

不同气氛条件下生物焦的热解路径及脱汞机理
贾里,郭晋荣,王彦霖,张永强,李泽鹏,刘丁赫,金燕
(太原理工大学 电气与动力工程学院,山西 太原,030024)
摘要:针对电厂烟气中的主要成分N2,O2和CO2,研究生物质在3种热解气氛条件下的热解过程,通过验证所提出的反应路径,揭示多气相组分条件下生物质的热解演变机理;在综合研究所生成生物焦的微观特性及单质汞吸附性能的基础上,利用程序升温脱附技术和吸附动力学揭示生物焦的单质汞吸附机理。研究结果表明:生物质在N2气氛条件下的热解过程可分为3个阶段;在O2条件下生物质主要有3种热解路径,且在5%~7%的O2体积分数之间存在临界阈值,该体积分数范围内生物质的氧化异相反应由氧的扩散过程控制,而超出该范围后,反应由动力学控制,且反应加速进行;CO2可通过Boudouard反应在750 ℃以后与生物焦直接发生气化反应,且随着CO2体积分数升高,气化反应提前且程度加强;生物质热解过程中存在大量平行进行的一级反应,构成了明显不同的复杂反应阶段,所对应的表观活化能E在30~300 kJ/mol范围内变化;相比N2气氛热解条件,O2会降低生物焦对Hg0的吸附能力;CO2则会提高生物焦对Hg0的吸附能力,其中20%体积分数的CO2作为热解气氛时,热解所形成的生物焦汞吸附能力最强;Hg0在吸附过程中先与生物焦表面的官能团结合,形成化学吸附;当化学吸附饱和后再进行物理吸附,通过不同方式所吸附的汞以一种混合形式赋存在生物焦表面,其中化学吸附的主要产物为Hg-OM和HgO。
关键词:热解气氛;生物焦;汞;反应路径;吸附机理
中图分类号:X51 文献标志码:A 开放科学(资源服务)标识码(OSID)
文章编号:1672-7207(2021)06-2011-12
Pyrolysis path and mercury removal mechanism of biochar in different atmospheres
JIA Li, GUO Jinrong, WANG Yanlin, ZHANG Yongqiang, LI Zepeng, LIU Dinghe, JIN Yan
(College of Electrical and Power Engineering, Taiyuan University of Technology, Taiyuan 030024, China)
Abstract: Pyrolysis process of biomass in three kinds of pyrolysis atmospheres was analysed, aiming at the main components of N2, O2 and CO2 in power plant flue gas. By verifying the proposed reaction path, the pyrolysis evolution mechanism of biomass under the condition of multi-phase components was revealed. Based on a comprehensive study of mercury adsorption characteristics and microscopic characteristics of biochar, the temperature-programmed desorption technique and the adsorption kinetics were employed to systematically explore the reaction mechanism of Hg0 adsorption. The results show that pyrolysis process of biomass can be divided into three stages in N2 atmosphere. Three pyrolysis reaction pathways are possible in O2 atmosphere. There is a critical threshold between 5%-7% O2 volume fraction. The heterogeneous reaction of biomass oxidation is controlled by the diffusion of O2 when the volume fraction is lower than the critical range. When the volume fraction exceeds the critical range, the reaction is accelerated and controlled by kinetics. Above 750 °C, CO2 can react directly with the biochar through Boudouard reaction. With the increase of CO2 volume fraction, the gasification reaction can proceed ahead of time and be intensified. There are a large number of parallel first-order reactions during the process of biomass pyrolysis, which constitute obviously different and complex reaction stages. The obtained apparent activation energy E value varies in the range of 30-300 kJ/mol. Compared with N2 pyrolysis atmosphere, Hg0 adsorption capacities of the biochars obtained in O2 pyrolysis atmosphere are worse. CO2 can improve the Hg0 adsorption capacity. When 20% CO2 volume fraction is selected as the pyrolysis atmosphere, biochar formed has the strongest mercury adsorption capacity. During the adsorption process, Hg0 firstly combines with the functional groups on the surface of biochar to form chemical adsorption. When the chemical adsorption is saturated, the physical adsorption occurs. The mercury adsorbed by various ways on the biochar surface is in a mixed form, among which the main products of chemical adsorption are Hg-OM and HgO.
Key words: pyrolysis atmosphere; biomass char; mercury; reaction path; adsorption mechanism
汞对人体健康危害极大,而煤炭燃烧后释放的汞占我国总汞排放量的50%以上。由于特殊的能源结构,中国火电厂燃烧的煤占全年产量的60%,同时,火电厂由于单台设备容量大、燃煤量多,所以,污染物的排放相对集中。现阶段尚无专门的控制汞排放装置,根据我国目前情况,能够与现有静电除尘器(ESP)和布袋除尘器(FF)等设备联用的吸附剂喷射法将成为具有发展前景的燃煤烟气汞排放控制技术[1-2],其中,活性炭喷射技术已得到应用,但其存在竞争吸附、成本高和温度域窄等问题,因此,开发高效廉价的可替代吸附剂具有重要意义。
生物焦作为生物质热解的固体产物,具有复杂的孔隙结构和良好的表面化学特性,国际上已开展利用生物焦脱除燃烧污染物的研究[3-4]。电厂锅炉煤燃烧后烟气所形成的高温,可以热解生物质,获得汞吸附剂,之后在温度较低的适宜区间高效脱除气态汞。但是,烟气中含有的O2和CO2会对生物质的热解过程产生影响,因此,研究不同热解气氛对生物焦单质汞吸附特性的影响是探索低费用脱汞工艺的必要前提。
现阶段人们对生物质热解的研究主要针对无氧气氛,通过考察热解过程对应的产物分布,获得生物质组分的热解反应规律及机理。其中,生物质热解过程反应复杂,主要以裂解反应和缩聚反应为主,而且在无氧条件下,原料种类、升温速率、热解温度、停留时间和颗粒粒径等热解条件均会对热解产物的产率和构成产生影响[5-7]。而生物质在不同气氛下的热解过程与无氧时存在较大差异。在高温热解条件下,热解气氛可以与热解过程中所形成的生物焦、释放的挥发分发生耦合反应,导致生物质热解过程变得复杂,进而影响热解产物生物焦的性质。但是,有关热解气氛对生物质热解过程的作用机理研究较少。煤的氧化性气氛不仅可以促进挥发性有机物的裂解,也可以与挥发性有机物发生反应进一步形成焦油及半焦,而还原性气氛则可以促进生成煤热解自由基[8-10]。
生物焦对汞的吸附与其特性有关,目前虽然已有关于相应气氛对其他碳基材料热解特性影响的研究,但由于相关影响会随热解物种类不同而存在较大差异,所获结论也不尽相同。另外,关于热解气氛对生物焦汞吸附特性影响和生物焦吸附汞的动力学研究也相对较少,相关机理解释不充分。本文在综合研究热解气氛对生物焦汞吸附特性影响的基础上,结合生物焦的微观特性,利用程序升温脱附技术和吸附动力学探究相应反应机理,以期为脱汞方法提供关键数据和理论依据。
1 研究对象与方法
1.1 生物焦的制备与表征
选取核桃壳(WS)作为料,利用破碎机进行破碎,并利用振筛机进行粒径分级,获得粒径为58~75 μm的核桃壳生物质。利用生物焦固定床制备实验系统,在不同气氛制备条件下热解10 min后放入干燥器中完成生物焦样品的制备,制备温度为800 ℃。
为了模拟电厂锅炉实际烟气环境,在O2气氛热解过程中,气氛分别设定为3%(体积分数,下同)O2+97% N2,5% O2+95% N2和7% O2+93% N2;在CO2气氛热解过程中,气氛分别设定为10% CO2+90% N2,15% CO2+85% N2和20% O2+80% N2。另外,还将生物质在N2气氛条件下进行1 h热处理(温度为900 ℃),去除生物焦表面的大部分官能团[11],记为WS-900N2。
通过TGA/SDTA851型热重分析仪获得生物焦的热解特性,其中,热解温度范围为50~1 000 °C,升温速率为10 °C/min,生物质填充量为100 mg;采用ASAP 2460型分析仪进行N2吸附和脱附实验,获得样品的孔隙结构参数;通过Vertex80V型傅里叶变换红外光谱分析仪,对生物焦的官能团进行分析。
1.2 固定床汞吸附/脱附实验方法
在固定床汞吸附实验过程中,汞蒸汽由放置在U形高硼硅玻璃管内的汞渗透管产生。为确保实验过程中Hg0质量浓度稳定,将U形管放置在恒温水浴箱内(42 ℃)。VM3000汞连续在线监测仪测量固定床出口汞质量浓度,采样间隔时间为1 s,生物焦吸附剂装填量为1 g。根据VM3000仪器自身的进气量要求,实验气体总流量设定为1.4 L/min,由载气和平衡气组成,且携带Hg0的N2流量为500 mL/min,在固定床入口处通入的平衡气(N2)流量为900 mL/min。
本文采用汞穿透率η和单位质量生物焦累积汞吸附量q研究生物焦的汞吸附性能。汞穿透率η为某一时刻ti固定床出口处烟气中Hg0质量浓度与固定床入口处Hg0初始质量浓度的比值,如式(1)所示。在相同实验条件下,同一时刻η越高,则对应生物焦样品的脱汞性能越差。
 (1)
(1)
式中: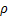 in为固定床入口Hg0初始质量浓度,ng/L;
in为固定床入口Hg0初始质量浓度,ng/L;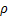 out为ti时刻固定床出口处烟气中Hg0质量浓度,ng/L。
out为ti时刻固定床出口处烟气中Hg0质量浓度,ng/L。
单位质量生物焦汞吸附量q是指从吸附开始到ti时刻为止,生物焦所吸附Hg0的总量,如式(2)所示。
 (2)
(2)
式中:q为0~ti时刻单位质量生物焦对Hg0的吸附总量,ng/g;F为流经生物焦的N2流量,L/s;m为生物焦装填量,ng;t为吸附时间,s。
在升温脱附实验过程中,将进行过单质汞吸附实验的生物焦样品填入固定床汞吸附实验装置中,并在总气流流量为1.4 L/min的N2气氛条件下,以10 ℃/min的升温速率将固定床反应器从室温升温到950 ℃,同时,利用VM3000检测出口汞质量浓度,从而获得吸附汞后生物焦样品随温度提高的汞释放量,进而获得汞在生物焦吸附剂中的赋存形态。
2 汞吸附特性及热解路径
2.1 汞吸附特性
图1所示为不同热解气氛条件下生物焦的汞吸附穿透率,图2所示为不同热解气氛条件下生物焦的单位累积汞吸附量。由图1~2可见:在180 min吸附时间内,WS-800N2样品的穿透率为64%,单位累积汞吸附量为1 715.4 ng/g;当O2体积分数分别为3%,5%和7%时,所制备样品在180 min吸附时间内的汞穿透率分别为93%,87%和96%,对应的单位累积汞吸附量分别为996.98,1 262.87和1 104.18 ng/g,但3个样品对Hg0的吸附能力均弱于WS-800N2样品的吸附能力。
随着CO2体积分数增大,生物焦样品的汞吸附能力呈整体逐渐增强的趋势,当CO2体积分数分别为10%,15%和20%时,所制备样品在180 min吸附时间内穿透率分别为75%,51%和41%,对应的单位累积汞吸附量分别为1 614.74,2 665.77和2 842.89 ng/g,且对Hg0的吸附能力均强于O2气氛热解获得的生物焦,但其中WS-10%CO2样品对Hg0的吸附能力弱于WS-800N2样品对Hg0的吸附能力。
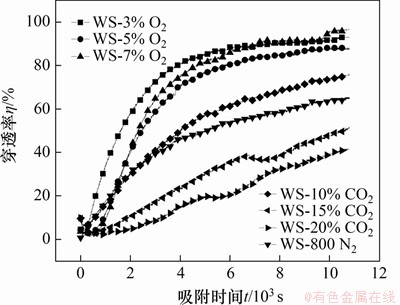
图1 不同热解气氛条件下生物焦的汞吸附穿透率
Fig. 1 Mercury adsorption penetration coefficients of biochars prepared in different pyrolysis atmospheres
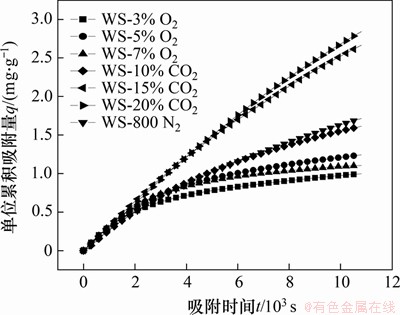
图2 不同热解气氛条件下生物焦的单位累积汞吸附量
Fig. 2 Total amount of mercury adsorbed per unit mass of biochars prepared in different pyrolysis atmospheres
表1 生物质的热解特性参数
Table 1 Characteristic pyrolysis parameters of biomass

2.2 热解特性
不同热解气氛条件下核桃壳生物质的热解曲线如图3所示,相关热解特性参数如表1所示。其中,T1为质量损失率约为10%时所对应温度,即挥发分的初始析出温度,℃;T2为热解第2阶段中挥发分最大析出速率所对应温度,℃;T3为热解第2阶段的终止温度,℃;T4为对应于(dw/dt)2max的温度,℃;(dw/dt)1max为热解过程中第1个质量损失峰所对应的最大质量损失速率,即DTG峰值,%/min;(dw/dt)2max为O2和CO2制备气氛下热解过程中第2个质量损失峰所对应的最大质量损失速率,%/min;V为热解过程中的总质量损失率,%。
2.2.1 N2热解气氛
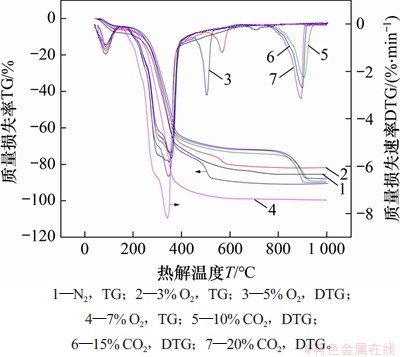
图3 生物质在不同热解气氛条件下的热解曲线
Fig. 3 Pyrolysis curves of biomass in the different atmospheres
核桃壳生物质在N2气氛下的热解过程大致可分为3个阶段。
1) 第1阶段(室温~210 ℃)主要发生了生物质的失水和内部解聚重组,同时为挥发分的析出和其他有机组分的分解做准备工作。
2) 第2阶段(210~550 ℃)为生物质中第一类和第二类有机物的析出和分解阶段,其中,第一类有机物相对分子质量较小,主要为具有挥发性和半挥发性的生物可降解物质(如半纤维素等),分子链短且带有支链,无定型结构。而第二类有机物的裂解和转化则需要更高热解温度,这类物质主要包括纤维素和木质素等,具有更稳定的晶体结构,化学键强度高。
3) 第3阶段(550 ℃~热解终温)为固定碳、其他含碳物质以及矿物质的分解阶段。由于生物质的灰分较低,所以最终质量损失率高达80%左右,说明此时可燃组分已基本完全热解,并最终形成热解产物即生物焦。
2.2.2 O2热解气氛
生物质在O2气氛下的热解过程则较复杂。研究发现,生物质中固定碳和挥发分的多相燃烧只能发生在其纯热解过程几乎完成之后[12],所以,生物质在含氧气氛中可能存在2种极端反应路径:1) 路径A+B表示生物质先热解为挥发分和固定碳,然后进行挥发分和固定碳的燃烧;2) 路径C表示生物质中的固定碳和挥发分同时发生多相氧化燃烧,产生相应的燃烧产物为CO2,CO和H2O等。
一般可燃物的有氧热解路径属于这2种情况中的一种或介于两者之间,主要取决于可燃物种类和反应条件(如粒度、燃烧温度和氧分压等)。由于生物质在N2气氛下的热解曲线只出现1个峰,根据以上热解路径假设,生物质在不同氧气体积分数气氛下的热解DTG曲线则有可能出现3种情况,如图4所示。
1) 热解过程中DTG曲线出现2个热解峰,其中第1个峰完全与生物质在N2热解气氛条件下所形成的热解峰重合,而第2个峰则为固定碳和挥发分的燃烧峰,即为典型的A+B热解路径,在该反应过程中,热解速度要比多相氧化速度快,且氧化气氛对热解过程的影响可以忽略。
2) DTG曲线仅出现1个较大的单峰,而且先于对应的N2热解质量损失峰出现,同时(dw/dt)max较大,即为典型的C热解路径,在该反应过程中,燃烧速度比热解速度快。
3) DTG曲线出现2个峰,第1个峰也是先于对应的N2热解质量损失峰出现,只是形状不同,而第2个在高温区出现的是固定碳和挥发分的燃烧峰,该热解模式是介于A+B路径和C路径之间,为纯热解和多项氧化的综合结果,在该反应过程中,生物质的多项氧化有利于化学键的断开和挥发分的产生。
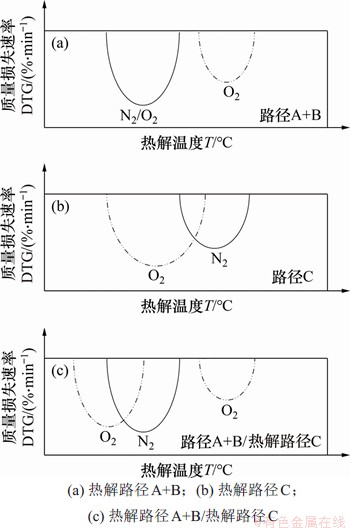
图4 O2气氛条件下生物质不同热解路径假设对应的DTG曲线
Fig. 4 DTG curves corresponding to different pyrolysis paths assumptions of biomass in O2 atmospheres
根据实验结果可知,生物质在3% O2制备体积分数下的热解路径为A+B,而且相比N2气氛热解条件,(dw/dt)1max较小,说明少量氧气会抑制挥发分的析出,主要是由于氧气参与了生物焦表面的交联反应[13]。随着O2体积分数增大到5%,于450 ℃开始出现第2个失重峰,所对应的(dw/dt)2max增大至-3.01%/min,促进了该温度区域内挥发分的析出,说明其热解路径介于A+B和C之间。而生物质在体积分数7% O2条件下,提前在150 ℃左右开始形成一个较大的单峰,且(dw/dt)1max大幅增大为-8.20%/min,说明其热解路径为C,均相及非均相反应程度都得到明显加强[14-15],燃烧所引起的质量损失对热解的抑制作用比氧气更显著。
所以,对于核桃壳生物质,5%~7%的O2可能存在临界体积分数,该体积分数以下生物质的氧化异相反应由氧的扩散过程控制;超出该体积分数时,反应由动力学控制,且反应加速进行[16],从而出现较大的氧化燃烧质量损失峰。
2.2.3 CO2热解气氛
在CO2热解气氛条件下,生物质在低于750 ℃时具有与在N2气氛条件下相似的质量损失规律,但整体质量损失率比N2气氛高,且T2向高温区移动,主要是由于CO2可以与焦油分子发生反应[17]。同时,第1个热解峰所对应的(dw/dt)1max较小,这是因为生物质热解所产生的挥发分在CO2中的扩散速率较慢,且CO2气氛下生物焦颗粒内部温度较低[18],而且CO2会抑制酚类和芳香物质的生成,其中酚类主要来自木质素[19],会抑制对汞的吸附[20]。所以,在该温度范围内,CO2作为热解气氛对挥发分的析出过程是不利的[21]。
当热解温度高于750 ℃时,生物质会在900 ℃附近出现在N2气氛下没有的质量损失峰,这是因为CO2还可通过Boudouard反应与生物焦直接发生气化反应(CO2+C→2CO),而且随着CO2体积分数升高,第2个热解峰所对应的(dw/dt)2max也随之增大,对应热解温度与反应温度区间也有减小的趋势,说明气化反应提前,且反应程度加剧。
2.2.4 热解动力学研究
由于生物质热解过程中存在大量平行进行的一级反应,构成了明显不同的复杂反应阶段,化学反应速率随之变化,因此,为了准确获得生物焦的热解特性及机理,采用分布活化能模型(如式(3)所示)研究不同热解阶段的反应动力学参数,结果如图5所示。图5中α为热解率,即某反应阶段的质量损失率与总质量损失率的比值。
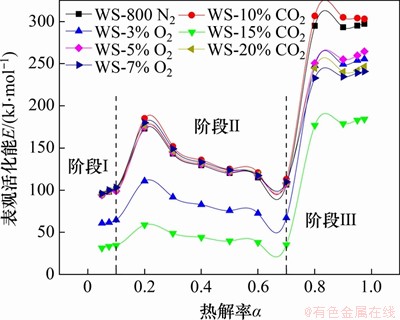
图5 不同热解气氛条件下生物质的热解反应动力学参数
Fig. 5 Kinetic parameters of pyrolysis reactions in different pyrolysis atmospheres
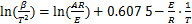 (3)
(3)
式中:T为热解温度,K;R为通用气体常数,8.314 J/(mol·K);β为升温速率,K/min;E为表观活化能,kJ/mol;A为对应于活化能E的频率因子,为量纲一变量。
在生物质整个热解过程中,表观活化能E在30~300 kJ/mol范围内变化。在热解第1阶段(α≤0.1),随着水分析出以及酚类和醇类物质分解,E略微增大。当热解过程进行到第2阶段时,反应初期对应的E较高,但逐渐下降,这是由于第一类和第二类有机物成分复杂,对应的裂解重组过程导致了化学反应能垒提高跃迁,但随着反应进行,样品的孔隙结构变得发达,利于热解过程中所形成的烟气及焦油类物质扩散,进而增强反应活性。当热解率大于0.7时,热解反应进行到第3阶段,活化能远高于其他阶段的活化能,且变化较小,这是由于虽然此时参与反应的物质种类趋于单一,且以含碳物质的分解为主,反应大多符合1级,但反应壁垒较高,需要较高的能量。
其中,对于WS-3%O2样品,由于O2所参与的交联反应抑制了挥发分的析出,所以,相比N2气氛条件下的热解过程,第2阶段和第3阶段的E较高。而随着O2体积分数提升至5%和7%时,由于均相及非均相反应程度得到了显著增强,所以,E大幅降低,热解过程加速进行。WS-7%O2样品所对应的E最低。同理,由于CO2作为热解气氛会抑制挥发分的析出和酚类物质的生成,所以,第1阶段和第2阶段对应的反应能垒略有提升。随着CO2体积分数升高,气化反应提前且程度加强,第3阶段所对应的表观活化能大幅降低。
2.3 孔隙结构
不同热解气氛条件下生物焦的孔隙结构参数如表2所示(其中,孔隙丰富度Z为单位容积下的比表面积)。
表2 不同热解气氛条件下生物焦的孔隙结构参数
Table 2 Pore structures of biochars prepared in different pyrolysis atmospheres
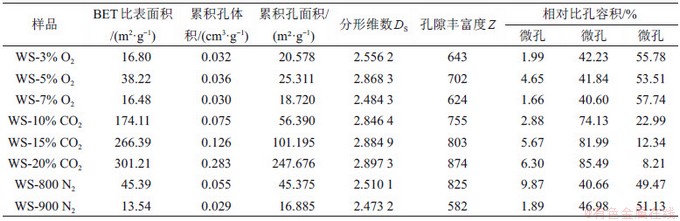
随着O2体积分数增加,生物焦的BET比表面积、累积孔体积、分形维数和孔隙丰富度均呈现先增高后减小的趋势,其中WS-5%O2样品的孔结构参数最大。一方面,随着扩散到生物焦本身所形成的初始孔隙中的O2增多,孔隙内部的反应增强,促进了挥发分的进一步析出与新孔隙的生成,利于孔隙结构的发展;另一方面,在5% O2下,生物质在热解过程中没有发生剧烈的燃烧反应,利于孔隙结构的保留,所以,相比其他在不同O2体积分数下所制备的生物焦,其微孔和介孔大量生成,且分形维数较高,表面结构无序、紊乱,所形成的孔较深,孔隙丰富度较高,孔隙发达,利于对汞的吸附[22]。但随着O2体积分数进一步升高至7%,扩散到孔隙内部和表面的O2与生物质发生了剧烈的均相及非均相反应,导致孔壁和表面的烧蚀程度增加,孔隙结构坍塌,甚至发生小孔互相贯通的现象,孔隙丰富度与比表面积大幅下降,分形维数降低,且孔结构有向大孔发展的趋势。由于当O2体积分数为3%时,会对生物质整个热解过程中挥发分的析出产生抑制作用,因此,所形成生物焦的孔隙结构参数较小,不利于对汞的吸附。
生物质在N2气氛条件下只发生热解碳化,该过程对生物焦孔隙结构影响有限,而在CO2气氛条件下,由于CO2本身作为一种活化剂[23],在高温下可以与生物焦发生相应活化反应,热解过程中的挥发分析出量较大,所以,随着CO2体积分数增大,热解所生成生物焦的BET比表面积和累积孔体积也逐渐增大,其中WS-15%CO2和WS-20%CO2样品具有大量利于汞吸附的微孔和介孔,孔隙发达,对汞的吸附能力远大于其他热解气氛生成的生物焦样品对汞的吸附能力。
另外,对于WS-900N2样品,由于热解温度较高,不利于孔隙结构的形成,所以,孔隙丰富度较低,同时,大孔的相对比孔容积高达51.13%。
2.4 表面化学特性
不同热解气氛条件下所获得生物焦的红外光谱图可分为4个主要区域:羟基振动区(3 600~3 000 cm-1)、脂肪CH振动区(3 000~2 700 cm-1)、含氧官能团振动区(1 800~1 000 cm-1)和芳香CH的面外振动区(900~700 cm-1),分别记为a,b,c和d。生物焦红外光谱图如图6所示。研究官能团种类和含量的过程中,选择合适的峰形函数(Gauss Amp型或Area型)组合进行最小二乘法迭代求解并拟合分峰面积,表征具体的官能团含量,拟合所得到的相关参数如表3所示。
表3 不同热解气氛条件下生物焦的官能团拟合结果
Table 3 Fitting results of biochars prepared in different pyrolysis atmospheres
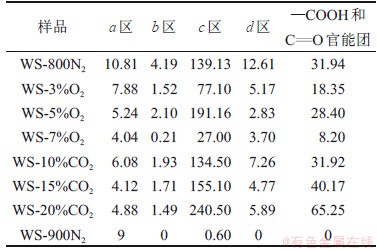
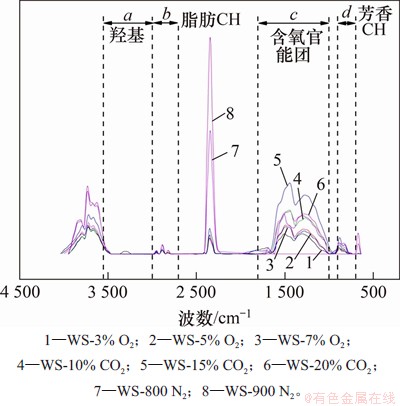
图6 不同热解气氛条件下生物焦的红外光谱图
Fig. 6 Infrared spectra of biochars prepared in different pyrolysis atmospheres
在不同气氛的热解过程中,生物焦的羟基含量均不同程度减小,说明生物质在热解过程中有—OH官能团脱落,反应析出了水分,并且对酚类和醇类物质的产生有抑制作用[24]。相比于N2气氛,O2和CO2体积分数增大促进了生物质中脂肪类结构和芳香甲基侧链的断裂,因此,脂肪—CH振动区中官能团的含量逐渐减少。
含氧官能团是生物质热解进行的活性基团[25],主要分布在2个振动频率段:
1) 1 150~1 350 cm-1频率段主要为C—O官能团的伸缩振动区,由于CO2气化作用,CO气体析出量增大,所以,相应含量减小,同时也说明生物焦分子中的醚、酯类结构相对含量减小[26];
2) 1 600 cm-1频率段附近的伸缩振动属于芳香族中芳核的C=C官能团,芳烃碳骨架是生物质分子中的主要结构[27],在热解过程中会发生聚合反应[28],而CO2在气化过程中,会破坏生物焦的相应结构[29],所以,其含量会随着CO2体积分数升高而逐渐降低。
另外,由于生物焦表面可以在高温条件下通过芳环裂解的方式和CO2反应,从而促进含氧官能团的生成,尤其是羰基和羧基,进而利于对汞的吸附,同时也说明CO2会促进酮类和杂环成分的生成,这也导致在CO2气氛条件下生物质挥发分析出量增加。但是10%CO2气氛条件对生物焦样品相关官能团的促进作用不明显,主要是由于CO2抑制了生物质在低温区的热解过程。
相比其他热解气氛,在7%O2条件下,生物质会发生剧烈的热解反应,所以,WS-7%O2样品的表面官能团含量大幅下降。WS-5%O2样品的相应含氧官能团保留情况较好,这是由于生物焦在热解过程中,其原始活性位可以提供O2在表面的吸附位点,O2又能促进生物焦表面含氧官能团的形成或补充含氧官能团所消耗的氧原子。对于WS-3%O2样品,由于热解过程受到抑制,不利于其表面官能团的形成,所以,相应含量比WS-800N2样品的含量低。WS-900N2样品由于在N2气氛条件下于900 ℃进行了1 h热处理,所以,表面大部分含氧官能团含量大幅下降,且脂肪CH振动区的相应官能团消失。
3 单质汞在生物焦表面的吸附动力学及机理研究
3.1 吸附动力学
综合评价吸附剂的脱汞性能时,初始汞吸附速率是需要考虑的一个重要参数,因为当吸附剂被喷射到锅炉烟道内后,在烟道内的停留时间仅3~5 s,在这较短的停留时间内,初始汞吸附速率f决定了吸附剂的喷射脱汞效率。其中,初始汞吸附速率可由准二级动力学方程获得,如式(4)所示。
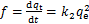 (4)
(4)
式中:qe为平衡时单位质量生物焦的吸附量,ng/g;k2为准二级速率常数,ng/(g·min)。
表4 初始汞吸附速率计算结果
Table 4 Results of initial mercury adsorption rates

随着O2体积分数增大,生物焦的初始汞吸附速率先增强再减弱,但均比WS-800N2样品的低;随着CO2体积分数增大,生物焦的初始汞吸附速率整体呈现逐渐增强的趋势,且均远大于O2气氛下所获得的生物焦,但其中WS-10%CO2样品的初始汞吸附速率低于WS-800N2样品的初始汞吸附速率;初始汞吸附速率与吸附剂的汞吸附容量呈整体正相关关系。以上结果验证了本文所获得的结论。
3.2 程序升温脱附
根据Mars-Maessen反应机理[30]和前面研究结果,单质汞在生物焦表面吸附的过程中,一部分发生物理吸附,另一部分则经由表面官能团反应生成不同种类的氧化汞和有机汞Hg-OM,因此,通过不同方式所吸附的汞是以混合形式赋存在生物焦表面。不同吸附方式中生物焦对汞的吸附结合能不同,物理吸附的汞一般可在较低温度脱附,化学吸附的汞则需要较高温度。这是因为物理吸附是由汞和生物焦分子间作用力所引起,因而结合力较弱,吸附热较小,脱附速度较快;化学吸附时,汞与生物焦表面官能团发生电子转移、交换或共有,通过相关化学键进行吸附,所涉及的力也远高于范德华力,因而脱附速率较慢,同时也需要更多能量。
在程序升温脱附过程中,根据生物焦表面不同赋存形态汞的键能不同,对应会有不同的分解曲线,所以,在相同升温条件下,通过热解曲线即可获得所吸附汞开始释放的温度及峰值温度,从而确定生物焦中汞的吸附方式和赋存形式。研究表明,在程序升温脱附过程中,生物焦通过物理作用所吸附的单质汞从160 ℃开始脱除[31],但是因为不同物质的孔隙结构具有差异,故无相应固定脱附峰值温度;在化学吸附中,羧基和羰基与Hg0进行异相反应生成的有机汞Hg-OM所对应脱附峰温度为210 ℃左右,而HgO所对应脱附峰的温度为300 ℃左右,其主要为生物焦表面其他含氧官能团对Hg0的氧化所致。
不同热解气氛条件下所制备生物焦吸附汞后的程序升温汞脱附结果如图7所示,由于在汞吸附脱附的平衡计算过程中会受到流速波动和测量误差的影响,所以,汞平衡率在70%~130%范围内,表明实验结果具有准确性,而本文的脱附结果满足相关要求。
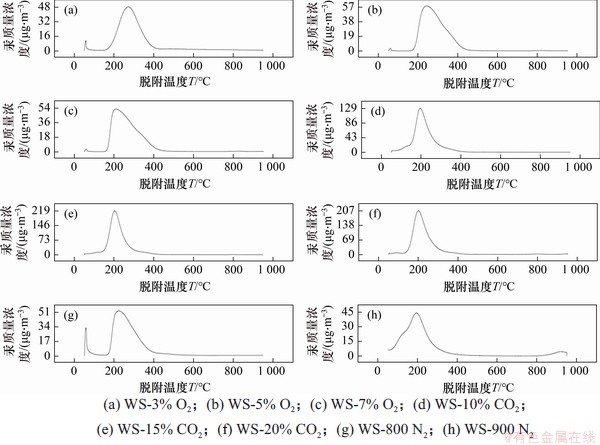
图7 吸附汞后生物焦的TPD曲线
Fig. 7 Temperature programmed decomposition profile of biochars after adsorption
由于WS-900N2样品没有相关化学吸附所需的官能团,所以,可以得到180 ℃即为核桃壳生物焦物理吸附Hg0的脱附峰对应温度,且脱附起始温度为140 ℃左右。CO2气氛条件下所制备生物焦的脱附峰温度为200 ℃左右,且脱附起始温度和终止温度均相应降低。一方面,由于CO2气氛条件下所制备生物焦的孔隙结构良好,利于所吸附的汞由生物焦颗粒内向外的扩散传质过程,进而导致物理吸附的单质汞脱附起始温度降低;另一方面,由于该样品具有的含氧官能团较多,所生成的有机汞Hg-OM含量较大,与物理吸附单质汞的脱附出现部分重叠。同时,由于低于350 ℃的脱附过程仍在进行,说明也有HgO脱附,但含量较少,未出现明显的脱除峰。对于WS-800N2样品,在吸附过程中,除了通过物理吸附的Hg0外,也生成了部分Hg-OM和HgO。
WS-7%O2样品对Hg0主要进行物理吸附,由于孔隙结构较差,所以,脱附峰值温度为205 ℃左右,且没有出现明显的有机汞Hg-OM脱附峰重叠现象。相比之下,WS-3% O2样品主要通过吸附位点对Hg0进行化学吸附,但所含有能够生成Hg-OM的羧基和羰基含量较小,所以,也未在210 ℃出现明显的有机汞Hg-OM脱附峰,同时,样品脱附峰温度为290 ℃左右,说明其在吸附过程中生成了HgO,这主要是由于Hg0在生物焦表面发生均相氧化,其机理[32]如式(5)和(6)所示。
O2+O2→O3+O(5)
Hg+O3→HgO+O2 (6)
而WS-5% O2样品的脱附峰温度为239 ℃,这是由于表面官能团含量较多,对Hg0主要进行化学吸附,所对应的主要脱附产物为Hg-OM。
3.3 生物焦对单质汞的吸附机理
在程序升温脱附过程中,生物焦所吸附的汞经历了解吸脱附过程,即为吸附的逆过程,因此,结合前面研究结果,并基于活性位点吸附机理和Mars-Maessen反应机理,可以获得不同热解气氛条件下所制备生物焦的汞吸附机理,如图8所示,吸附过程主要通过“活性位点”发生。
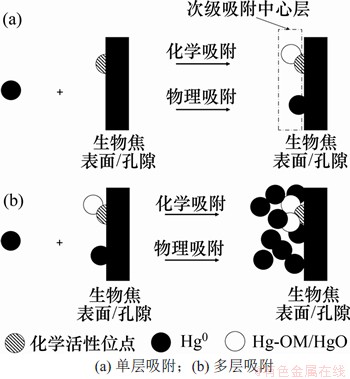
图8 单质汞在生物焦表面的物理与化学吸附机理
Fig. 8 Mechanism of physical and chemical Hg0adsorption by biochar
在生物焦对汞的吸附过程中,小部分单质汞以化学吸附的方式直接被以羰基和羧基为主的官能团吸附在化学活性位点上,同时,形成单层或多层的“次级吸附中心层”,其他单质汞可以进一步吸附在“次级吸附中心层”外,每一层之间都具有一定能量差。生物焦孔隙结构越发达,孔体积越大,越利于多层吸附的发生。而大部分单质汞与生物焦表面接触,发生碰撞,通过范德华力(包括色散力和静电力等)吸附在生物焦表面和孔隙结构中。当脱附温度较低时,还不足以破坏范德华力,只能使得吸附在生物焦最外层表面的小部分汞脱离。当温度继续升高时,这种物理吸附被完全破坏,并伴随着化学吸附中相关吸附键的断裂,导致生物焦中大部分汞脱附,因此,生物焦中汞的脱附过程在一个温度区间内持续进行。同时,随着化学活性位点在相应温度的破坏,“次级吸附中心层”也随之消失,由于没有多层吸附作用,其他通过物理吸附的汞随之脱附。另外,也可以推断单质汞与生物焦表面接触后,先与官能团结合,形成化学吸附,当化学吸附饱和后再进行物理吸附。
4 结论
1) 相比N2气氛热解条件,O2会降低生物焦对Hg0的吸附能力,CO2则会提高生物焦对Hg0的吸附能力,且随着CO2体积分数增大,其汞吸附能力逐渐增强。
2) 在3% O2,生物质先热解为挥发分和固定碳,而固定碳和挥发分会在7% O2下同时发生剧烈的多相氧化燃烧,相比之下,生物质在5% O2下的热解反应则为纯热解和多项氧化的综合结果;生物质在CO2气氛下的热解过程中,CO2可通过Boudouard反应在750 ℃以后与生物焦直接发生气化反应,从而改变生物质的挥发分析出和生物焦形成过程,促进孔隙结构和表面官能团的发展。
3) 在生物焦对汞的吸附过程中,小部分单质汞以化学吸附方式直接被以羰基和羧基为主的官能团吸附在化学活性位点上,同时形成单层或多层的“次级吸附中心层”,其他单质汞可以进一步吸附在“次级吸附中心层”外。
4) 单质汞在生物焦表面吸附的过程中,通过不同方式所吸附的汞以混合形式赋存在生物焦表面,其中生物焦通过化学吸附的主要产物为Hg-OM和HgO。
参考文献:
[1] 张波, 仲兆平, 丁宽, 等. 凹凸棒土的吸附脱汞特性[J]. 中南大学学报(自然科学版), 2015, 46(2): 723-727.
ZHANG Bo, ZHONG Zhaoping, DING Kuan, et al. Adsorption removal of mercury by attapulgite sorbent[J]. Journal of Central South University(Science and Technology), 2015, 46(2): 723-727.
[2] 李立清, 吴绍康, 李海龙, 等. CeO2-TiO2催化剂低温协同控制燃煤NOx与汞[J]. 中南大学学报(自然科学版), 2016, 47(3): 1049-1057.
LI Liqing, WU Shaokang, LI Hailong, et al. Simultaneous removal of NOx and mercury over CeO2-TiO2 catalyst at low flue gas temperature[J]. Journal of Central South University(Science and Technology), 2016, 47(3): 1049-1057.
[3] 王静, 邵龙义, 耿春梅, 等. 燃煤排放PM10的氧化性损伤能力[J]. 中南大学学报(自然科学版), 2014, 45(6): 2137-2143.
WANG Jing, SHAO Longyi, GENG Chunmei, et al. Oxidative capacity of PM10 emitted by burning coals[J]. Journal of Central South University(Science and Technology), 2014, 45(6): 2137-2143.
[4] 郭学益, 肖彩梅, 梁莎, 等. 改性柿子粉吸附剂对Cd2+的吸附性能[J]. 中南大学学报(自然科学版), 2012, 43(2): 412-417.
GUO Xueyi, XIAO Caimei, LIANG Sha, et al. Adsorption of Cd2+ by chemically modified persimmon powder[J]. Journal of Central South University(Science and Technology), 2012, 43(2): 412-417.
[5] DAI Leilei, WANG Yunpu, LIU Yuhuan, et al. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass[J]. Science of the Total Environment, 2020, 749: 142386.
[6] KOSTETSKYY P, BROADBELT L J. Progress in modeling of biomass fast pyrolysis: a review[J]. Energy & Fuels, 2020, 34(12): 15195-15216.
[7] MUIGAI H H, CHOUDHURY B J, KALITA P, et al. Co-pyrolysis of biomass blends: Characterization, kinetic and thermodynamic analysis[J]. Biomass and Bioenergy, 2020, 143: 105839.
[8] WANG Pengqian, WANG Chang'an, WANG Chaowei, et al. Synergistic effects in rapid co-pyrolysis of semi-coke and coal at high temperature[J]. Fuel, 2020, 282: 118795.
[9] ZHANG Kang, LU Peng, GUO Xutao, et al. High-temperature pyrolysis behavior of two different rank coals in fixed-bed and drop tube furnace reactors[J]. Journal of the Energy Institute, 2020, 93(6): 2271-2279.
[10] DONG Liang, WANG Ziming, ZHANG Yadong, et al. Study on pyrolysis characteristics of coal and combustion gas release in inert environment[J]. Journal of Chemistry, 2019, 2019: 1-9.
[11] SHIN S, JANG J, YOON S H, et al. A study on the effect of heat treatment on functional groups of pitch based activated carbon fiber using FTIR[J]. Carbon, 1997, 35(12): 1739-1743.
[12] GONG Zhiqiang, WANG Zhentong, WANG Zhenbo, et al. Study on pyrolysis characteristics of tank oil sludge and pyrolysis char combustion[J]. Chemical Engineering Research and Design, 2018, 135: 30-36.
[13] BORREGO A G, ALVAREZ D. Comparison of chars obtained under oxy-fuel and conventional pulverized coal combustion atmospheres[J]. Energy & Fuels, 2007, 21(6): 3171-3179.
[14] SENNECA O, CHIRONE R, SALATINO P, et al. Patterns and kinetics of pyrolysis of tobacco under inert and oxidative conditions[J]. Journal of Analytical and Applied Pyrolysis, 2007, 79(1/2): 227-233.
[15] OHLEMILLER T J, KASHIWAGI T, WERNER K. Wood gasification at fire level heat fluxes[J]. Combustion and Flame, 1987, 69(2): 155-170.
[16] FANG M X, SHEN D K, LI Y X, et al. Kinetic study on pyrolysis and combustion of wood under different oxygen concentrations by using TG-FTIR analysis[J]. Journal of Analytical and Applied Pyrolysis, 2006, 77(1): 22-27.
[17] GUIZANI C, ESCUDERO SANZ F J, SALVADOR S. Effects of CO2 on biomass fast pyrolysis: Reaction rate, gas yields and char reactive properties[J]. Fuel, 2014, 116: 310-320.
[18] MOLINA A, SHADDIX C R. Ignition and devolatilization of pulverized bituminous coal particles during oxygen/carbon dioxide coal combustion[J]. Proceedings of the Combustion Institute, 2007, 31(2): 1905-1912.
[19] CARLSON T R, JAE J, LIN Yuchuan, et al. Catalytic fast pyrolysis of glucose with HZSM-5: The combined homogeneous and heterogeneous reactions[J]. Journal of Catalysis, 2010, 270(1): 110-124.
[20] ZHAO Shilin, PUDASAINEE D, DUAN Yufeng, et al. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies[J]. Progress in Energy and Combustion Science, 2019, 73: 26-64.
[21] BAI Yonghui, WANG Yulong, ZHU Shenghua, et al. Structural features and gasification reactivity of coal chars formed in Ar and CO2 atmospheres at elevated pressures[J]. Energy, 2014, 74: 464-470.
[22] ZHAO Shilin, DUAN Yufeng, YAO Ting, et al. Study on the mercury emission and transformation in an ultra-low emission coal-fired power plant[J]. Fuel, 2017, 199: 653-661.
[23] JIA Li, FAN Baoguo, HUO Ruipeng, et al. Study on quenching hydration reaction kinetics and desulfurization characteristics of magnesium slag[J]. Journal of Cleaner Production, 2018, 190: 12-23.
[24] ZHAO Shilin, DUAN Yufeng, LI Yaning, et al. Emission characteristic and transformation mechanism of hazardous trace elements in a coal-fired power plant[J]. Fuel, 2018, 214: 597-606.
[25] LAURENDEAU N M. Heterogeneous kinetics of coal char gasification and combustion[J]. Progress in Energy and Combustion Science, 1978, 4(4): 221-270.
[26] JIA Li, FAN Baoguo, LI Ben, et al. Effects of Pyrolysis Mode and Particle Size on the Microscopic Characteristics and Mercury Adsorption Characteristics of Biomass Char[J]. Bioresources, 2018, 13(3): 5450-5471.
[27] DELA PUENTE G, IGLESIAS M J, FUENTE E, et al. Changes in the structure of coals of different rank due to oxidation—effects on pyrolysis behaviour[J]. Journal of Analytical and Applied Pyrolysis, 1998, 47(1): 33-42.
[28] ARENILLAS A, PEVIDA C, RUBIERA F, et al. Characterisation of model compounds and a synthetic coal by TG/MS/FTIR to represent the pyrolysis behaviour of coal[J]. Journal of Analytical and Applied Pyrolysis, 2004, 71(2): 747-763.
[29] RATHNAMR K, ELLIOTTLK, WALLTF, et al. Differences in reactivity of pulverised coal in air (O2/N2) and oxy-fuel (O2/CO2) conditions[J]. Fuel Processing Technology, 2009, 90(6): 797-802.
[30] YANG Jianping, ZHU Wenbing, ZHANG Shibo, et al. Role of flue gas components in Hg0 oxidation over La0.8Ce0.2MnO3 perovskite catalyst in coal combustion flue gas[J]. Chemical Engineering Journal, 2019, 360: 1656-1666.
[31] JIA Li, FAN Baoguo, YAO Yuxing, et al. Study on the elemental mercury adsorption characteristics and mechanism of iron-based modified biochar materials[J]. Energy & Fuels, 2018, 32(12): 12554-12566.
[32] YANG Jianping, LI Qin, LI Min, et al. In situ decoration of selenide on copper foam for the efficient immobilization of gaseous elemental mercury[J]. Environmental Science & Technology, 2020, 54(3): 2022-2030.
(编辑 秦明阳)
收稿日期: 2020 -10 -06; 修回日期: 2020 -12 -12
基金项目(Foundation item):国家自然科学基金资助项目(U1510135,U1810126,U1910214);山西省高等学校科技创新项目(2020L0073)(Projects(U1510135, U1810126, U1910214) supported by the National Natural Science Foundation of China; Project(2020L0073) supported by the Science and Technology Innovation Program of Colleges and Universities of Shanxi Province)
通信作者:金燕,博士,教授,从事燃煤污染物控制、污泥能源化利用等研究;E-mail:jinyan@tyut.edu.cn
DOI: 10.11817/j.issn.1672-7207.2021.06.031
引用格式:贾里, 郭晋荣, 王彦霖, 等. 不同气氛条件下生物焦的热解路径及脱汞机理[J]. 中南大学学报(自然科学版), 2021, 52(6): 2011-2022.
Citation:JIA Li, GUO Jinrong, WANG Yanlin, et al. Pyrolysis path and mercury removal mechanism of biochar in different atmospheres[J]. Journal of Central South University(Science and Technology), 2021, 52(6): 2011-2022.