L)-phenylalanine with copper(Ⅱ) N-decyl-(L)-hydroxy-proline[J]. Chemical Engineering Science, 1997, 52: 337-386.
[8] 宁凤容, 黄可龙, 焦飞鹏. HPCD手性流动相HPLC法拆分萘普生对映体的色谱保留机制和拆分机理研究[J].化学通报, 2006, 69(6): 425-429.
NING Feng-rong, HUANG Ke-long, JIAO Fei-peng. Study on retention characteristics and separation mechanism of enantiomers of naproxen with HPCD as a mobile phase modifier in HPLC[J]. Chemistry Online, 2006, 69(6): 425-429.
[9] Shehatta I, El-Askalany A H, Gomaa E A. Thermodynamic parameters of transfer and solution of oxalic acid in dimethylsulphoxide-water media[J]. Thermochimica Acta, 1993, 219: 65-72.
[10] Calabrò M L, Tommasini S, Donate P, et al. Effects of α- and β-cyclodextrin complexation on the physico-chemical properties and antioxidant activity of some 3-hydroxyflavones[J]. Journal of Pharmaceutical and Biomedical Analysis, 2004, 35(2/16): 365-377.
[11] Bettinetti G P, Mura P, Liguori A, et al. Solubilization and interaction of naproxen with cyclodextrins in aqueous solution and in the solid state[J]. Farmaco, 1989, 44: 195-213.
[12] Li P, Tabibi E, Yalkowsky S H. Combined effect of complexation and pH on solubilization[J]. Journal of Pharmaceutical Sciences, 1998, 87(12): 1535-1537.
[13] Zerrouk N, Corti G, Ancillotti S, et al. Influence of cyclodextrins and chitosan, separately or in combination, on glyburide solubility and permeability[J]. Journal of Pharmaceutical and Biomedical Analysis, 2006, 62(3): 241-246.
收稿日期:2007-08-10;修回日期:2007-10-06
基金项目:国家自然科学基金资助项目(20776038);中国博士后科学基金资助项目(2004035650)
通信作者:刘佳佳(1963-),男,湖南隆回人,教授,从事药物的生物有机合成与制备工作;电话:0731-8836834;E-mail: liujj0903@163.net