文章编号:1004-0609(2007)06-0997-05
废锂离子电池中集流体与活性物质的分离
卢毅屏,夏自发,冯其明,龙 涛,欧乐明,张国范
(中南大学 资源加工与生物工程学院,长沙 410083)
摘 要:针对废旧锂离子电池回收工艺中电极集流体的分离问题,根据集流体、活性物质、粘结剂的物理化学性质差异,对高温焙烧法、物理擦洗法和稀酸浸出?搅拌擦洗法分离集流体与活性物质进行研究。结果表明:高温焙烧与物理擦洗法都不能完全使集流体分离出来,而通过稀酸溶解?搅拌擦洗联合作用分离效果良好,在硫酸浓度为0.5 mol/L、固液比1?10、搅拌速度200 r/min、反应时间为40 min的条件下,可以实现正负极活性物质与集流体的分离,铝箔和铜箔可直接作为产品回收,只有极少部分进入浸出液,浸出渣用硫酸再浸,可以使钴、锂全部溶出,净化除杂后可回收钴和锂。
关键词:废锂离子电池;集流体;活性物质;稀酸浸出
中图分类号:TM 912.9;X 705 文献标识码:A
Separation of current collectors and active materials from spent lithium-ion secondary batteries
LU Yi-ping, XIA Zi-fa, FENG Qi-ming, LONG Tao, OU Le-ming, ZHANG Guo-fan
(School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The separation and recovery of valuable metals from spent lithium-ion battery were investigated. Based on different physical and chemical properties among the current collectors, active materials and binder, three methods, high-temperature calcination, stirring scrubbing and dilute acid leaching with stirring scrubbing, were used to study the separation of active materials from current collectors. Factors influencing this process such as leaching concentration, reaction time and the ratio of liquid to solid were studied. The results show that the high-temperature calcination and stirring scrubbing are invalid because the current collectors and active materials can not be separated. The dilute acid leaching with stirring scrubbing is the most optimum method. The optimum separation can be obtained in 0.5 mol/L sulfuric acid solution with ratio of liquid to solid of 1?10, and stirring at 200 r/min for 40 min at room temperature. The active materials containing LiCoO2 and C are leached out by H2SO4 and can be used for the further recovery of cobalt and lithium. The aluminum foil and copper foil are obtained as product by cleaning current collectors.
Key words: spent lithium-ion secondary battery; current collectors; active materials; diluted acid leaching
锂离子电池由于工作电压高、体积小、质量轻、比能量高、无记忆效应、无污染、自放电小、循环寿命长等优点成为21世纪发展起来的理想能源[1]。目前锂离子电池的制造成本不断降低,应用不断拓展,消费量不断提高,到2002年,我国超过1亿只,2004年达到7.6亿只[2]。最近,锂离子电池又开始向动力电池发展,这又将大大促进锂离子电池的进一步发展。
锂离子二次电池的使用寿命通常在几百次至 1 000次左右,但因为其巨大的消耗量和应用前景,废弃问题仍然是不容忽视的,由此带来的环境污染和资源浪费问题也日益突出。因此对锂离子电池中Co、Ni、Li、Al、Cu和钢铁等资源进行回收[3],既防止了丢弃电池对环境造成的污染,同时还使有限的资源得以利用。这不仅具有经济效益,在环保方面也具有重要意义。
现有的各种锂离子电池的一般回收方法是:用强酸溶解铝钴膜,使得集流体和活性物质钴酸锂全部溶解进入溶液,再分离回收各种金属元素。该处理方式工艺简单,但是浸出液复杂的成分无疑对后续的净化及产品生产过程提出较高的要求。因此,预先分离集流体与活性物质对各种有价金属的回收是十分有利的。
本文作者针对废锂离子电池回收中集流体与活性物质的分离问题,研究高温焙烧法、物理擦洗法、稀酸浸出法对集流体与活性物质的分离效果并提出了可行的回收方案。
1 实验
1.1 原料
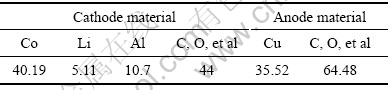
小型实验所需的废锂离子二次电池从手机维修店收集,额定容量范围为550~1 250 mA?h。将废旧锂离子二次电池除去包装及外壳,取出电芯,分离出正极、负极材料。表1所列为实验原料的成分分析结果。
表1 实验原料的组成
Table 1 Composition of spent anode and cathode material (mass fraction, %)
1.2 原理
在锂离子电池制造工艺中,PVDF(聚偏二氟乙烯),VF2(偏二氟乙烯)的均聚物和VF2的共聚物VF2/HFP(六氟丙烯)是常用的粘结剂,在调制电极浆料时,将粘结剂用NMP溶解,与正极活性物质或负极活性物质混合调成浆料,再涂覆在金属集流体上制成薄膜电极[4]。
由电极中各材料的物理化学性质及制备方法可知,将正极材料中的复合氧化物与铝箔进行分离主要有以下3条途径:1) 高温分解或溶解粘结剂;2) 溶解集流体;3) 破坏接触界面。
有研究通过加热处理正极材料,使粘结剂分解挥发[5],再采用浮选法或重选法分离出正极活性物质。Contestabile等[6]和秦毅红等[7]采取有机溶剂溶解锂钴膜上粘结剂PVDF,直接回收钴酸锂、金属铜和铝。由于LiCoO2不溶于碱,钟海云等[8]和吴芳等[9]在酸浸之前用NaOH预先除去大部分铝,而钴全部留在碱浸渣中。但以上方法都有其局限性,如高温焙烧能耗高,设备投资大,并且会产生有毒的含氟气体;而有机溶剂价格昂贵且使用量大,生产成本高,回收系统投资大,对生态环境污染大,对生产人员的身体健康也有一定的危害;而碱溶除铝难以回收有价值的纯铝箔,通过选择性沉淀回收铝,分离效果不够理想。
锂离子电池在使用过程中,粘结剂受到极性有机溶剂电解液的溶剂化侵蚀而发生溶解和溶胀,使得电极中复合氧化物与集流体的接触变得疏松,甚至脱落,因此尝试采用物理擦洗的方法将两者进行分离。
纯铝箔在稀硫酸中的溶解速度很慢,纯铜箔不溶于稀硫酸,而涂覆在铝箔表面的正极活性物质钴酸锂可溶于稀硫酸,因此可以通过稀酸局部溶蚀集流体表面和正极活性物质,造成表面缺陷,再通过搅拌擦洗作用使活性物质钴酸锂、乙炔黑、石墨化碳等形成混合黑色粉末从电极集流体上脱落,以实现其与正负极集流体分离。铝箔、铜箔经洗涤、干燥后可直接作为产品回收,而混合黑色粉末可经进一步处理回收有价组分。其稀酸浸出示意图如图1所示。

图1 稀酸浸出示意图
Fig.1 Sketch map of dilute acid leaching
稀酸浸出过程发生反应如下:

2 结果与讨论
2.1 高温焙烧电极材料的分离效果
将试样置于马弗炉中,分别在450、500、550、600、650、700和750 ℃焙烧120 min。实验结果表明:当温度≤500 ℃时,正极材料完好,当温度≤600 ℃时,部分正极材料上的黑色粉末从铝箔上脱落,而其余正极材料完好,当温度≥650 ℃时,正极脆化,铝箔溶解为细小的白色颗粒(铝的熔点为660 ℃),与正极活性物质混合在一起,难于分离。隔膜在450 ℃时很快分解挥发,并放出刺激性气味。在450~750 ℃范围内,负极材料都变成黑色且脆化。随着电池制造技术的发展,已经有很多种粘结剂应用于锂离子电池中[10],而有些粘结剂的实际挥发温度接近或高于铝的熔点[11],从而使得焙烧法并不适合于分离铝箔与活性物质。
经高温焙烧后的正极原料,再用硫酸浸出,研究焙烧温度对钴、铝浸出率的影响。实验条件如下:焙烧时间120 min;浸出剂H2SO4浓度2 mol/L,每克正极原料添加3 mL H2O2,浸出温度80 ℃,浸出时间90 min,固液比1?20,搅拌速度400 r/min。实验结果如图2所示。
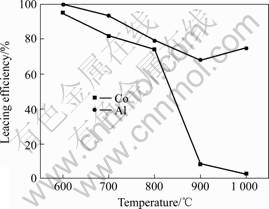
图2 焙烧温度对钴、铝浸出率的影响
Fig.2 Effects of roasting temperature on leaching rate of cobalt and aluminum
从图2可以看出,随着焙烧温度升高,钴和铝的浸出率都降低,当焙烧温度在900~1 000 ℃时,钴的浸出率明显降低,这是因为高温焙烧使LiCoO2转化为更难溶于硫酸的Co3O4[12],而铝箔焙烧后熔化成小颗粒的铝滴而分散于渣中,而且各铝滴上还粘附了许多钴渣,限制硫酸的扩散而使铝的浸出率降低。
2.2 物理擦洗对活性物质的脱除效果
将尺寸约2 cm×2 cm的正极原料8 g加入到装有320 mL水的烧杯中,常温下在Eurostar搅拌机中搅拌一定时间后用孔径1.2 mm的筛子筛分分离,视筛下产物为脱下的锂钴膜。固定搅拌时间为120 min,搅拌强度对锂钴膜脱除率的影响如图3所示;当其它条件不变,搅拌强度为400 r/min时,搅拌时间对锂钴膜脱除率的影响图4所示。

图3 搅拌强度对锂钴膜脱除率的影响
Fig.3 Effects of agitation rate on removal rate of Co-Li film
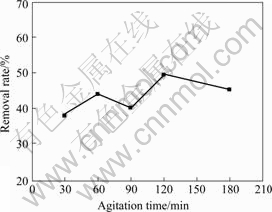
图4 搅拌时间对锂钴膜脱除率的影响
Fig.4 Effects of agitation time on removal rate of Co-Li film
由图3和4可知,通过物理擦洗的方法可以脱除一部分的锂钴膜,增加搅拌强度和搅拌时间,钴膜脱除率增加很少。这是由于粘结剂使锂钴膜与铝箔粘结紧密,单纯的物理擦洗不能完全将其脱除。对于负极来说,碳材料与铜箔的粘接极为疏松,在拆解电池时,大部分电池的负极活性物质完全从集流体上脱落。
2.3 稀硫酸浸出直接分离集流体与活性物质
将电极材料剪成大小约2 cm×3 cm,每次取5 g正极原料或5 g正极原料和4 g负极原料的混合原料投入到一定浓度的稀硫酸溶液中,在空气振荡器中进行实验。控制实验温度为25 ℃,转速为200 r/min,研究硫酸浓度、固液比、浸出时间对钴酸锂与集流体分离的影响。反应结束后过滤,用热水多次淋洗渣相,滤液和洗液合并后回收有价组分,渣相在水中用孔径2.1 mm的筛子进行筛分,筛上产物为铝箔和铜箔,筛下产物过滤、干燥得到含钴酸锂、乙炔黑、石墨化碳等的混合黑色粉末。不同反应条件对正极原料和正极+负极混合原料溶解性的影响如表2和3所示。
表2 不同反应条件对正极试样溶解性的影响
Table 2 Effects of different reactive conditions on solubility of anode sample
“?” The sample can not be separated.
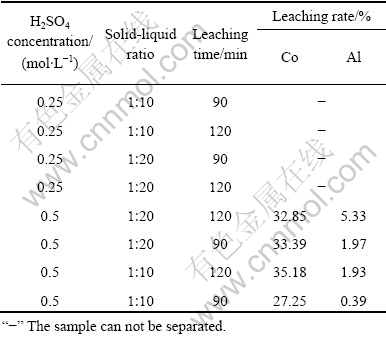
表3 不同反应条件对正极+负极混合试样溶解性的影响
Table 3 Effects of different reactive conditions on solubility of anode and cathode samples
“?” The sample can not be separated.
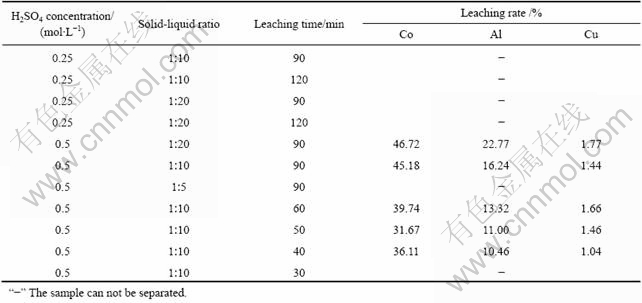
从表2可以看出,当硫酸浓度较低时,正极原料分离难以完全。控制一定的条件,可以使钴酸锂以粉末形式从铝箔上脱落,铝箔的溶解量很少且可直接筛分出来。表明硫酸溶解与物理擦洗共同作用可以实现正极活性物质与绝大部分铝箔的分离。
由表3可以看出,在一定的条件下,正极+负极的混合原料中活性物质与铝箔、铜箔可以有效分离,最佳分离条件为:硫酸浓度 0.5 mol/L、固液比1?10、反应时间 40 min。正极+负极混合原料的浸出过程中,除了有少量铝浸出外,还有少量的铜浸出。这可能是由于铜箔上的石墨化碳很容易脱落,铜箔表面被氧化生成氧化铜而溶于稀硫酸。单独浸出正极原料时,铝的溶解量比浸出正极+负极混合原料时要小,这可能是体系组成了原电池,铜箔促进了铝箔的溶解。
增加正负极原料用量在最佳酸浸条件下进行实验,正极原料+负极原料=437 g+306.63 g=743.63 g、硫酸浓度为0.5 mol/L、固液比为1?10、反应时间为40 min,实验结果如表4所示。
表4 稀酸浸出实验结果
Table 4 Experimental results of dilute acid leaching
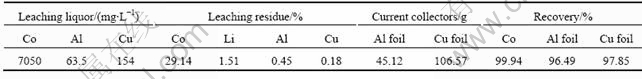
向稀酸浸出液中添加硫酸,用来再浸稀酸浸出渣,可以使钴、锂全部溶出,净化除杂后回收钴和锂[13]。
3 结论
1) 高温焙烧不能使铝箔与LiCoO2完全分离,酸溶焙烧渣实验结果表明,随着焙烧温度升高,钴和铝的浸出率都降低。
2) 物理擦洗可以脱除一部分的锂钴膜,增大搅拌强度和增加搅拌时间,正极材料脱除率仅略微上升。
3) 采用稀酸溶解与物理擦洗共同作用可以实现电极活性物质与集流体的分离,铝箔和铜箔可直接作为产品回收,其回收率可达96%以上。
4) 使用本工艺可直接回收铝箔和铜箔,与其它工艺相比,流程短,操作简单,成本低,产品价值高,不失为废锂离子电池集流体回收的新途径。
REFERENCES
[1] 郭炳琨,李新海,杨松青. 化学电源?电池原理及制造技术[M]. 长沙:中南工业大学出版社, 2000: 314?315.
GUO Bing-kun, LI Xin-hai, YANG Song-qing. Chemical power sources-battery’s theory and fabrication technology[M]. Changsha: Central South University of Technology Press, 2000.
[2] 戴永年,杨 斌,姚耀春,马文会,李伟宏. 锂离子电池的发展状况[J]. 电池,2005, 35(3): 193?195.
DAI Yong-nian, YANG Bin, YAO Yao-chun, MA Wen-hui, LI Wei-hong. Development status of Li-ion batteries[J]. Battery Bimonthly, 2005, 35(3): 193?195.
[3] 欧秀芹,孙新华,程耀丽. 废锂离子电池的综合处理方法[J]. 中国资源综合利用,2002, 6: 8?19.
OU Xiu-qin, SUN Xin-hua, CHENG Yao-li. Study on the comprehensive treatment of spent lithium-ion secondary batteries[J]. China Resources Comprehensive Utilization, 2002, 6: 18?19.
[4] 吴宇平,戴晓兵,马军旗,程预江. 锂离子电池-应用与实践[M]. 北京:化学工业出版社,2004, 344?348.
WU Yu-ping, DAI Xiao-bing, MA Jun-qi, CHENG Yu-jiang. Lithium-ion battery-application and practice[M]. Beijing: Chemistry Industry Press, 2004.
[5] 欧秀芹,孙新华,赵庆云,范 飞. 锂离子废电池资源化技术进展[J]. 无机盐工业,2005, 37(9): 11?14.
OU Xiu-qin, SUN Xin-hua, ZHAO Qing-yun, FAN Fei. Progress in recovery technology of waste lithium ion battery[J]. Inorganic Chemicals Industry, 2005, 37(9): 11?14.
[6] Contestabile M, Panero S, Scrosati B. A laboratory-scale lithium-ion battery recycling process[J]. Journal of Power Sources, 2001, 92: 65?69.
[7] 秦毅红, 齐 申. 有机溶剂分离法处理废旧锂离子电池[J]. 有色金属(冶炼部分), 2006(1): 13?16.
QIN Yi-hong, QI Shen. The treatment of waste lithium-ion batteries by organic solvent partition process[J]. Nonferrous Metals, 2006(1): 13?16.
[8] 钟海云, 李 荐, 柴立元. 从锂离子二次电池正极废料——铝钴膜中回收钴的工艺研究[J]. 稀有金属与硬质合金, 2001(1): 1?4.
ZHONG Hai-yun, LI Jian, CHAI Li-yuan. Study on technology for recycling cobalt from Li-ion secondary battery anode scrap of Al-Co film[J]. Rare Metal and Cemented Carbides, 2001(1): 1?4.
[9] 吴 芳. 从废旧锂离子二次电池中回收钴和锂[J]. 中国有色金属学报, 2004, 14(4): 697?701.
WU Fang. Recovery of cobalt and lithium from spent lithium-ion secondary batteries[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 697?701.
[10] 汪国杰,潘慧铭. 锂离子电池用胶粘剂[J]. 粘接, 2000, 21(5): 6?8.
WANG Guo-jie, PAN Hui-ming. Review on binders for Li ion battery[J]. Technology on Adhesion & Sealing, 2000, 21(5): 6?8.
[11] 化工百科全书编委会. 树脂与塑料-化工百科全书[M]. 北京:化学工业出版社, 2002: 723?790.
Committee of the Encyclopaedia of Chemical Industry. Resins and plastics-the encyclopaedia of chemical industry[M]. Beijing: Chemistry Industry Press, 2002: 723?790.
[12] 曹笃盟,李志友,周科朝. 锂离子电池正极材料热稳定性研究进展[J]. 材料导报, 2003, 17(9): 51?53.
CAO Du-meng, LI Zhi-you, ZHOU Ke-chao. Developments in research on thermal stability of positive materials for lithium-ion batteries[J]. Materials Review, 2003, 17(9): 51?53.
[13] 南俊民,韩东梅,崔 民,左晓希. 溶剂萃取法从废旧锂离子电池中回收有价金属[J]. 电池,2004, 34(4): 309?311.
NAN Jun-min, HAN Dong-mei, CUI Min, ZUO Xiao-xi. Recycling of valuable metal from spent Li-ion batteries by solvent extraction[J]. Battery Bimonthly, 2004, 34(4): 309?311.
收稿日期:2006-09-29;修订日期:2006-12-14
通讯作者:卢毅屏,副教授;电话:0731-8830913;E-mail:luyp309@sohu.com
(编辑 龙怀中)