纳米Fe3O4包覆结构及其磁流体稳定性
崔升1, 2,林本兰1, 3,沈晓冬1
(1. 南京工业大学 材料科学与工程学院,江苏 南京,210009;
2. 佐治亚理工学院 土木与环境学院,美国 亚特兰大,30332;
3. 南京工业大学 学报编辑部,江苏 南京,210009)
摘要:采用化学共沉淀法制备纳米Fe3O4粉体,通过机械球磨的方法研究阴离子表面活性剂十二烷基苯磺酸纳(SDBS)的用量对纳米Fe3O4的包覆结构及其磁流体分散效果的影响。对制得的样品经过XRD,HRTEM,FT-IR和XPS等进行表征。在50 mL水溶液中, 当纳米Fe3O4质量为10 g,pH为4.5和球磨时间为5 h时,SDBS最佳用量为0.8 g;SDBS以化学和物理吸附在尖晶石结构的Fe3O4纳米颗粒表面,形成了Fe-O-S化学键使得纳米颗粒表面的包覆结构很难被打破,制得的磁流体具有较强的稳定性。
关键词:Fe3O4磁流体;十二烷基苯磺酸钠;包覆结构;分散稳定性
中图分类号:TQ138.1 文献标志码:A 文章编号:1672-7207(2011)06-1593-06
Covering structure and stability of nanosized Fe3O4 magnetic liquid
CUI Sheng1, 2, LIN Ben-lan1, 3, SHEN Xiao-dong1
(1. College of Materials Science and Technology, Nanjing University of Technology, Nanjing 210009, China;
2. School of Civil and Environmental Engineering, Georgia Institute of Technology, Atlanta 30332, United States;
3. Journal Editorial Board, Nanjing University of Technology, Nanjing 210009, China)
Abstract: Fe3O4 nanoparticles were prepared with Chemical co-precipitation method. The covering structure and stability of nanosized Fe3O4 magnetic liquid effected by the dosage of sodium dodecyl benzene sulfonate (SDBS) were investigated with ball milling method. The samples were measured by X-ray diffraction (XRD), high-resolution retransmission electron microscopy (HRTEM), Fourier transform infrared spectrometry (FT-IR) and X-ray photoelectron spectroscopy(XPS). The results show that when the mass of Fe3O4 is 10 g in 50 mL water, pH is 4.5 and the ball milling time is 5 h, the better dosage of SDBS is 0.8 g. SDBS is adsorbed on the surface of Fe3O4 nanoparticles with spinel structure by multi-layer chemisorption and physisorption. And the Fe-O-S chemical bond makes the encasing structure difficult to be broken, and the magnetic liquid is stable.
Key words: Fe3O4 magnetic liquid; sodium dodecyl benzene sulfonate (SDBS); covering structure; dispersion stability
纳米颗粒的表面效应和界面效应导致纳米颗粒表面具有较大的自由能,因此,颗粒发生团聚从而降低自由能是一种自发的过程[1-3]。表面活性剂常用于纳米颗粒表面修饰和分散的过程中,用于制得稳定分散的纳米浆料[4]。表面活性剂一般由亲水基和疏水基组成,在油性溶液中表面活性剂的极性官能团吸附到纳米颗粒表面,非极性的官能团与油性介质相溶合[5-6]。反之,在水溶液中,人们认为表面活性剂的非极性亲油基吸附到颗粒表面,而极性的亲水基团与水相溶,达到分散的目的[7-8]。Vladimirov等[9-11]认为:亲水基吸附在纳米颗粒表面, 疏水基伸向溶剂产生空间位阻效应,改善分散状况。然而,以纳米Fe3O4颗粒在水溶液中分散为例,发现阴离子表面活性剂十二烷基苯磺酸纳(SDBS)在纳米Fe3O4表面的包覆情况随着SDBS用量(质量,下同)的变化发生很大的变化;同时,SDBS还作为分散剂对浆料的分散稳定性起很大的作用。在此,本文作者首先采用化学共沉淀法制得纳米Fe3O4粒子,然后,通过机械球磨法研究SDBS用量对纳米Fe3O4颗粒表面包裹结构以及纳米Fe3O4磁流体分散效果的影响。
1 实验
1.1 实验原料和仪器
(1) 实验原料:FeCl3?6H2O,分析纯,上海分析厂生产;FeCl2?4H2O,分析纯,上海分析厂生产;十二烷基苯磺酸钠,上海凌峰化学试剂有限公司生产;盐酸,分析纯,上海化学试剂有限公司生产;氨水,分析纯,上海化学试剂有限公司生产。
(2) 仪器:501A型超级恒温器,上海实验仪器厂有限公司制造;XQM-2L变频行星式球磨机,南京科析实验仪器研究所制造;TGL-16M型高速台式冷冻离心机,长沙湘仪离心机仪器有限公司制造;PHS-2S型数显pH计,上海精密科学仪器有限公司制造;JY98-3D型超声波细胞粉碎机,宁波新芝生物科技股份有限公司制造;722s可见光分光光度计,上海棱光技术有限公司制造;X’TRAX-射线衍射仪,瑞士制造;Nexus670型富氏变换红外-拉曼光谱仪,美国Nicolet公司制造;ESCALAB MK-II光电子能谱测试仪,英国VG公司制造;Zetasizer3000电位粒径分布仪,英国Malven公司制造;JSM-2010UHR型高分辨透射电镜,日本电子公司制造。
1.2 纳米Fe3O4磁流体的制备
采用化学共沉淀法制备纳米Fe3O4水基磁流 体[12]。将FeCl2?4H2O和FeCl3?6H2O混合,并使物质的量比n(Fe2+):n(Fe3+)=2:1,加入到200 mL蒸馏水中,在强烈搅拌的同时缓慢滴加沉淀剂NH3?H2O,至pH=9。然后,将溶液移入三口烧瓶中,恒温水浴晶化 1 h。用磁铁分离出磁性颗粒,用去离子水多次洗涤,于50 ℃烘箱烘干,得到黑色磁性颗粒。
采用机械球磨法,研究SDBS用量对纳米Fe3O4的包覆结构及其磁流体分散效果的影响。取蒸馏水 50 mL加入纳米Fe3O4,使其固含量(质量浓度)为 5 g/mL,加入HCl调节溶液的pH为4.5, SDBS用量分别取0,0.30,0.50,0.65,0.80,1.00,1.30,1.50,2.00,3.00和4.00 g。在300 r/min转速下球磨时间5 h,分别制得纳米Fe3O4磁流体。
1.3 纳米Fe3O4颗粒及其磁流体的表征
将稳定分散的纳米Fe3O4磁流体经多次离心洗涤、干燥,制得表面处理的纳米Fe3O4颗粒。采用X线衍射分析(XRD)、高分辨透射电镜(HRTEM)、傅里叶转换红外光谱分析(FT-IR)和X线光电子能谱(XPS)对SDBS表面处理前后纳米Fe3O4颗粒的结构、形貌、表面基团和表面化学组成等进行表征。
采用分光光度计法、静态沉降高度法、器皿法和表面Zeta法表征纳米Fe3O4磁流体的分散稳定性能。分光光度计是利用“溶液的质量浓度越大,对光的散射越强,光透过率越低,对应的吸光度越大”的原理来评价溶液中质量浓度。在相同的工艺条件下,得到磁流体中稳定分散的Fe3O4质量浓度越大,说明磁流体分散性能越好。因此,可以通过分光光度计测量磁流体对光的透过性来评价磁流体的分散稳定性能。在实验过程中首先确定检测波长。图1所示为不同检测波长下试样对光的透过率。从图1可见:试样对400 nm左右的光吸收最强(即透过率最低)。同时,将SDBS直接溶于水溶液中,测各个波长处的吸光度,结果发现吸光度基本保持不变且为0。因此,选择检测波长为400 nm,对采用分光光度计法测得的纳米Fe3O4磁流体的分散稳定性能进行研究。

图1 Fe3O4磁流体的透过率与波长的关系
Fig.1 Relationship between transparence and wavelength of Fe3O4 magnetic liquid
静态沉降法是指具有可比性的溶液在相同的具有密集刻度的容器中静置一段时间后,根据溶液中固体悬浮物的沉降高度来判断溶液的分散稳定性。把沉降的粉体堆积试管底部的高度记为沉降高度。沉降高度越小,说明沉降的粉体质量越小。溶液中稳定分散的粉体质量越大,则溶液的分散稳定性越强。固体悬浮物在溶液中的沉降速度与固体粒子的粒径、密度、溶剂的黏度以及固体粒子和溶剂间的相互作用力相关。在其他条件相同时,固体粒子间排斥力和固体粒子与溶剂间相互作用力越大,粒子的沉降速度越慢,沉降高度越小,即溶液的分散稳定性越好。因此,可以根据静置后溶液的沉降高度来判断溶液的分散稳定性。
2 结果与讨论
2.1 纳米Fe3O4磁流体的分散性能
在检测波长为400 nm时,测得Fe3O4磁流体吸光度与SDBS用量关系曲线,见图2(a)。将SDBS不同添加量的磁流体静置50 d,测得纳米Fe3O4磁流体的沉降高度与SDBS用量的关系,见图2(b)。器皿法是指将制得的磁流体经高速离心后,取上清液于蒸发器皿中,于高温(100 ℃左右)烘干,测得蒸发皿的质量变化量即为溶液中残留没有包覆在Fe3O4颗粒表面的SDBS质量,样品测试结果见图2(c)。根据上清液SDBS的质量计算SDBS在粉体和溶液中的质量。其中,S0,S1和S2分别为加入SDBS的总质量、上清液中SDBS质量和颗粒表面吸附的质量。
每种分散剂都有一最佳加入量,并不是分散剂加入越多越好。当分散剂的用量较小时,颗粒包覆不完全,部分颗粒间发生团聚生成大颗粒;当分散剂恰好将颗粒表面包裹时,分散剂能够最大发挥其位阻稳定和静电稳定作用;当分散剂用量再增大时,多余的高分子长链在体系中可能会相互交连而导致絮凝,使悬浮液体系的稳定性变差[4]。
图2(a)中,L0,L1和L2分别为磁流体未稀释、稀释时和稀释并静置沉降后检测出的吸光度曲线。从图2可看出:当SDBS用量小于0.5 g时,静置50 d后,溶液吸光度几乎为0,沉降高度较高(图2(b));加入的SDBS几乎全部吸附于颗粒表面,但由于加入的SDBS总量小,颗粒表面无法充分包覆形成有效的空间位阻阻止纳米Fe3O4团聚,同时,上清液中几乎无SDBS(图2(c)中S1曲线),不能形成很好的包覆结构阻止纳米粒子接触聚沉,磁流体不稳定。
当SDBS用量增加至约0.5 g时,沉降高度变低(图2(b)),上清液中仍几乎无分散剂(图2(c) 中S1曲线),但颗粒表面的吸附量增加且达到1个峰值,溶液吸光度较大(图2(a)曲线L0);但将液体稀释后,溶液变混浊,测其吸光度陡然增大(见曲线L1),混浊的液体静置数分钟后,颗粒几乎聚集沉降,测其吸光度几乎为0(见曲线L2)。这可能是由于纳米Fe3O4表面形成了如图3(a)所示的单层吸附结构,由于复合微球间表面较强的负电排斥作用阻止了纳米粒子的团聚,稀释溶液时,吸附在颗粒表面的部分SDBS溶于溶液中,使得颗粒表面包覆不完全,纳米粒子间由于布朗运动不停地互相撞击,继而在不完全包覆点形成羟桥进而团聚沉降。
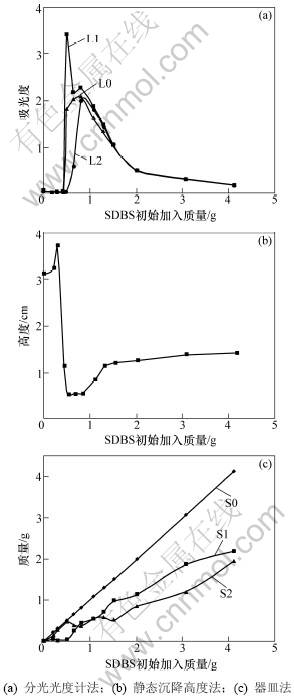
图2 在不同方法下不同SDBS用量对纳米Fe3O4磁流体稳定性的影响
Fig.2 Effect of various SDBS additions on stability of nanosized Fe3O4 magnetic liquid with different methods
当SDBS用量增加至0.8 g左右时,磁流体被水稀释30倍后,仍未发现有聚沉的现象,且此时上清液中SDBS质量与颗粒表面吸附量相当(图2(c))。说明此时一部分SDBS在颗粒表面形成较强的化学吸附,一部分在颗粒表面形成较弱的物理吸附甚至以游离形式存在溶液中形成如图3(b)所示的双层吸附结构。稀释溶液时,颗粒表面吸附较弱的SDBS溶于溶液中,但由于在处理过程中SDBS与颗粒表面形成了较强的化学吸附,较强空间位阻作用阻止了颗粒之间靠近团聚,因此,多次稀释未见溶液混浊。在实验过程中还发现未添加SDBS的磁流体Zeta电位为+7 mV,而SDBS用量为1.0 g时测得Zeta电位为-18.7 mV,这说明Fe3O4颗粒表面正电荷被中和后又继续吸附SDBS使得Fe3O4颗粒表面带负电荷,同时,空间位阻效应也增强,较强的静电和空间位阻效应使得磁流体非常稳定。
当SDBS用量大于0.8 g时,随着SDBS用量的增大,纳米磁流体的稳定性反而下降。这是由于过多的高分子长链相互交连,导致絮凝,因而系统稳定性降低。
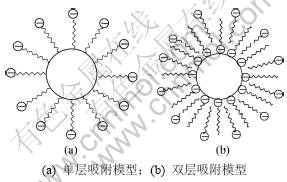
图3 纳米Fe3O4颗粒在水溶液中的分散模型
Fig.3 Dispersion models of Fe3O4 nanoparticles in water
2.2 XRD分析
图4所示为SDBS表面处理前、后纳米Fe3O4颗粒的XRD谱。由图4可见:表面处理前、后的颗粒均为尖晶石结构Fe3O4,由谢乐公式计算出处理前、后Fe3O4晶体颗粒平均粒径分别为17.9 nm和18.1 nm,晶粒粒径相差很小,说明表面改性过程对颗粒的晶粒粒度几乎没有影响。
2.3 HRTEM形貌分析
图5(a)和(b)所示为经SDBS处理后的纳米Fe3O4磁流体显微图像,图5(c)所示为未经表面处理的纳米Fe3O4磁流体显微图像。图中呈网格状明暗相间晶格条纹的为Fe3O4晶体颗粒,且晶粒粒径为10~20 nm。图5(b)中晶格条纹之间和边缘存在无定型物质,起到了空间位阻作用;而图5(c)中没有无定型物质,颗粒间容易发生聚集团聚。从图5(a)还可看出:浆料中纳米Fe3O4颗粒基本呈球形,颗粒粒径很小,单个晶粒粒径为十几纳米,粒径分布均匀。

图4 表面处理前后纳米Fe3O4颗粒的XRD谱
Fig.4 XRD patterns of unmodified and modified Fe3O4 nanoparticles

图5 表面处理前后纳米Fe3O4颗粒的HRTEM像
Fig.5 HRTEM images of unmodified and modified Fe3O4 nanoparticles
2.4 红外光谱分析
不同SDBS添加量对应的纳米Fe3O4颗粒的红外光谱如图6所示。从图6可见:在2 960~2 850,1 650~ 1 450和900~650 cm-1处出现了吸收峰,分别对应烷烃的C—H伸缩振动、苯环的C—C伸缩振动以及苯环的C—H同位相面外弯曲振动;在1 300~1 000 cm-1之间的吸收带是O=S=O和S—O振动引起的。且随着SDBS的加入量增加,这些峰的吸收强度相对增加,说明HRTEM图中的无定形物质为SDBS。通过XPS表面元素分析,得表面改性后纳米Fe3O4颗粒表面S质量分数为3.7%。
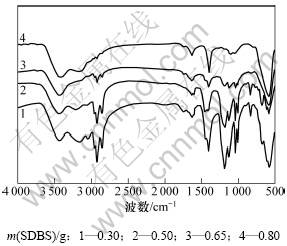
图6 纳米Fe3O4颗粒FT-IR谱
Fig.6 FT-IR spectra of Fe3O4 nanoparticles
2.5 XPS分析
采用XPS对SDBS改性前、后的纳米Fe3O4颗粒进行表征,结果见图7~9。由于Fe元素的电负性远小于S元素的电负性,S通过O使Fe与O之间的电子云偏向于S与O之间,造成Fe周围的电子密度急剧减小,屏蔽效应相应减小,因而使得Fe元素电子结合能由711 eV增加至713.45 eV(见图8);同时,O元素电子结合能由529.90 eV增加至530.50 eV,偏移了0.60 eV(见图9)。这2个吸收峰偏移说明SDBS在Fe3O4颗粒表面形成了Fe—O—S键,使得SDBS加入总质量为0.8 g时制得磁流体在稀释30倍的过程中没有发生混浊和聚沉现象;但这2个吸收峰都较弱,这是SDBS包裹层的厚度很薄(从HRTEM可以看出Fe3O4颗粒表面包裹层为2 nm左右,而且其中有部分为物理吸附在Fe3O4颗粒表面的SDBS),而XPS的探测深度为5 nm左右,所以,导致2个偏移的吸收峰都较弱。这也说明SDBS在Fe3O4颗粒表面是通过化学键合和物理吸附相结合,在水溶液中达到分散改性的目的。
由于纳米Fe3O4颗粒表面带有较多的—OH,在酸性条件下极容易吸附H+并在Fe3O4颗粒表面形成 Fe—OH2+。当SDBS加入水溶液中时,水解出十二烷基苯磺酸根离子,该离子极易通过静电作用吸附在 Fe—OH2+的表面。当外加一高能如高能机械力处理时,使其越过其反应势垒,而释放出H2O,从而在纳米Fe3O4表面形成了很强的化学键Fe—O—S键;因此,SDBS在纳米Fe3O4颗粒表面以多层化学和物理吸附的形式通过静电作用和空间位阻作用有效防止纳米粒子团聚,制得稳定分散的纳米Fe3O4磁流体,其静置50 d后无明显沉降现象。
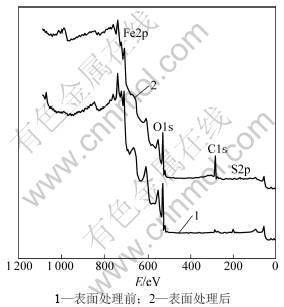
图7 表面处理前、后纳米Fe3O4颗粒的XPS宽程扫描谱
Fig.7 XPS width spectra of unmodified and modified Fe3O4 nanoparticles
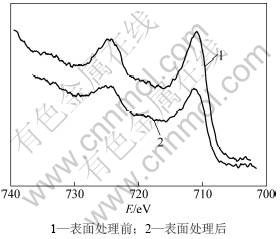
图8 表面处理前、后的Fe2p3/2的电子结合能变化
Fig.8 Variations of binding energy of unmodified and modified Fe2p3/2

图9 表面处理前、后的O1s的电子结合能变化
Fig.9 Variations of binding energy of unmodified and modified O1s
3 结论
(1) 磁流体中纳米Fe3O4的包覆结构和磁流体的稳定性受表面活性剂SDBS用量的影响很大。在SDBS用量很小(<0.5 g)时,颗粒不能被完全包覆,纳米颗粒碰撞团聚;增大SDBS用量(0.5 g)且当颗粒形成单层包覆时,磁流体稳定性迅速增大,但此时稀释磁流体,部分物理吸附的SDBS溶于溶液中,使得纳米颗粒表面包覆变得不完全,磁流体变得不稳定;继续增大SDBS用量(0.8 g),纳米粒子表面形成稳定的多层化学和物理吸附,在稀释过程中仅有少量物理吸附SDBS脱吸附,但还有大量SDBS以多层化学和物理吸附在纳米颗粒表面,使得纳米颗粒的包覆结构不被破坏,此时,稀释作用对磁流体稳定性影响很小。
(2) 表面处理前、后的颗粒均为尖晶石结构Fe3O4颗粒,其晶粒平均粒径均为18 nm左右。纳米Fe3O4颗粒为球形,表面处理后纳米Fe3O4颗粒间无明显团聚现象。
(3) 纳米粒子表面形成了Fe—O—S键,SDBS以化学吸附于颗粒表面,并以多层化学和物理的形式通过静电作用和空间位阻作用有效防止纳米颗粒团聚,制得稳定分散的纳米Fe3O4磁流体,其静置50 d后无明显沉降现象。
参考文献:
[1] Viota J L, Vicente J, Durán J D G., et al. Stabilization of magnetorheological suspensions by polyacrylic acid polymers[J]. Journal of Colloid and Interface Science, 2005, 284(2): 527-541.
[2] Yuary O E, Ahmed Y G. Destabilizing effect of time-dependent oblique magnetic fluid on magnetic fluids streaming in porous media[J]. Journal of Colloid and Interface Science, 2004, 269(1): 224-239.
[3] Gravina P P, Bakuzis A F, Neto K S, et al. Investigation of the pH effect on the stability of biocompatible magnetic fluids using time-dependent birefringence measurements[J]. Journal of Magnetism and Magnetic Materials, 2005, 289(3): 448-451.
[4] 柴立元, 程明明, 彭兵, 等. 十二烷基苯磺酸钠对抗菌陶瓷釉料分散稳定性的影响[J]. 中南大学学报: 自然科学版, 2007, 38(6): 1107-1111.
CHAI Li-yuan, CHENG Ming-ming, PENG Bing, et al. Effect of dodecylbenzenesulfonate on dispersion stability of antibacterial ceramic glaze[J]. Journal of Central South University: Science and Technology, 2007, 38(6): 1107-1111.
[5] Pop L M, Buioca C D, Iusan V, et al. Long-term stability of magnetic fluids in low-gradient magnetic fields[J]. Journal of Magnetism and Magnetic Materials, 2002, 252(11): 46-48.
[6] 顾中强, 王海泉, 陈莉, 等. PMMA/SiO2纳米复合膜表面性能的研究[J]. 南京工业大学学报: 自然科学版, 2007, 29(3): 1-6.
GU Zhong-qiang, WANG Hai-quan, CHEN Li, et al. Surface properties of PMMA/SiO2 nanocomposite films[J]. Journal of Nanjing University of Technology: Natural Science Edition, 2007, 29(3): 1-6.
[7] 王德志, 吴壮志, 梁汛, 等. 表面活性剂对制备纳米 MoS2 颗粒的影响[J]. 中南大学学报: 自然科学版, 2009, 40(3): 676-680.
WANG De-zhi, WU Zhuang-zhi, LIANG Xun,et al. Effect of surfactants on preparation of MoS2 nanoparticles[J]. Journal of Central South University :Science and Technology, 2009, 40(3): 676-680.
[8] Ahmed E R. Magneto-selfgravitational stability of triple superposed fluids layers of different densities[J]. Applied Mathematics and Computation, 2003, 141(2/3): 401-413.
[9] Vladimirov V A, Moffatt H K, Davidson P A, et al. On the stability of a rigid body in a magnetostatic equilibrium[J]. European Journal of Mechanics B/Fluids, 2003, 22(5): 511-523.
[10] 余姗姗, 沈晓冬, 崔升. 均匀沉淀法制备ZAO纳米棒[J]. 南京工业大学学报: 自然科学版, 2009, 31(4): 60-64.
YU Shan-shan, SHEN Xiao-dong, CUI Sheng. Preparation of ZAO nanorods by homogeneous precipitation method[J]. Journal of Nanjing University of Technology: Natural Science Edition, 2009, 31(4): 60-64.
[11] B?nnemann H, Brijoux W, Brinkmann R, et al. A size-selective synthesis of air stable colloidal magnetic cobalt[J]. Inorganica Chimica Acta, 2003, 350(4): 617-624.
[12] 崔升, 沈晓冬, 林本兰. 利用BP神经网络算法优化纳米Fe3O4的合成工艺[J]. 功能材料与器件学报, 2005, 11(4): 435-439.
CUI Sheng, SHEN Xiao-dong, LIN Ben-lan. Optimization of the preparation condition of Fe3O4 nanoparticle by BP nerve network[J]. Journal of Functional Materials and Devices, 2005, 11(4): 435-439.
(编辑 陈灿华)
收稿日期:2010-05-10;修回日期:2010-07-28
基金项目:江苏省自然科学基金重点资助项目(BK2010082)
通信作者:崔升(1980-),男,江苏泗洪人,博士,副教授,从事纳米材料研究;电话:025-83587235;E-mail:scui@njut.edu.cn